Introduction
Androgen action is essential for the development of
the prostate and also for the development of prostate cancer.
Androgen deprivation therapy is a fundamental treatment for
metastasised prostate cancer. The activity of androgens is mediated
mainly via the androgen receptor (AR), although other androgen
responsive activation mechanisms may exist, especially in
hormone-refractory prostate cancer (1). Androgen-regulated gene expression has
been investigated in numerous studies using prostate cancer cell
lines. However, to the best of our knowledge, only a few studies
have used human tissues to evaluate androgen-regulated genes. One
study reported gene expression changes following castration using
prostatectomy samples after three months of androgen deprivation
therapy (combination of anti-androgen and chemical castration)
(2). In another study, a
comparison was made of the expression profiles of untreated and
androgen-independent prostate cancer (3). Mostaghel et al (4) studied gene expression changes in
human prostate tissue at different time points up to nine months
after castration for the treatment of localised prostate cancer.
The same group further examined the effect of the 5-α reductase
inhibitor dutasteride on prostate gene expression (5). The aim of the present study was to
identify androgen-regulated genes in the human prostate. Gene
expression analysis was performed on human prostate tissue samples
from benign and malignant prostate tissue and from prostate biopsy
samples obtained three days after surgical castration.
Materials and methods
Patient samples
Prostate samples were collected from three patients
undergoing radical prostatectomy for the treatment of prostate
cancer. Prostate biopsy samples were obtained from another three
patients three days after surgical castration performed as
treatment for prostate cancer. These male individuals had newly
diagnosed prostate cancer with no previous hormonal treatments. The
biopsies were extracted using a biopsy gun technique and 18 G
needles. Six biopsies were obtained and a single prophylactic dose
of ciprofloxacin (500 mg) was administered. Written informed
consent was obtained from every patient giving tissue samples for
the study. The study was approved by the Ethics Council of the
Northern Ostrobothnia Hospital District. RNA from the samples was
isolated using the QuickPrep Total RNA Extraction kit (Amersham
Biosciences, Piscataway, NJ, USA) according to the manufacturer's
instructions. The RNA from the samples was individually labelled
and used for the GeneChip array. Three samples were microdissected
and histologically confirmed as benign prostate tissue from radical
prostatectomy specimens, and three samples were microdissected and
histologically confirmed as Gleason 3+3 prostate cancer tissue from
radical prostatectomy specimens and three samples were from
biopsies taken following surgical castration performed as a
therapeutic procedure for prostate cancer.
Cell culture
The prostate cancer cell line LNCaP (CRL-1740) was
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). LNCaP cell cultures were maintained in
RPMI-1640 (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10
mM HEPES, 1 mM sodium pyruvate and 2.5 g/l D-glucose or DMEM
(Sigma-Aldrich) supplemented with 4,500 mg/l glucose, L-glutamine
and 1% penicillin-streptomycin (Invitrogen-Gibco, Carlsbad, CA,
USA). The cell cultures were supplemented with 10% foetal bovine
serum (FBS) (HyClone, Logan, UT, USA) at 37°C in a humidified
atmosphere of 5% CO2. FBS was substituted with
charcoal-treated FBS in the hormone-induction experiments. The
LNCaP cells were plated at 1×106 cells/plate 72 h prior
to the experiments. The cells were treated with 10 nM R1881
(PerkinElmer, Boston, MA, USA) or an equal volume of ethanol for 0,
6, 24, or 48 h. Following incubation, the cells were collected,
washed with phosphate-buffered saline and used directly for the
isolation of RNA.
GeneChip protocol
Experimental procedures for GeneChip were performed
according to the Affymetrix GeneChip Expression Analysis Technical
Manual following the microarray experiment guidelines. In brief,
using 8 μg of total RNA as a template, double-stranded DNA was
synthesised using the One-Cycle cDNA Synthesis kit (Affymetrix,
Santa Clara, CA, USA) and T7-(dT)24 primers. The DNA was purified
using a GeneChip Sample Cleanup Module (Qiagen, Venlo, The
Netherlands). In vitro transcription was performed to
produce biotin-labeled cRNA using an in vitro transcription
labeling kit (Affymetrix), according to the manufacturer's
instructions. Biotinylated cRNA was cleaned with a GeneChip Sample
Cleanup Module (Qiagen), fragmented to 35–200 nucleotides and
hybridised to Affymetrix Human Genome U133 Plus 2 arrays that
contained ~55,000 human transcripts. After being washed, the array
was stained with streptavidin-phycoerythrin (Molecular Probes,
Eugene, OR, USA). The staining signal was amplified using
biotinylated anti-streptavidin (Vector Laboratories, Burlingame,
CA, USA) and a second staining was performed with
streptavidin-phycoerythrin before the array was scanned on a
GeneChip Scanner 3000. The expression data were analysed using
Affymetrix GeneChip Operating System Software. Signal intensities
of all probe sets were scaled to the target value of 500.
Quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA from LNCaP cells for quantitative RT-PCR
measurements was isolated with TRIzol reagent (Invitrogen-Gibco)
according to the manufacturer's instructions. Using the
First-Strand cDNA synthesis kit (Amersham Biosciences) the
first-strand cDNA was synthesised with 1 μg of RNA and pd(N)6
random hex deoxynucleotides according to the manufacturer's
instructions. The mRNA levels for LNCaP cells were measured by
quantitative RT-PCR analysis (ABI 7700, Applied Biosystems, Foster
City, CA, USA) as described previously (6). The forward and reverse primers for
for the detection of dual specificity phosphatase 1 (DUSP1) mRNA
were 5′-TCCTTCTTCGCTTTCAACGC-3′ and 5′-ACGATGGTGCTGAAGCGC-3′,
respectively. Amplicons were detected using the fluorogenic probe
5′-FAM-CACA TCGCCGGCTCTGTCAACG-TAMRA-3′. The primers and probe for
the 18 S amplicon were 5′-TGGTTGCAAAGCTGA AACTTAAAG-3′ (forward),
5′-AGTCAAATTAAGCCGCA GGC-3′ (reverse) and 5′-VIC-CCTGGTGGTGCCCTTCCG
TCA-TAMRA-3′, respectively.
Analysis of gene expression profiles
The expression profiles from the GeneChip array were
analysed using Chipster software (http://chipster.csc.fi). The GeneChips were normalised
using the robust multiarray average (RMA) method and gene
expression intensity estimates were received in log2-transformed
values. The pathways involved were identified using Chipster
software utilising data from ConsensusPathDB (7). The data discussed in this study have
been deposited in the NCBI's Gene Expression Omnibus (8) and are accessible through GEO Series
accession number GSE32982 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE32982).
Statistical analysis
Statistical analysis of the levels of DUSP1 mRNA in
LNCaP cells was performed using SPSS version 15.0 (SPSS, Chicago,
IL, USA). The Student's t-test was used for comparison between the
two groups. Values were presented as the means ± SD.
Results
Genes differentially expressed in prostate cancer
and benign prostate tissue, and genes differentially expressed in
benign prostate tissue or prostate cancer and castrated prostate
cancer are listed in Table I.
These genes contain several well-known androgen-regulated genes and
prostate cancer-associated genes, including MSMB, SORD, CRISP3 and
PCGEM1. The consensus pathways involved between benign and
malignant tissue and between benign tissue and castrated prostate
cancer are shown in Table II.
 | Table IDifferentially expressed genes in
benign prostate tissue samples, prostate cancer samples and samples
obtained following surgical castration. a |
Table I
Differentially expressed genes in
benign prostate tissue samples, prostate cancer samples and samples
obtained following surgical castration. a
| | | Fold
overexpression |
|---|
| | |
|
|---|
| Symbol | Description | P-value | Cancer vs.
benign | Benign/cancer vs.
castrated |
|---|
| CRISP3 | Cysteine-rich
secretory protein 3 | <0.001 | 5.78 | −3.08 |
| FOS | FBJ murine
osteosarcoma viral oncogene homologue | <0.001 | | 4.76 |
| PCGEM1 | Prostate-specific
transcript 1 (non-protein coding) | <0.001 | | 4.42 |
| MYBPC1 | Myosin binding
protein C, slow type | <0.001 | | 4.30 |
| PCA3 | Prostate cancer
antigen 3 (non-protein coding) | <0.001 | 4.18 | 3.74 |
| OR51E2 | Olfactory receptor,
family 51, subfamily E, member 2 | <0.001 | 4.01 | 3.29 |
| ANPEP | Alanyl (membrane)
aminopeptidase | <0.001 | | 3.84 |
| DPP4 | Dipeptidyl-peptidase
4 | 0.005 | | 3.79 |
| GCNT2 | Glucosaminyl
(N-acetyl) transferase 2, I-branching enzyme (I blood group) | 0.001 | | 3.73 |
| HOXC6 | Homeobox C6 | 0.003 | 3.69 | −2.62 |
| AGR3 | Anterior gradient
homologue 3 (Xenopus laevis) | 0.003 | 3.63 | 3.00 |
| RGS1 | Regulator of
G-protein signalling 1 | 0.002 | | 3.55 |
| NR4A2 | Nuclear receptor
subfamily 4, group A, member 2 | 0.002 | | 3.50 |
| DUSP1 | Dual specificity
phosphatase 1 | 0.011 | | 3.48 |
| AMACR | α-methylacyl-CoA
racemase | 0.008 | 3.40 | 3.00 |
| LOC728606 | Hypothetical
LOC728606 | 0.015 | | 3.36 |
| DLX1 | Distal-less homeobox
1 | 0.011 | 3.30 | |
| VEGFA | Vascular endothelial
growth factor A | 0.004 | | 3.30 |
| MSMB | Microseminoprotein,
β- | 0.006 | | 3.21 |
| TDO2 | Tryptophan
2,3-dioxygenase | 0.025 | | 3.19 |
| NCAPD3 | Non-SMC condensin II
complex, subunit D3 | 0.008 | | 3.14 |
| EGR1 | Early growth response
1 | 0.027 | | 3.14 |
| CD38 | CD38 molecule | 0.008 | | 3.13 |
| C15orf48 | Chromosome 15 open
reading frame 48 | 0.022 | 3.11 | |
| RASD1 | RAS,
dexamethasone-induced 1 | 0.030 | | 3.09 |
| AGR2 | Anterior gradient
homologue 2 (Xenopus laevis) | 0.023 | 3.07 | |
| SORD | Sorbitol
dehydrogenase | 0.012 | | 3.02 |
| C12orf56 | Chromosome 12 open
reading frame 56 | 0.037 | | 3.01 |
| SFTPA2 | Surfactant protein
A2 | 0.037 | | 2.99 |
| ST6GALNAC1 | ST6
(α-N-acetyl-neuraminyl-2,3-β-galactosyl-1,3)-N-acetylgalactosaminide
α-2,6-sialyltransferase 1 | 0.038 | | 2.98 |
| ACADL | Acyl-CoA
dehydrogenase, long chain | 0.013 | | 2.98 |
| EGR3 | Early growth
response 3 | 0.044 | | 2.94 |
| ERG | V-ets
erythroblastosis virus E26 oncogene homologue (avian) | 0.045 | 2.90 | |
| FOSB | FBJ murine
osteosarcoma viral oncogene homologue B | 0.016 | | 2.90 |
| SCD | Stearoyl-CoA
desaturase (δ-9-desaturase) | 0.024 | | 2.80 |
| GNMT | Glycine
N-methyltransferase | 0.028 | | 2.76 |
| CCK |
Cholecystokinin | 0.028 | | 2.75 |
| PEBP4 |
Phosphatidylethanolamine-binding protein
4 | 0.031 | | 2.72 |
| ATF3 | Activating
transcription factor 3 | 0.031 | | 2.71 |
| HLA-DQA1 | Major
histocompatibility complex, class II, DQ α 1 | 0.032 | | 2.70 |
| SELE | Selectin E | 0.035 | | 2.66 |
| PENK | Proenkephalin | 0.043 | | 2.61 |
| CD177 | CD177 molecule | 0.048 | | 2.58 |
| MALAT1 |
Metastasis-associated lung adenocarcinoma
transcript 1 (non-protein coding) | 0.032 | | −2.69 |
| ASPN | Asporin | 0.029 | | −2.74 |
| PSPH | Phosphoserine
phosphatase | 0.027 | | −2.77 |
| SLC14A1 | Solute carrier
family 14 (urea transporter), member 1 (Kidd blood group) | 0.048 | −2.88 | −3.05 |
| HBB | Haemoglobin, β | 0.016 | | −2.89 |
| SCGB1A1 | Secretoglobin,
family 1A, member 1(uteroglobin) | 0.045 | −2.91 | |
| KRT14 | Keratin 14 | 0.032 | −2.99 | |
| NEFH | Neurofilament,
heavy polypeptide | 0.022 | −3.10 | |
| TMEM45B | Transmembrane
protein 45B | 0.007 | | −3.16 |
| GPM6A | Glycoprotein
M6A | 0.027 | | −3.17 |
| RLN1 | Relaxin 1 | 0.016 | −3.21 | |
| GREM1 | Gremlin 1 | 0.004 | | −3.29 |
| MME | Membrane
metallo-endopeptidase | 0.004 | −3.57 | |
| CXCL13 | Chemokine (C-X-C
motif) ligand 13 | 0.003 | −3.67 | |
| CYP3A5 | Cytochrome P450,
family 3, subfamily A, polypeptide 5 | 0.001 | −3.92 | |
| WIF1 | WNT inhibitory
factor 1 | <0.001 | −4.14 | |
 | Table IIPathways potentially active in the
transition from benign to malignant prostate tissue and during
surgical castration based on gene-expression differences.a |
Table II
Pathways potentially active in the
transition from benign to malignant prostate tissue and during
surgical castration based on gene-expression differences.a
| | P-value |
|---|
| |
|
|---|
| Pathway | Database | Benign tissue vs.
cancer | Castrated tissue
vs. benign tissue |
|---|
|
AP-1_transcription_factor_network | PID | 0.007 | <0.001 |
|
Beta1_integrin_cell_surface_interactions | PID | <0.001 | |
|
Bevacizumab_Pathway | SMPDB | 0.006 | |
|
Cholesterol_biosynthesis | Wikipathways | | <0.001 |
|
Cholesterol_biosynthesis | Reactome | | 0.002 |
|
cholesterol_biosynthesis_I | HumanCyc | | 0.003 |
|
cholesterol_biosynthesis_II_(via_24,25-dihydrolanosterol) | HumanCyc | | 0.003 |
|
cholesterol_biosynthesis_III_(via_desmosterol) | HumanCyc | | 0.003 |
|
Collagen_adhesion_via_alpha_2_beta_1_glycoprotein | Reactome | 0.001 | <0.001 |
|
ECM-receptor_interaction_-_Homo_sapiens_(human) | KEGG | <0.001 | |
|
Fatty_Acyl-CoA_Biosynthesis | Reactome | | 0.007 |
| Focal_Adhesion | Wikipathways | 0.004 | |
|
Focal_adhesion_-_Homo_sapiens_(human) | KEGG | 0.003 | |
|
GPCR_signalling-cholera_toxin | INOH | 0.004 | 0.003 |
|
GPCR_signalling-G_alpha_i | INOH | | 0.006 |
|
GPCR_signalling-pertussis_toxin | INOH | | 0.006 |
|
HIF-1-alpha_transcription_factor_network | PID | | 0.008 |
|
Immunoregulatory_interactions_between_a_
Lymphoid_and_a_non-Lymphoid_cell | Reactome | <0.001 | <0.001 |
| Integrin | INOH | 0.006 | |
|
Integrins_in_angiogenesis | PID | 0.004 | |
| Ketogenesis | HumanCyc | | 0.006 |
|
Neurophilin_interactions_with_VEGF_and_VEGFR | Reactome | 0.005 | |
|
Platelet_degranulation_ | Reactome | 0.006 | |
|
Prostaglandin_Synthesis_and_Regulation | Wikipathways | 0.007 | |
|
Protein_digestion_and_absorption_-_Homo_
sapiens_(human) | KEGG | 0.002 | |
|
Response_to_elevated_platelet_cytosolic_Ca2+ | Reactome | 0.007 | |
|
Signalling_by_PDGF | Reactome | <0.001 | |
|
Signalling_by_VEGF | Reactome | 0.008 | |
|
Smooth_Muscle_Contraction | Reactome | 0.003 | |
|
Steroid_Biosynthesis | SMPDB | | <0.001 |
|
Steroid_biosynthesis_-_Homo_sapiens_(human) | KEGG | | 0.002 |
|
Superpathway_of_cholesterol_biosynthesis | HumanCyc | | <0.001 |
|
Syndecan-1-mediated_signalling_events | PID | 0.001 | |
|
Valine_degradation_I | HumanCyc | | 0.009 |
|
Vatalanib_Pathway | SMPDB | 0.006 | |
|
VEGF_ligand-receptor_interactions | Reactome | 0.008 | |
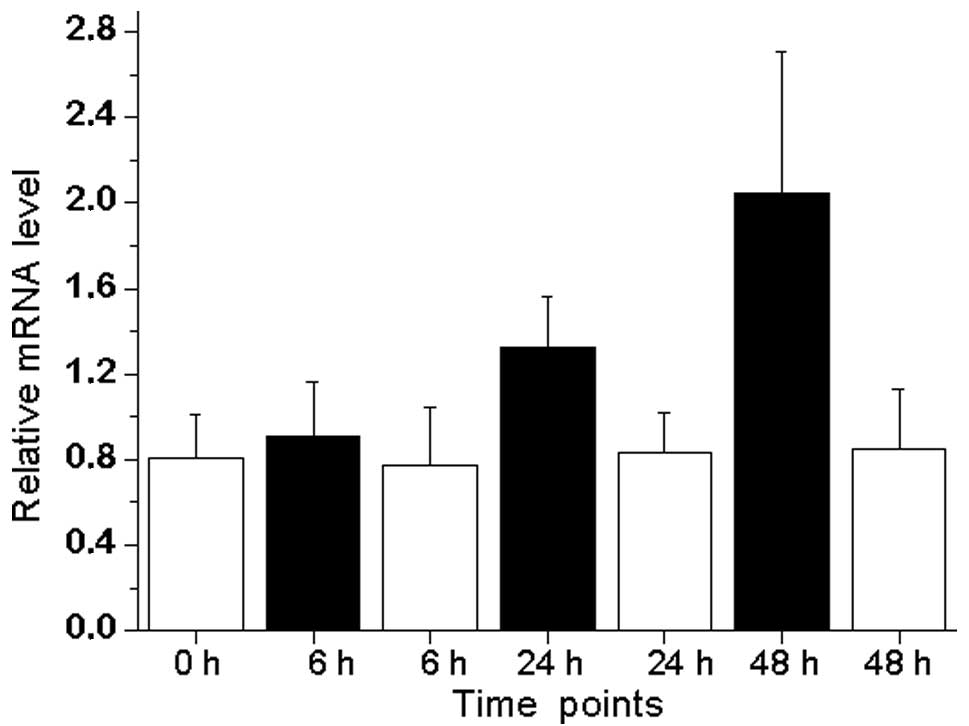
The androgen-regulated expression of DUSP1 in LNCaP
prostate cancer cell lines was also evaluated (Fig. 1). The treatment of LNCaP cells with
synthetic androgen R1881 led to an increased expression of DUSP1
mRNA, with a peak of 2.6-fold expression following 48 h of
treatment compared with androgen-depleted conditions.
Discussion
To identify androgen-regulated genes in the human
prostate, we used human prostate tissue mRNAs in a GeneChip array.
The main limitation of widely used human prostate cancer cell lines
such as LNCaP, PC-3 and DU-145 is that they do not represent the
prostate per se, but are isolated from a prostate cancer
metastasis. We evaluated the gene expression profiles from
microdissected tissue samples from freshly prepared radical
prostatectomy samples and from transrectal prostate biopsies
obtained three days after surgical castration performed as a
treatment for prostate cancer.
Certain known androgen-regulated genes were
identified, including MSMB (9) and
SORD (10). Furthermore, a number
of previously identified prostate cancer-associated genes, such as
CRISP3 (11), PCGEM1 (12), PCA3 (13) and OR51E2 (also known as PSGR)
(14) were also differentially
expressed.
Cholesterol biosynthesis has several correlations
with prostate cancer. Low serum cholesterol levels have been
correlated with a lower risk of high-grade prostate cancer
(15). Furthermore, the use of
cholesterol-lowering drugs, such as statins, is associated with a
lower risk of advanced prostate cancer (16). Increased cholesterol synthesis may
serve as a precursor for intratumoural androgen synthesis in
castration-resistant prostate cancer (17).
Focal adhesion pathways are involved in
gene-expression changes between benign and malignant prostate
tissue. Cell adhesion is a significant process in cancer (18). The steroid biosynthesis pathway was
found to be active between the castrated and benign tissue, as
expected.
DUSP1 (also known as mitogen-activated protein
kinase phosphatase 1, MKP1) is androgen-regulated in the rat
prostate (19). DUSP1 expression
was detected in prostate cancer with a decreased expression in
poorly differentiated carcinomas. Moreover, DUSP1 expression was
downregulated in androgen-depleted clinical prostate cancer samples
and the expression of DUSP1 was inversely correlated with apoptosis
(20). In prostate cancer
specimens, the expression of DUSP1 was low in hormone-refractory
prostate cancer, whereas it was high in benign prostatic
hyperplasia samples and in non-hormone-treated prostate cancer
(21). The results of our study
confirm those of previous results as DUSP1 expression was 3.48
times lower in castrated samples compared with benign or malignant
prostate samples (Table I). We
also provide data for the androgen-mediated induction of DUSP1 in
androgen-sensitive LNCaP prostate cancer cell lines (Fig. 1). Taken together, it appears that
DUSP1 is not important in hormone-refractory prostate cancer, as
increased apoptosis detected in androgen-depleted prostate cancer
by Magi-Galluzzi et al (20) may be explained by the low
expression of DUSP1 under androgen-depleted conditions and high
apoptotic indices under the same conditions in hormone-sensitive
prostate cancer. The low expression of DUSP1 in hormone-refractory
prostate cancer (21) may be
explained by the androgen-dependent expression of DUSP1 shown in
our study and previously by Leav et al (19), as castration is an ongoing process
in hormone-refractory prostate cancer.
Of note are the genes that are differentially
expressed in benign tissue and cancer, which are also overexpressed
or underexpressed following castration, meaning that the gene is
regulated by androgens and potentially involved in prostate
carcinogenesis. These genes are CRISP3, PCA3, OR51E2, HOXC6, AGR3,
AMACR and SLC14A1 (Table I). To
the best of our knowledge, the association between prostate cancer
and AGR3 or SLC14A1 has yet to be established. A high level of the
immunoexpression of AGR3 has been linked with prolonged survival in
ovarian cancer (22) and AGR3
expression has previously been detected in breast tumours (23). SLC14A1 was recently described as a
urinary bladder cancer susceptibility gene (24).
Certain well-known androgen-regulated genes, such as
kallikreins (25,26), are not shown in Table I. This is due to the variation in
gene expression levels in different samples leading to increased
P-values. Thus genes, such as kallikreins, did not exceed the given
threshold level of P<0.05 for genes presented in Table I (data not shown). The number of
samples analysed in our study was limited (three samples per group)
and the biopsies obtained from castrated patients were random
biopsies, thus these samples may represent cancer or benign tissue,
or both. Despite these limitations, our data may be valuable for
further studies in prostate cancer.
In conclusion, we have described the identification
of androgen-regulated genes in the human prostate, some of which
are potential new diagnostic or therapeutic targets in prostate
cancer. Particular attention should be paid to AGR3 and SLC14A1 for
their roles in prostate cancer. Furthermore, these gene expression
profiles may be useful in sophisticated gene expression analyses
utilising expression profiles from several different sources.
Acknowledgements
We are grateful to Ms. Mirja Mäkeläinen, Ms. Tarja
Piispanen and Ms. Marja Tolppanen for technical assistance. Dr Mika
Ilves performed the quantitative RT-PCR experiments. Dr Jussi
Vuoristo performed the GeneChip arrays. The computations presented
in this document were performed by the Centre for Scientific
Computing (CSC) environment. CSC is the Finnish IT centre for
science and is owned by the Ministry of Education. This study was
supported by grants from the Finnish Medical Fund, the Päivikki and
Sakari Sohlberg Foundation and the Urologic Research Foundation,
Finland.
References
|
1
|
Bennett NC, Gardiner RA, Hooper JD,
Johnson DW and Gobe GC: Molecular cell biology of androgen receptor
signalling. Int J Biochem Cell Biol. 42:813–827. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holzbeierlein J, Lal P, LaTulippe E, Smith
A, Satagopan J, Zhang L, Ryan C, Smith S, Scher H, Scardino P, et
al: Gene expression analysis of human prostate carcinoma during
hormonal therapy identifies androgen-responsive genes and
mechanisms of therapy resistance. Am J Pathol. 164:217–227. 2004.
View Article : Google Scholar
|
|
3
|
Best CJ, Gillespie JW, Yi Y, Chandramouli
GV, Perlmutter MA, Gathright Y, Erickson HS, Georgevich L, Tangrea
MA, Duray PH, et al: Molecular alterations in primary prostate
cancer after androgen ablation therapy. Clin Cancer Res.
11:6823–6834. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mostaghel EA, Page ST, Lin DW, Fazli L,
Coleman IM, True LD, Knudsen B, Hess DL, Nelson CC, Matsumoto AM,
et al: Intraprostatic androgens and androgen-regulated gene
expression persist after testosterone suppression: therapeutic
implications for castration-resistant prostate cancer. Cancer Res.
67:5033–5041. 2007. View Article : Google Scholar
|
|
5
|
Mostaghel EA, Geng L, Holcomb I, Coleman
IM, Lucas J, True LD and Nelson PS: Variability in the androgen
response of prostate epithelium to 5alpha-reductase inhibition:
implications for prostate cancer chemoprevention. Cancer Res.
70:1286–1295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Majalahti-Palviainen T, Hirvinen M,
Tervonen V, Ilves M, Ruskoaho H and Vuolteenaho O: Gene structure
of a new cardiac peptide hormone: a model for heart-specific gene
expression. Endocrinology. 141:731–740. 2000.PubMed/NCBI
|
|
7
|
Kamburov A, Wierling C, Lehrach H and
Herwig R: ConsensusPathDB--a database for integrating human
functional interaction networks. Nucleic Acids Res. 37:D623–D628.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edgar R, Domrachev M and Lash AE: Gene
expression Omnibus: NCBI gene expression and hybridization array
data repository. Nucleic Acids Res. 30:207–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dahlman A, Edsjö A, Halldén C, Persson JL,
Fine SW, Lilja H, Gerald W and Bjartell A: Effect of androgen
deprivation therapy on the expression of prostate cancer biomarkers
MSMB and MSMB-binding protein CRISP3. Prostate Cancer Prostatic
Dis. 13:369–375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szabo Z, Hamalainen J, Loikkanen I,
Moilanen AM, Hirvikoski P, Vaisanen T, Paavonen TK and Vaarala MH:
Sorbitol dehydrogenase expression is regulated by androgens in the
human prostate. Oncol Rep. 23:1233–1239. 2010.PubMed/NCBI
|
|
11
|
Bjartell A, Johansson R, Bjork T,
Gadaleanu V, Lundwall A, Lilja H, Kjeldsen L and Udby L:
Immunohistochemical detection of cysteine-rich secretory protein 3
in tissue and in serum from men with cancer or benign enlargement
of the prostate gland. Prostate. 66:591–603. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Srikantan V, Zou Z, Petrovics G, Xu L,
Augustus M, Davis L, Livezey JR, Connell T, Sesterhenn IA, Yoshino
K, et al: PCGEM1, a prostate-specific gene, is overexpressed in
prostate cancer. Proc Natl Acad Sci USA. 97:12216–12221. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Kok JB, Verhaegh GW, Roelofs RW,
Hessels D, Kiemeney LA, Aalders TW, Swinkels DW and Schalken JA:
DD3(PCA3), a very sensitive and specific marker to detect prostate
tumors. Cancer Res. 62:2695–2698. 2002.
|
|
14
|
Xu LL, Stackhouse BG, Florence K, Zhang W,
Shanmugam N, Sesterhenn IA, Zou Z, Srikantan V, Augustus M, Roschke
V, et al: PSGR, a novel prostate-specific gene with homology to a G
protein-coupled receptor, is overexpressed in prostate cancer.
Cancer Res. 60:6568–6572. 2000.PubMed/NCBI
|
|
15
|
Platz EA, Till C, Goodman PJ, Parnes HL,
Figg WD, Albanes D, Neuhouser ML, Klein EA, Thompson IM Jr and
Kristal AR: Men with low serum cholesterol have a lower risk of
high-grade prostate cancer in the placebo arm of the prostate
cancer prevention trial. Cancer Epidemiol Biomarkers Prev.
18:2807–2813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murtola TJ, Tammela TL, Lahtela J and
Auvinen A: Cholesterol-lowering drugs and prostate cancer risk: a
population-based case-control study. Cancer Epidemiol Biomarkers
Prev. 16:2226–2232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leon CG, Locke JA, Adomat HH, Etinger SL,
Twiddy AL, Neumann RD, Nelson CC, Guns ES and Wasan KM: Alterations
in cholesterol regulation contribute to the production of
intratumoral androgens during progression to castration-resistant
prostate cancer in a mouse xenograft model. Prostate. 70:390–400.
2010.
|
|
18
|
Mol AJ, Geldof AA, Meijer GA, van der Poel
HG and van Moorselaar RJ: New experimental markers for early
detection of high-risk prostate cancer: role of cell-cell adhesion
and cell migration. J Cancer Res Clin Oncol. 133:687–695. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leav I, Galluzzi CM, Ziar J, Stork PJ, Ho
SM and Loda M: Mitogen-activated protein kinase and
mitogen-activated kinase phosphatase-1 expression in the Noble rat
model of sex hormone-induced prostatic dysplasia and carcinoma. Lab
Invest. 75:361–370. 1996.PubMed/NCBI
|
|
20
|
Magi-Galluzzi C, Mishra R, Fiorentino M,
Montironi R, Yao H, Capodieci P, Wishnow K, Kaplan I, Stork PJ and
Loda M: Mitogen-activated protein kinase phosphatase 1 is
overexpressed in prostate cancers and is inversely related to
apoptosis. Lab Invest. 76:37–51. 1997.PubMed/NCBI
|
|
21
|
Rauhala HE, Porkka KP, Tolonen TT,
Martikainen PM, Tammela TL and Visakorpi T: Dual-specificity
phosphatase 1 and serum/glucocorticoid-regulated kinase are
downregulated in prostate cancer. Int J Cancer. 117:738–745. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
King ER, Tung CS, Tsang YT, Zu Z, Lok GT,
Deavers MT, Malpica A, Wolf JK, Lu KH, Birrer MJ, Mok SC, et al:
The anterior gradient homolog 3 (AGR3) gene is associated with
differentiation and survival in ovarian cancer. Am J Surg Pathol.
35:904–912. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fletcher GC, Patel S, Tyson K, Adam PJ,
Schenker M, Loader JA, Daviet L, Legrain P, Parekh R, Harris AL and
Terrett JA: hAG-2 and hAG-3, human homologues of genes involved in
differentiation, are associated with oestrogen receptor-positive
breast tumours and interact with metastasis gene C4.4a and
dystroglycan. Br J Cancer. 88:579–585. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rafnar T, Vermeulen SH, Sulem P,
Thorleifsson G, Aben KK, Witjes JA, Grotenhuis AJ, Verhaegh GW,
Hulsbergen-van de Kaa CA, Besenbacher S, et al: European
genome-wide association study identifies SLC14A1 as a new urinary
bladder cancer susceptibility gene. Hum Mol Genet. 20:4268–4281.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Henttu P and Vihko P: Prostate-specific
antigen and human glandular kallikrein: two kallikreins of the
human prostate. Ann Med. 26:157–164. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Young CY, Andrews PE and Tindall DJ:
Expression and androgenic regulation of human prostate-specific
kallikreins. J Androl. 16:97–99. 1995.PubMed/NCBI
|