Introduction
Although the therapeutic outcomes of patients with
gastric cancer continue to improve, it remains the second leading
cause of cancer-related mortality worldwide (1,2).
Even when undergoing curative resection, almost half of patients
with advanced cancers eventually die of peritoneal recurrence
(3,4), a fact that insinuates the peritoneal
metastatic cascade of gastric cancer and contributes significantly
to gastric cancer-related mortality. To date, no mechanism has been
specified by which gastric carcinoma undergoes peritoneal
carcinomatosis.
Stephen Paget’s ‘seed and soil’ theory of tumor
metastasis may provide a clue useful for further research on
peritoneal carcinomatosis of gastric cancer. This proposes, that
the sites where metastasis occurs are defined not only by the tumor
cells (seed) but also by the local microenvironment of the
metastatic site (soil) (5). This
is to say that the interactions between tumor cells and the local
microenvironment at the secondary site are no less important than
the biological activities of free cancer cells shed from primary
gastric cancer in this process (6). Therefore, peritoneal carcinomatosis
may occur as the peritoneal stroma environment promotes tumor cell
attachment to the peritoneal mesothelium (7). This process requires the interactions
between extracellular matrix proteins and signals produced by
mesothelial cells and the corresponding adhesion molecules from
tumor cells (8).
In our previous study, we demonstrated that
transforming growth factor (TGF-β1) levels in peritoneal lavage
fluid were correlated with the peritoneal metastasis of gastric
cancer and TGF-β1 plays a key role in induction of peritoneal
fibrosis, which in turn affected the adhesion and metastasis of
gastric cancer cells.
βig-h3 is a TGF-β-induced extracellular matrix (ECM)
protein, consisting of 4 fasciclin-1 (fas-1) homologous domains and
an RGD motif at the C-terminus. The fas-1 domain is well-conserved
in several proteins from different species, and has motifs
interacting with the α3β1, α5β3 and α5β5 integins, through which it
mediates adhesion and migration in several cell types (9,10).
The βig-h3 transcript has been detected in a variety of human and
mouse tissues including uterine tissue, heart, breast, prostate,
skeletal muscle, testes, thyroid, kidney, liver and stomach
(11). Some studies have revealed
that βig-h3 expression is substantially elevated in colon and
pancreatic cancers in comparison with corresponding normal tissues
(12,13). The overexpression of βig-h3 in
colon cancer cells promotes tumor metastasis, while the suppression
of βig-h3 expression significantly decreases their metastatic
potential in vitro (13).
In this study, we aimed to confirm whether TGFβ-1
induces peritoneal mesothelium cells to express βig-h3 and whether
βig-h3 is involved in generating a suitable microenvironment for
peritoneal dissemination and whether it induces gastric cancer cell
migration to peritoneal tissue.
Materials and methods
Reagents and instruments
βig-h3 antibodies and secondary antibodies were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The
human TGF-β1, epidermal growth factor (EGF), RT-PCR kit, dimethyl
sulfoxide (DMSO), fibronectin (FN), bovine serum albumin (BSA) and
trypsin were purchased from Sigma (St. Louis, MO, USA). DMEM,
streptomycin and other cell culture supplies were from Gibco BRL
(Grand Island, NY, USA). Fetal bovine serum (FBS) was obtained from
HyClone (Logan, UT, USA). MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltrazolium bromide] was
obtained from Fluka (Ronkonkoma, NY, USA). Human recombinant βig-h3
proteins and a βig-h3 ELISA kit were purchased from R&D
(Minneapolis, MN, USA). A phase contrast microscope (Japan Nikon,
Japan) and a spectrofluorometer (Japan Olympus, Japan) were used.
Other laboratory reagents were obtained from Sigma.
Cell lines and culture
A human peritoneal mesothelial cell line HMrSV5 was
kindly provided by Professor Youming Peng of the Second Hospital,
Zhongnan University, Changsha, China and Professor Pierre Ronco,
Hospital Tenon, Paris, France. This cell line was established after
the infection of a fully characterized primary culture of human
peritoneal mesothelial cells with an amphotropic recombinant
retrovirus that encodes SV40 large-T Ag under the control of a
Moloney virus long terminal repeat. A human gastric carcinoma cell
line, SGC-7901, was obtained from the Cancer Research Institute of
Beijing, China. These cell lines were cultivated in T75 tissue
culture flasks in DMEM supplemented with 10% FBS, 100 U/ml
penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, and 20 mM
hydroxyethyl-piperazineethanesulfonic acid (HEPES). Cultures were
grown at 37°C in a humidified 5% CO2 and 95% air
incubator.
Tissue samples
Human peritoneum tissue samples were obtained from
75 gastric cancer patients and 14 benign disease patients, who
underwent surgery at the First Affiliated Hospital of the China
Medical University from May to October 2011. The benign diseases
included ileus, appendicitis and gastric adenomas. These tissue
specimens were obtained from the lower anterior abdominal wall. No
patients had received any form of radiation or chemotherapy prior
to surgery. The local institutional review board approved our
protocol for using patient samples; all patients provided written
consent prior to participation in the study. All histology slides
were staged and classified according to the UICC new TNM staging
(7th edition) (14) and the serosa
was classified according to Sun et al (15). The peritoneal tissues were directly
obtained from the surgical site and immediately fixed in 10%
buffered formalin and then embedded in paraffin. Sections (5 μm)
were prepared for immunohistochemical staining.
Immunohistochemistry
To reveal the antigens, sections were placed in a
1-mM Tris solution (pH 9.0) supplemented with 0.5 mM EGTA
[ethylenglycol-bis(β-amino-ethylether)-N,N, N′,N′-tetraacetic acid]
and heated using a microwave oven for 10 min. The non-specific
binding of immunoglobulin was prevented by incubating the sections
in 50 mM NH4Cl for 30 min, followed by blocking in PBS
supplemented with 1% BSA, 0.05% saponin and 0.2% gelatin. The
sections were incubated overnight at 4°C with an immune serum
diluted in PBS supplemented with 0.1% BSA and 0.3% Triton X-100
(1:3,000), and labeling was visualized using a horseradish
peroxidase-conjugated secondary antibody. Negative controls
included omitting the primary antibody with normal rabbit IgG at an
equivalent protein concentration.
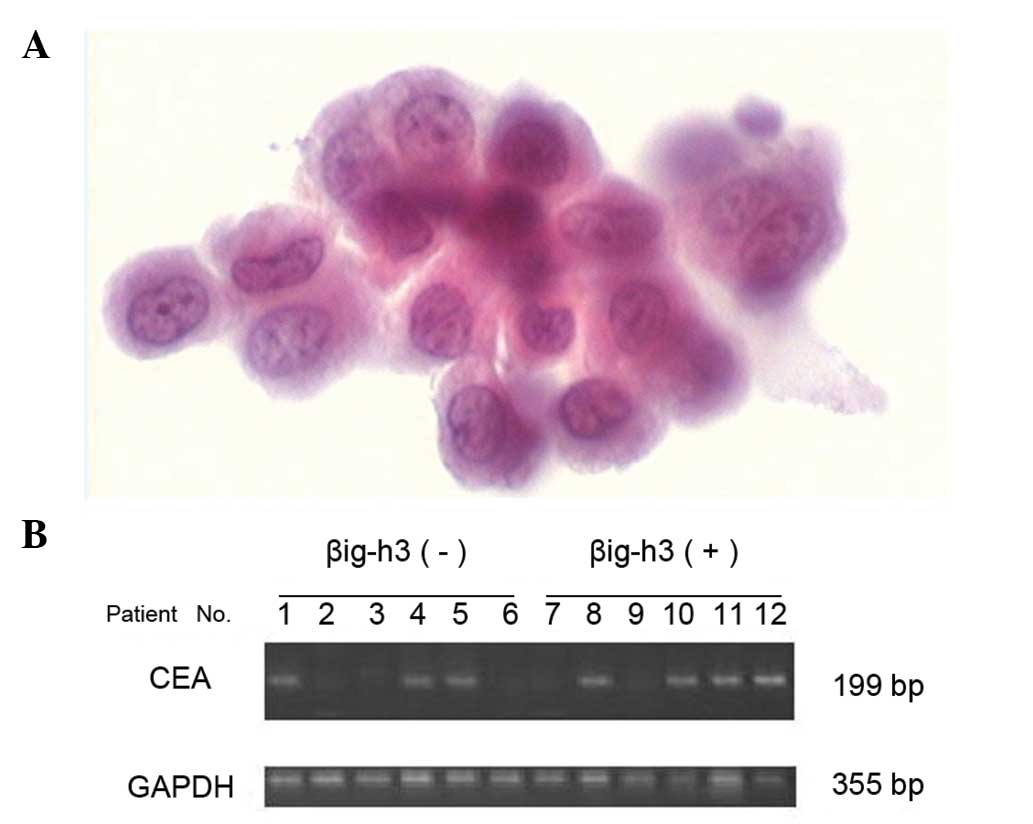
Peritoneal lavage cytological examination
and RT-PCR detection of CEA mRNA levels in the peritoneal lavage
fluid
The peritoneal lavage fluid was collected from each
patient. Briefly, during laparotomy, 100 ml of physiological saline
was injected into the right upper quadrant or the Douglas pouch and
approximately 60 ml was retrieved. The peritoneal lavage sample was
immediately centrifuged at 2000 rpm for 10 min at room temperature.
Approximately one half of each sample was used for cytopathological
examination after conventional Papanicolaou staining; the remainder
was dissolved in Isogen RNA extraction buffer and stored at −80°C
until use. Total RNA was isolated from these cells using the TRIzol
reagent according to the manufacturer’s instructions. A total
amount of 1 mg of the total cellular RNA was then
reverse-transcribed into cDNA for PCR amplification using a kit
from Sigma. The sequences of the primers used were as follows: A:
5′-CATCATGATTGGAGTGCTGGTTG-3′; B: 5′-CACGATGTTGGCTAGGATGGTC-3′.
Amplification consisted of an initial 5-min incubation at 95°C and
then 30 cycles of amplification using 30 sec of denaturation at
95°C, 30 sec at 56°C and 60 sec at 72°C. The final extension was
set for 10 min at 72°C. Samples with visible 199-bp bands were
designated as positive.
ELISA detection of βig-h3 levels in the
supernatants
After the HMrSV5 cells were grown and treated with
or without TGF-β1, the supernatants were collected and immediately
centrifuged at 2000 rpm for 10 min at room temperature and stored
at −80°C until use. The βig-h3 levels were then assayed with a
human βig-h3 ELISA kit according to the manufacturer’s
instructions. Data on the βig-h3 protein levels were expressed as
the means ± SD.
Protein extraction and western
blotting
After the HMrSV5 cells were grown and treated with
or without TGF-β1, the total cellular protein was extracted using a
lysis buffer and quantified by using protein quantification
reagents from Bio-Rad. Next, 50 μg of the protein were suspended in
a 5X reducing sample buffer, boiled for 5 min, electrophoresed on
10% SDS-PAGE gels and then transferred to a polyvinylidene
difluoride membranes by electroblotting. The membrane was blocked
in 1% BSA/0.05% Tween/PBS solution overnight at 4°C, followed by
incubation with the primary antibody for 24 h. A horseradish
peroxidase-labelled goat anti-mouse IgG was used as the secondary
antibody. The blots were then developed by incubation in a
chemiluminescence substrate and exposed to X-ray films.
Immunofluorescence staining
The expression of βig-h3 in HMrSV5 cells was also
analyzed by immunofluorescence microscopy. In brief, after the
cells were grown and treated with or without TGF-β1, they were
cultured on collagen-coated glass coverslips to confluency and then
fixed in 4% paraformaldehyde in 20 mM HEPES (pH 7.4) and 150 mM
NaCl for 20 min. The glas coverslips were rinsed 3 times and
permeabilized with 1.2% Triton X-100 for 5 min, rinsed 3 times
again and then incubated with 1% BSA/0.05% Tween/PBS for 1 h.
Staining for the expression of βig-h3 was carried out with a
primary rabbit antibody anti-βig-h3 (1:200) and then with a
secondary antibody conjugated with FITC. The DNA dye To-PRO-3
(blue) was used for counterstaining. The stained cells were viewed
under an immunofluorescence microscope.
Tumor cell adhesion assay
The cell adhesion assay was performed as described
previously (16). Briefly, 96-well
plates were coated with BSA, βig-h3 and FN (20 μg/ml) diluted in
PBS at 4°C overnight. Then the plates were rinsed with PBS and the
uncoated surfaces were blocked with 2% BSA for 1 h. The SGC-7901
cells were suspended in medium at a density of 4×103
cells/200 μl and added to each well of the coated plates. After
incubation for 1 h at 37°C, unattached cells were removed by
rinsing with PBS, and the absorbance was measured at 570 nm in a
Bio-Rad model 550 microplate reader. Experiments were repeated in
triplicate. Data are reported as the mean ± SD.
Tumor cell migration assay
A cell migration assay was performed using Transwell
plates. The undersurface of the membrane was coated with BSA,
βig-h3 and FN (20 μg/ml) diluted in PBS, at 4°C overnight. Then the
plates were rinsed with PBS and uncoated surfaces were blocked with
2% BSA for 1 h. The SGC-7901 cells (4×104) per well in
200 μl complete medium were seeded in the upper compartment of the
plates. After 6 h of migration, cells in the upper chamber of the
filter were removed and non-migrating cells on the top of the
filters were removed with a cotton swab. SGC-7901 cells on the
lower side of the filter were fixed with 8% glutaraldehyde and then
stained with 0.25% crystal violet in 20% methanol. Each cell
experiment was repeated in triple-wells and for each well the
numbered cells were counted in 9 randomly selected microscopic
high-power fields.
Proliferation assay
A total of 24-well culture plates were coated with
BSA, βig-h3 and FN (20 μg/ml) diluted in PBS at 4°C overnight. Then
the plates were rinsed three times in PBS and uncoated surfaces
were blocked with PBS containing 2% BSA for 1 h at 37°C. The plates
were rinsed again and 5×103 SGC-7901 cells were added to
each well in 1 ml culture medium. Although the initial cell
adhesion efficiency was different depending on the substrates, most
of the cells became adherent within a few hours, thus giving the
same cell numbers. Then, we subjected SGC-7901 cells to serum
starvation for 24 h, which should have brought most of the cells
into the G0 phase of the cell cycle. After incubation for 24 h,
SGC-7901 cell proliferation was assessed by counting cells after
trypsinization using a hematocytometer at 24 h intervals. Cell
numbers at 0 h indicate the numbers at 24 h after the initial cell
seeding, showing that there was no difference in the initial cell
numbers at the 0 h point under different conditions. Experiments
were repeated in triplicates. Data are reported as the mean ±
SD.
Statistical analysis
All the statistical analyses were carried out with
the SPSS 16.0 statistical package (SPSS Inc., Chicago, IL, USA).
All data were summarized as the mean ± SD, where appropriate. The
two-tailed χ2 test or Student’s t-test was performed in
order to compare the different groups. Differences were considered
statistically significant at a p-value ≤0.05.
Results
βig-h3 expression of peritoneal
mesothelial cells in gastric cancer patients and its relation to
pathological factors
Histological sections were examined to localize
βig-h3 expression in peritoneum tissue. Immunohistochemical
staining showed that there was a positive staining in the
peritoneal mesothelial cells (Fig.
1). βig-h3 was confirmed positive in the peritoneal tissue in
29 patients with gastric cancer and in 1 with benign lesions, the
difference being significant (p=0.03). In the gastric cancer group,
the positive rate of βig-h3 was significantly higher in the more
invasive and advanced serous type subgroups (Table I).
 | Table IComparison of βig-h3 expression and
various clinicopathologic features of the gastric cancer cases. |
Table I
Comparison of βig-h3 expression and
various clinicopathologic features of the gastric cancer cases.
| Clinicopathological
features | Expression of βig-h3
in peritoneal mesothelial cells | p-value |
|---|
|
| |
|---|
| − | + | |
|---|
| Histologic grade | | | 0.555 |
| Differentiated | 19 | 10 | |
|
Undifferentiated | 27 | 19 | |
| Lauren grade | | | 0.362 |
| Intestinal | 22 | 17 | |
| Diffuse | 24 | 12 | |
| Invasive depth | | | 0.016 |
| T1, T2, T3 | 32 | 12 | |
| T4 | 14 | 17 | |
| Lymph node
metastasis | | | 0.338 |
| Negative | 21 | 16 | |
| Positive | 25 | 12 | |
| Types of serosa | | | |
| Normal and reactive
type | 13 | 3 | 0.037 |
| Nodular type | 19 | 9 | |
|
Tendonoid/color-diffused type | 14 | 17 | |
Association of βig-h3 expression and
various factors indicating peritoneal metastasis
In the gastric cancer group, there were 13 patients
with visible peritoneal metastasis, 20 with PLC(+) and 32 with CEA
mRNA(+) (Fig. 2). The positive
rate of βig-h3 was significantly higher in the subgroups with
visible peritoneal metastasis, PLC(+) or CEA mRNA(+) (p<0.05)
(Table II).
 | Table IIAssociation between the expression of
βig-h3 and various factors indicating peritoneal metastasis. |
Table II
Association between the expression of
βig-h3 and various factors indicating peritoneal metastasis.
| Factors indicating
peritoneal metastasis | Expression of βig-h3
in peritoneal mesothelial cells | p-value |
|---|
|
| |
|---|
| − | + | |
|---|
| Visible peritoneal
metastasis at surgery | | | 0.002 |
| Negative | 43 | 19 | |
| Positive | 3 | 10 | |
| PLC | | | 0.005 |
| Negative (−) | 39 | 16 | |
| Positive (+) | 7 | 13 | |
| CEA mRNA | | | 0.027 |
| Negative (−) | 31 | 12 | |
| Positive (+) | 15 | 17 | |
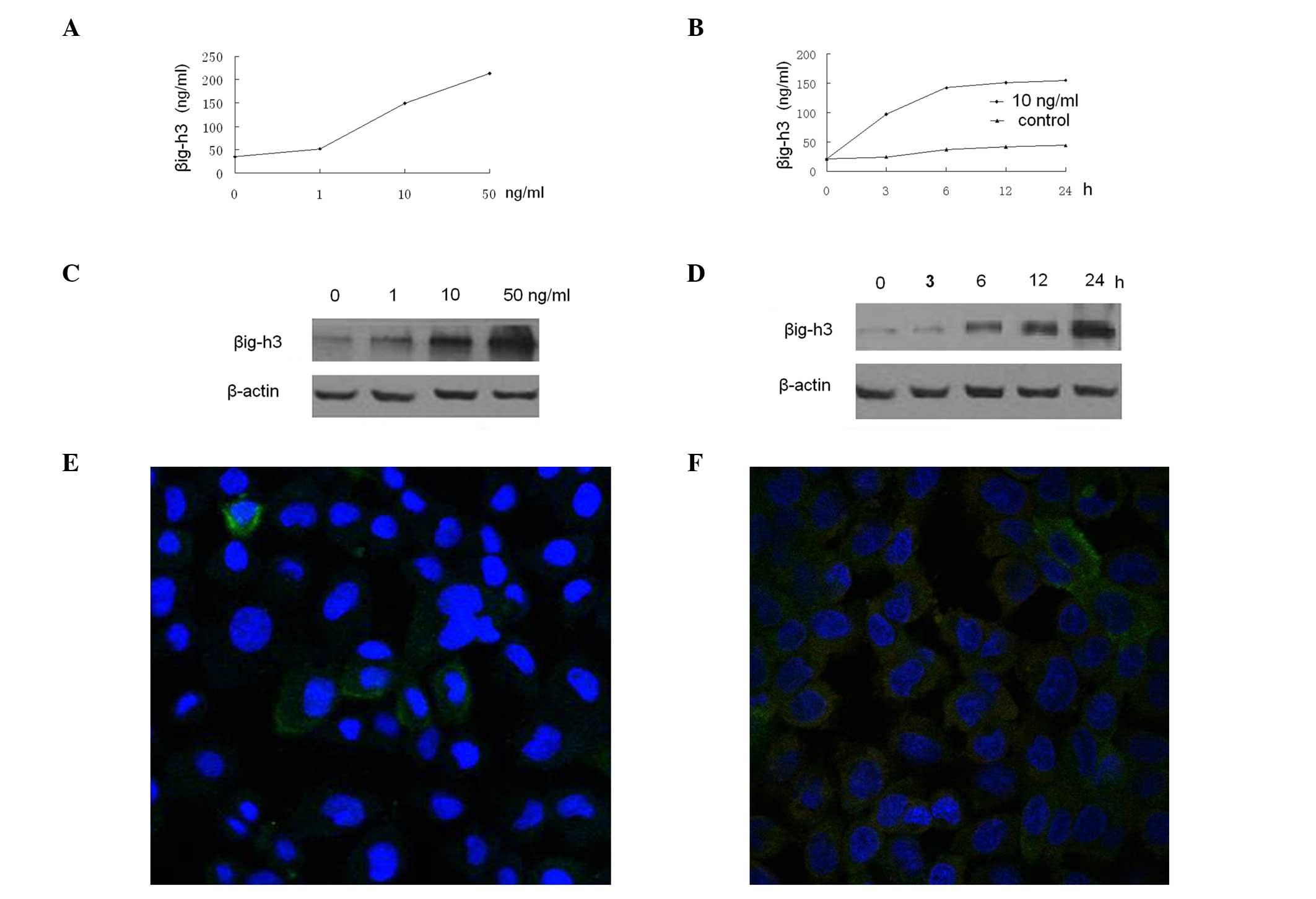
Expression of βig-h3 is induced by TGF-β1
in HMrSV5 cells
βig-h3 is known as one of the target genes of TGF-β.
To determine whether TGF-β1 induces βig-h3 production in HMrSV5
cells, the βig-h3 protein levels were measured by ELISA in the
culture supernatants of HMrSV5 cells incubated with various
concentrations of TGF-β1 for different period of time. TGF-β1
increased the βig-h3 protein levels in the culture supernatants in
dose- and time-dependent manners (Fig.
3A and B). The maximum increase was observed at 50 ng/ml and 24
h. A similar result was observed in the western blot analysis of
HMrSV5 cells (Fig. 3C and D). We
assessed whether EGF affected βig-h3 expression in HMrSV5 cells.
Different concentrations of recombinant human EGF (Sigma) ranging
from 1 to 50 ng/ml were added and incubation was carried out for 48
h, resulting in no effect on the βig-h3 production by HMrSV5 cells
at any concentration (data not shown). In addition, we used an
immunofluorescence microscopy to confirm the protein data; the
TGF-β1-treated HMrSV5 cells exhibited a higher level of βig-h3 than
the control (Fig. 3E and F).
βig-h3 supports the adhesion, migration,
and proliferation of HMrSV5 cells
Since βig-h3 mediates the adhesion of several cell
types (10,11,16),
its ability to mediate the adhesion of HMrSV5 cells was examined by
using a recombinant βig-h3 protein. In addition, the effects of
βig-h3 on the migration and proliferation of the SGC-7901 cells
were also examined. For the cell adhesion assay, a cell culture
plate with recombinant βig-h3 protein was used. As shown in
Fig. 4A, βig-h3 significantly
increased SGC-7901 cell adhesion compared to BSA, and there was no
significant difference between βig-h3 and FN. Subsequently, the
ability of βig-h3 to mediate the SGC-7901 cells proliferation was
tested. The βig-h3 had the strongest ability to induce
proliferation, followed by FN and BSA in descending order (Fig. 4B) and the difference was
significant. In the migration assay, βig-h3 and FN significantly
increased the SGC-7901 cell migration compared to BSA (Fig. 4C–E). Cells seeded on βig-h3-coated
culture plates showed a marked increase in number compared to those
seeded on BSA-coated plates.
Discussion
In the present study, we demonstrated, that βig-h3
was expressed in mesothelial cells, especially in patients with
advanced gastric cancer. The positive rate of βig-h3 was
significantly higher in the more invasive and advanced serous-type,
with visible peritoneal metastasis, in PLC(+) and CEA mRNA(+)
subgroups. All the data indicated the expression of βig-h3 in
mesothelial cells were closely related to peritoneal metastasis.
Our study subsequently showed, that the expression of βig-h3
increased gradually with elevated TGF-β1 concentrations and in a
time- and dose-dependent manner. βig-h3 induced HMrSV5 cell
adhesion and significantly increased its migration and
proliferation.
Stephen Paget’s ‘seed and soil’ theory of tumor
metastasis has been adopted by most scholars. To date, however,
most of the relevant studies focus more on the ‘seed’ rather than
the ‘soil’. It is generally believed, that gastric cancer cells
first acquire some particular ability to readily physically invade
the peritoneal cavity occupying a unique position to eventually
metastasize to the peritoneum. However, a more complicated process
may be involved. For example, peritoneal mesothelial cells may also
change in favor of implantation of gastric cancer cells to
peritoneal tissue (7). In this
study, we observed the expression of βig-h3 in peritoneal
mesothelial cells and the positive rate of βig-h3 was significantly
higher in the more invasive and advanced serous-type subgroups.
Previously, the depth of invasion and the serosal changes were
reported to be significant risk factors for the prediction of
peritoneal recurrence (15,17).
Thus, we proposed the hypothesis that the βig-h3 expression in
peritoneal mesothelial cells in gastric cancer patients may be a
marker of the biological behavior of gastric cancer and could
predict peritoneal metastasis. To confirm our hypothesis, we
further evaluated the relationship between the expression of βig-h3
and the other factors indicating peritoneal metastasis. The
cytologic examination of the lavage fluid obtained during surgery
is a conventional method to detect free cancer cells in the
peritoneal space and is considered a gold standard for predicting
peritoneal metastasis (18). In
recent years, however, some investigations have demonstrated that
the CEA RT-PCR analysis of peritoneal lavage fluids was more
sensitive than conventional cytology (17,19).
Thus, both examinations were performed. Our study demonstrated,
that the positive rate of βig-h3 was significantly higher in the
visible peritoneal metastasis PLC(+) and CEA mRNA(+) subgroups. The
results further support our hypothesis.
βig-h3 is an extracellular matrix protein, which was
first identified as a gene induced in A549 cells, after treatment
with TGF-β1, and was subsequently reported to be present in several
cell types including skin fibroblasts (6), corneal epithelial cells, and
chondrocytes (9–11). We first proved it was present in
human peritoneal mesothelial cells by immunohistochemical staining.
To further confirm our conclusion, an in vitro experiment
was performed. The result showed that TGF-β1 increased the βig-h3
protein levels in the culture supernatants of HMrSV5 cells in a
dose- and time-dependent manner. A similar result was observed in
western blot analysis and immunofluorescence staining in evaluating
the βig-h3 protein levels in HMrSV5 cells. Our previous study
showed, that TGF-β1 levels in the peritoneal wash-fluid were
significantly higher in patients with gastric cancer than in those
with benign disease and increased along with the development of the
disease (20–22). Our current study indicated that the
TGF-β1 levels in the peritoneal wash fluid might play a key role in
promoting peritoneal mesothelial cells to express βig-h3. We have
also noted that the concentration of TGF-β1 in the peritoneal
wash-fluid was lower than the one used in vitro to treat
mesothelial cells. This may be attributed to the natural
differences between in vivo and in vitro experiments.
In addition, another substance in the peritoneal wash-fluid,
secreted by gastric cancer cells, may have also contribute to this
effect.
It is understood that the attachment of malignant
cells to the peritoneal mesothelium is a critical step in the
peritoneal dissemination of a disease (5,7).
Previous studies have suggested that this process is mediated by
the interaction between the extracellular matrix and the
corresponding adhesion molecules from the gastric cancer cells
(8). Moreover, the extracellular
matrix may serve to anchor the cancer cells (7,23,24).
Although the biological roles of the βig-h3 are largely unknown,
the most extensive literature regarding βig-h3 to date suggests
that it acts as a cell adhesion substrate, regulates cell growth,
interconnects other matrix components and transduces TGF-β-mediated
signaling. Several recent studies have revealed, that βig-h3
regulates cell growth and migration in colorectal and pancreatic
cancer cells (12,13). However, little is known about the
effect of βig-h3 on gastric cancer cells. Our results showed that
βig-h3 induces gastric cancer cell adhesion, migration and
proliferation. βig-h3 activities on HMrSV5 cell adhesion and
migration were comparable with those of FN, although the activity
of βig-h3 was somewhat lower than that of FN. Notably, the βig-h3
exhibited a stronger ability to induce SGC-7901 cell proliferation
than FN. All the above findings indicate a possible role for βig-h3
in the development of gastric cancer, promoting a suitable
environment for gastric cancer cell adhesion, migration,
proliferation and finally peritoneal metastasis.
In conclusion, peritoneal mesothelial cells do
express βig-h3. TGF-β1 increased the βig-h3 protein levels both in
the culture supernatants and in the HMrSV5 cells in a dose- and
time-dependent manner in vitro. βig-h3 induced gastric cell
adhesion, migration and proliferation. These data suggest, that
βig-h3 expression in peritoneal mesothelial cells in gastric cancer
patients may be a marker of biological behavior of gastric cancer
and play an important role in the process of peritoneal
carcinomatosis. These data provide a sound scientific rationale for
further investigation into the use of βig-h3 as a therapeutic
target for peritoneal metastasis of gastric cancer.
Acknowledgements
This study was financed by the National Natural
Science Foundation of China (nos. 30873043, 30901419 and 81071956).
The authors thank Professor Feng Li for the technical assistance
and the precious advice.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar
|
|
2
|
Goggins WB and Wong GK: Poor survival for
US Pacific Islander cancer patients: evidence from the
Surveillance, Epidemiology, and End Results database: 1991 to 2004.
J Clin Oncol. 25:5738–5741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
D’Angelica M, Gonen M, Brennan MF,
Turnbull AD, Bains M and Karpeh MS: Patterns of initial recurrence
in completely resected gastric adenocarcinoma. Ann Surg.
240:808–816. 2004.PubMed/NCBI
|
|
4
|
Roviello F, Marrelli D, Manzoni G,
Morgagni P, Di Leo A, Saragoni L and De Stefano A: Italian Research
Group for Gastric cancer: Prospective study of peritoneal
recurrence after curative surgery for gastric cancer. Br J Surg.
90:1113–1119. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paget S: The distribution of secondary
growths in cancer of the breast. Cancer Metastasis Rev. 8:98–101.
1989.PubMed/NCBI
|
|
6
|
Chau I, Norman AR and Cunningham D:
Multivariate prognostic factor analysis in locally advanced and
metastatic esophago-gastric cancer-pooled analysis from three
multicenter, randomized, controlled trials using individual patient
data. J Clin Oncol. 22:2395–2403. 2004. View Article : Google Scholar
|
|
7
|
Yashiro M, Chung YS, Nishimura S, Inoue T
and Sowa M: Fibrosis in the peritoneum induces by scirrhous gastric
cancer cells may act as ‘soil’ for peritoneal dissemination.
Cancer. 77:1668–1675. 1996.PubMed/NCBI
|
|
8
|
Rieppi M, Vergani V, Gatto C, Zanetta G,
Allavena P, Taraboletti G and Giavazzi R: Mesothelial cells induce
the motility of human ovarian carcinoma cells. Int J Cancer.
80:303–307. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Skonier J, Bennett K, Rothwell V, Kosowski
S, Plowman G, Wallace P, Edelhoff S, Disteche C, Neubauer M,
Marquardt H, et al: βeta ig-h3: a transforming growth
factor-beta-responsive gene encoding a secreted protein that
inhibits cell attachment in vitro and suppresses the growth of CHO
cells in nude mice. DNA Cell Biol. 13:571–584. 1994.
|
|
10
|
Kawamoto T, Noshiro M, Shen M, et al:
Structural and phylogenetic analyses of RGD-CAP/betaig-h3, a
fasciclin-like adhesion protein expressed in chick chondrocytes.
Biochim Biophys Acta. 1395:288–292. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Skonier J, Neubauer M, Madisen L, Bennett
K, Plowman GD and Purchio AF: cDNA cloning and sequence analysis of
betaig-h3, a novel gene induced in a human adenocarcinoma cell line
after treatment with transforming growth factor-beta. DNA Cell
Biol. 11:511–522. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bhowmick NA, Neilson EG and Moses HL:
Stromal fibroblasts in cancer initiation and progression. Nature.
432:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma C, Rong Y, Radiloff DR, Datto MB,
Centeno B, Bao S, Cheng AW, Lin F, Jiang S, Yeatman TJ and Wang XF:
Extracellular matrix protein betaig-h3/TGFBI promotes metastasis of
colon cancer by enhancing cell extravasation. Genes Dev.
22:308–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
National Comprehensive Cancer Network.
http://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.
|
|
15
|
Sun Z, Xu YY, Wang ZN, Zhu Z, Zhang H,
Huang BJ, Xu Y, Chen JQ and Xu HM: Macroscopic serosal
classification predicts peritoneal recurrence for patients with
gastric cancer underwent potentially curative surgery. Ann Surg
Oncol. 18:1068–1080. 2011. View Article : Google Scholar
|
|
16
|
Ha SW, Bae JS, Yeo HJ, Lee SH, Choi JY,
Sohn YK, Kim JG, Kim IS and Kim BW: TGF-beta-induced protein
betaig-h3 is upregulated by high glucose in vascular smooth muscle
cells. J Cell Biochem. 88:774–782. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roukos DH, Lorenz M, Karakostas K,
Paraschou P, Batsis C and Kappas AM: Pathological serosa and
node-based classification accurately predicts gastric cancer
recurrence risk and outcome, and determines potential and
limitation of a Japanese-style extensive surgery for Western
patients: a prospective with quality control 10-year follow-up
study. Br J Cancer. 84:1602–1609. 2001.
|
|
18
|
Bando E, Yonemura Y, Takeshita Y,
Taniguchi K, Yasui T, Yoshimitsu Y, Fushida S, Fujimura T,
Nishimura G and Miwa K: Intraoperative lavage for cytological
examination in 1,297 patients with gastric carcinoma. Am J Surg.
178:256–262. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kodera Y, Nakanishi H, Yamamura Y, Shimizu
Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T, Kito T and
Tatematsu M: Prognostic value and clinical implications of
disseminated cancer cells in the peritoneal cavity detected by
reverse transcriptase-polymerase chain reaction and cytology. Int J
Cancer. 79:429–433. 1998. View Article : Google Scholar
|
|
20
|
Lv ZD, Na D, Liu FN, Du ZM, Sun Z, Li Z,
Ma XY, Wang ZN and Xu HM: Induction of gastric cancer cell adhesion
through transforming growth factor-beta1-mediated peritoneal
fibrosis. J Exp Clin Cancer Res. 29:129–139. 2010.PubMed/NCBI
|
|
21
|
Na D, Liu F, Miao Z, Du Z and Xu H:
Destruction of gastric cancer cells to mesothelial cells by
apoptosis in the early peritoneal metastasis. J Huazhong Univ Sci
Technolog Med Sci. 29:163–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lv ZD, Na D, Ma XY, Zhao C, Zhao WJ and Xu
HM: Human peritoneal mesothelial cell transformation into
myofibroblasts in response to TGF-β1 in vitro. Int J Mol Med.
27:187–193. 2011.PubMed/NCBI
|
|
23
|
Matsuoka T, Hirakawa K, Chung YS, Yashiro
M, Nishimura S, Sawada T, Saiki I and Sowa M: Adhesion polypeptides
are useful for the prevention of peritoneal dissemination of
gastric cancer. Clin Exp Met. 16:381–388. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahmed N, Riley C, Rice G and Quinn M: Role
of integrin receptors for FN, collagen and laminin in the
regulation of ovarian carcinoma functions in response to a matrix
microenvironment. Clin Exp Metastasis. 22:391–402. 2005. View Article : Google Scholar : PubMed/NCBI
|