Introduction
Due to the advancements in pediatric surgery, the
chance for neonatal exposure to anesthetics has increased. Previous
experimental studies have suggested that early exposure to
anesthetic agents, prior to the completion of synaptogenesis, may
result in widespread apoptotic neuronal degeneration and late
learning disability (1–3). Therefore, it is necessary to examine
the effects of anesthetics on neuronal apoptosis associated with
neurohistopathologic changes. All clinically used general
anesthetics enhance GABAA receptors, block N-methyl-d-aspartate
(NMDA) receptors, or both. In contrast to the mature brain,
however, it has recently been discovered that the transient
pharmacological blockade of NMDA receptors in the developing rodent
brain causes excessive neuronal apoptosis (4).
Sevoflurane, one of the most frequently used
volatile anesthetics, is particularly useful for infants and
children, as it allows for rapid induction and recovery, and is
less irritative to the airway (5).
Sevoflurane has been shown to enhance GABAA receptors (6) and block NMDA receptors (7). Although there are certain studies
that have demonstrated through in vivo and in vitro
experiments that sevoflurane may effect cell survival and
potentiate neuronal apoptosis (8,9),
studies on the effect of sevoflurane on the hippocampus of the
developing brain are yet to be conducted.
In the brain, nitric oxide (NO), produced mainly by
neuronal nitric oxide synthase (nNOS), behaves as an intercellular
and intracellular diffusible messenger involved in multiple
functions from developmental neural plasticity to the control of
neurotransmitter release and memory consolidation (10,11).
Studies have demonstrated that the sustained inhibition of NO
production triggers apoptosis in differentiated cerebellar granule
neuron cultures (12). NO seems to
have distinct functions during the different stages of ontogenesis
of the forebrain, midbrain and cerebellum in rats (13), which is also reflected in the nNOS
expression.
Caspases are crucial mediators of programmed cell
death (apoptosis). Among them, caspase-3 is a frequently activated
death protease, catalyzing the specific cleavage of many key
cellular proteins (14). In
multiple cell types, caspase-3 is required for certain typical
nuclear and other morphological changes associated with the
completion of apoptosis and the formation of apoptotic bodies
(15–17).
In this study, to investigate the possible
neurotoxicity induced by sevoflurane, we exposed neonatal rats to
sevoflurane and assessed the morphological changes, as well as the
expression of nNOS and cleaved caspase-3 in the hippocampus.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats obtained from the
Experimental Animal Center of the Sun Yat-sen University Guangzhou,
China, were used in this study. The use of aimals in this study was
approved by the Institutional Animal Care and Use Committee of Sun
Yat-sen University. All efforts were made to minimize the number of
animals used as well as their suffering. The room was illuminated
with a 12-h light-dark cycle (light from 07:00–19:00), and the room
temperature was maintained at 21±1°C.
Sevoflurane exposure
The SD rats at postnatal day (P)7 (weight, 16–17 g)
were randomly divided into an air-treated control group and a
sevoflurane-treated group. Rats in the sevoflurane group were
placed in a plastic container and exposed to 2.3% sevoflurane for 6
h continuously, using air as a carrier with a total gas flow of 2
l/min. During the sevoflurane exposure, the container was heated to
38°C using a heating device (NPS-A3 heated device; Midea Co.,
Guangdong, China). The levels of sevoflurane, oxygen and carbon
dioxide were monitored in the chamber, using a gas monitor
(Detex-Ohmeda, Louisville, CO, USA). Sevoflurane administration was
terminated 6 h later and the rats were exposed to air solely. When
the rats were once again moving freely, they were placed back into
the maternal cage. During exposure to sevoflurane, the respiratory
frequency and skin color of the rats were monitored. In case of
apnea or hypoxia, the rat was immediately exposed to air and
excluded from the experiment. Rats (at P7) in the control group
were placed into the same container as the rats in the sevoflurane
group, but were exposed to air alone for 6 h.
Histopathological examination
Sevoflurane-exposed rats as well as rats from the
control group (16–17 g) were sacrificed at 6 h (n=3), after a 6-h
exposure. Tissue blocks (0.5 cm thick) from the hippocampus were
embedded in paraffin, sliced in 5-mm-thick sections and stained
with hematoxylin and eosin (H&E). The results were examined in
detail under a light microscope so as to determine morphological
changes in parts of the CA1 and CA3 regions.
Immunofluorescence
Sevoflurane-exposed rats as well as rats (16–17 g)
from the control group (n=3/group) were deeply anesthetized with
chloral hydrate at 6 h after a 6-h exposure, and then perfused with
4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) in PBS (pH
7.4) through the left cardiac ventricle, in order to assess the
sevoflurane exposure-induced changes on nNOS and caspase-3 levels
in the hippocampus. Brains were dissected out and placed in the
same fixative solution overnight. After postfixation, the brains
were soaked in 30% sucrose for an additional 24 h.
Coronal sections (20 μm) were cut using a sliding
microtome (Scientific Instruments, Palm Beach, FL, USA) and
subsequently processed for immunofluorescence analysis. Briefly,
the floating sections were blocked with a solution containing 1%
BSA and 0.4% Triton X-100 for 2 h at room temperature and incubated
with mouse anti-nNOS (diluted 1:3000; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) and rabbit cleaved caspase-3 (diluted
1:15000; Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C
overnight. After washing with PBS, the sections were incubated with
anti-mouse IgG tetrarhodamine isothiocyanate (TRITC) conjugate
(diluted 1:800; Sigma-Aldrich) and anti-rabbit IgG FITC conjugate
(diluted 1:400; Sigma-Aldrich) in the dark. Finally, the sections
were rinsed with PBS, mounted on gel-coated slides and observed
under a microscope (Axio Imager Z1). Each experiment was repeated
independently at least 3 times.
Results
Inhalation of sevoflurane for 6 h
decreases the number of eosinophilic cells and causes morphological
changes in parts of CA1 and CA3 regions
In the control group, eosinophilic cells in the CA1
region were arranged in neat order and observed under a microscope
(Fig. 1A). At high magnification,
at least 5 layers of cells with cell size uniformity were detected
(Fig. 1B). In the sevoflurane
group, the cells in the CA1 region were arranged in a disorganized
manner and were not so closely attached (Fig. 1E). At high magnification, several
dark neurons with destroyed nuclei were observed. Moreover, the
number of the cell layers decreased (Fig. 1F). In the control group, a dense
radiating cluster of eosinophilic cells in the CA3 region were
arranged in neat order (Fig. 1C and
D). In the sevoflurane group, however, morphological changes
could be observed, while in the CA3 region various dark neurons
were detected. Some nuclei were not in the center of the
eosinophilic cells, indicating that apoptosis may occur due to
sevoflurane inhalation (Fig. 1G and
H).
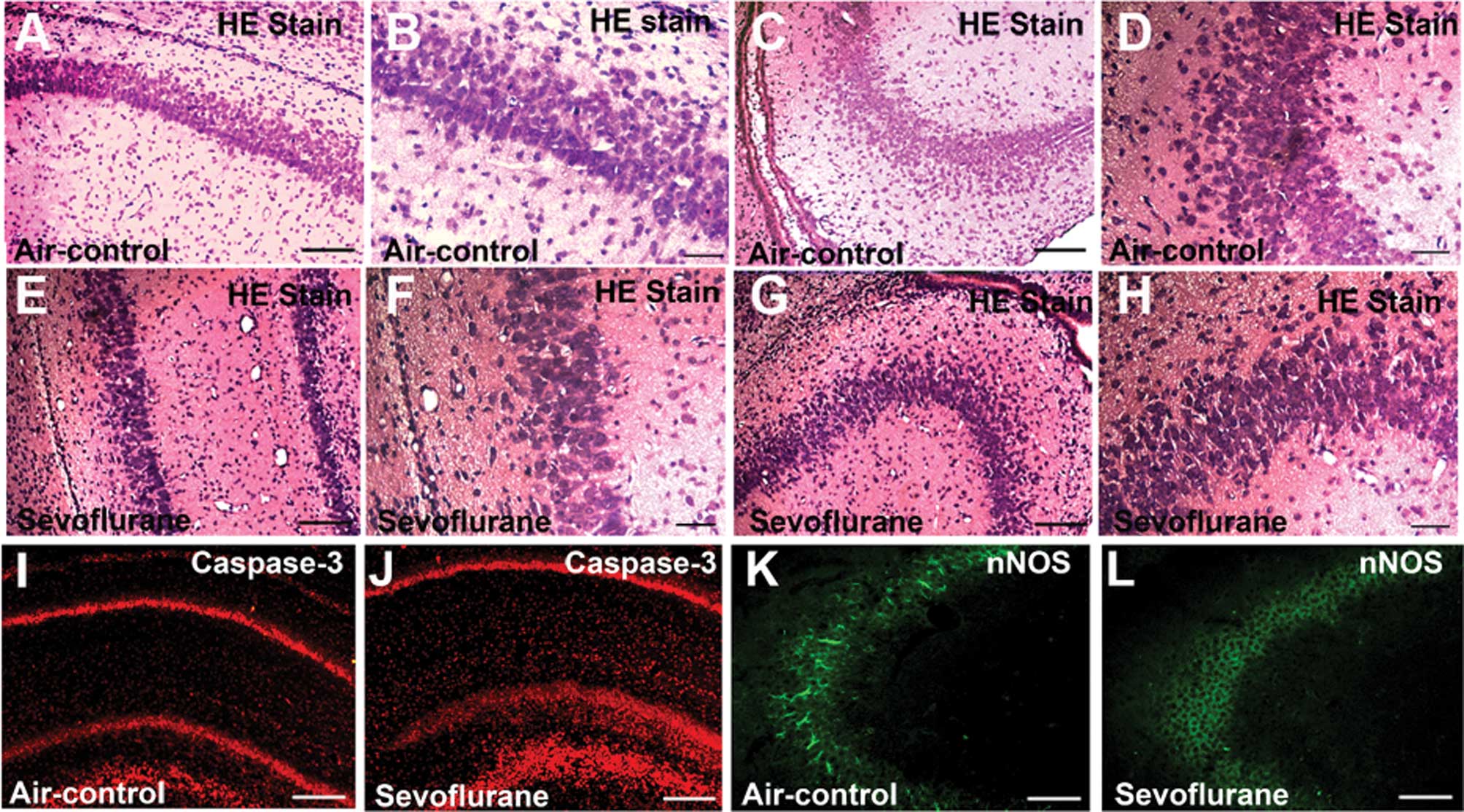 | Figure 1Sevoflurane inhalation caused
morphological changes in parts of the CA1 and CA3 regions, and it
also significantly activated caspase-3 and decreased neuronal
nitric oxide synthase (nNOS) levels in both CA1 and CA3 regions 6 h
after exposure. (A–D) Control group; (E–H) sevoflurane group. (A,
B, E and F) Low-magnification images of the CA1 region (A and E
bar, 100 μm) and high-magnification images of the CA1 region (B and
F bar, 20 μm). (C, D, G and H) Low-Magnification images of the CA3
region (C and G bar,100 μm) and high-magnification images of the
CA3 region (D and H bar, 20 μm). Morphological changes in the
sevoflurane group can be observed. In the sevoflurane group (E–H),
the cells were disorganized and not that closely ranked, and some
nuclei were not in the center of the eosinophilic cells. (I–L) nNOS
(green) and caspase-3 (red) immunofluorescence staining of the
hippocampus at the 6-h point after treatment. (I) CA1 field of the
control group; (J) CA1 field of the sevoflurane group; (K) CA3
field of the control group; (L) CA3 field of the sevoflurane group.
The images show that the nNOS-positive cells in the sevoflurane
group were less than the nNOS-positive cells in the control group,
in both the CA1 and CA3 fields. The images also show that caspase-3
levels in the sevoflurane group were more increased compared to the
control group in the CA1 field. (I–L bar, 100 μm). |
Exposure to sevoflurane-induces
expression of cleaved caspase-3 and reduces expression of nNOS in
neonatal rat hippocampus
The effects of sevoflurane on cleaved caspase-3 adn
nNOS expression are presented in Fig.
1I-L. Both cleaved caspase-3 (Fig.
1I) and nNOS (Fig. 1K) were
expressed in the hippocampus of the control rats. In the CA1 region
of the hippocampus, cleaved-caspase immunostaining greatly
increased at the 6-h point, after sevoflurane inhalation.
nNOS-positive cells were found in the CA3 region of rats in the
control group (Fig. 1K). However,
after the 6-h exposure to sevoflurane, nNOS immunostaining
decreased (Fig. 1L). The images
show that nNOS-positive cells in the sevoflurane group were less
than the nNOS-positive cells in the control group in the CA3
field.
Discussion
Due to the advances in fetal surgery, there is an
increase in the duration and complexity of anesthesia. Sevoflurane
is particularly useful for infants and children, as it allows for
rapid induction and recovery, and is less irritative to the airway
(5). There is evidence indicating
that exposure to sevoflurane results in apoptosis in the developing
brain (18). The hippocampus is
part of the limbic system of the brain, highly associated with
neuronal synaptic plasticity, learning and memory functions, and it
is easily damaged due to its structure (19). In the present study, we selected
2.3% sevoflurane, as this was the highest concentration not
inhibiting respiration and circulation in rat pups under our
experimental conditions and was comparable to the concentration
used in clinical settings.
In our study, H&E staining results demonstrated
that a single 6-h sevoflurane exposure at P7 caused morphological
changes in the hippocampus. Compared with the air-control groups
both in the CA1 and CA3 fields, the cells were disorganized and not
that closely attached. Under high magnification it was observed
that in the sevoflurane group, the nuclei in certain cells were not
in the center of the cytoplasm.
The immunofluorescence results showed that exposure
to sevoflurane induced the expression of cleaved caspase-3 and
reduced the expression of nNOS in the neonatal rat hippocampus.
Caspase-3, when activated by proteolytic cleavage, is one of the
apoptotic effectors responsible for the breakdown of cellular
components. Activated caspase-3 is widely used as a marker for
apoptotic cells (20). In our
study, the results indicated that a 6-h exposure to 2.3%
sevoflurane induced apoptosis in the hippocampus. The nNOS is the
predominant NOS isoform in the nervous system and can be
transcriptionally induced under certain circumstances, such as
neuronal development (21). In the
brain, NO produced mainly by nNOS, plays an important role in
central nervous system (CNS) functions, including apoptosis,
neurogenesis, neuronal differentiation and development (22). We show that a 6-h exposure to 2.3%
sevoflurane induces a decrease in nNOS levels, which may
participate in neuronal apoptosis. However, additional
investigations are required to determine whether this decrease will
continue through to adulthood.
In conclusion, neonatal exposure to 2.3% sevoflurane
for 6 h causes neurohistopathological changes, apoptosis and
decreases nNOS protein levels in the rat hippocampus.
Acknowledgements
The authors gratefully acknowledge the financial
support of the National Science Foundation Council of China
(31140050), Guangdong Science Foundations (2010B031600037) and
Guangdong Science and Technology Planning Project (2010B031600207;
2011B050400024).
References
|
1
|
Anand KJ: Anesthetic neurotoxicity in
newborns: should we change clinical practice? Anesthesiology.
107:2–4. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wiklund A, Granon S, Faure P, Sundman E,
Changeux JP and Eriksson LI: Object memory in young and aged mice
after sevoflurane anaesthesia. Neuroreport. 20:1419–1423. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jevtovic-Todorovic V, Hartman RE, Izumi Y,
et al: Early exposure to common anesthetic agents causes widespread
neurodegeneration in the developing rat brain and persistent
learning deficits. J Neurosci. 23:876–882. 2003.
|
|
4
|
Perouansky M: General anesthetics and
long-term neurotoxicity. Handb Exp Pharmacol. 182:143–157. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lerman J, Sikich N, Kleinman S and Yentis
S: The pharmacology of sevoflurane in infants and children.
Anesthesiology. 80:814–824. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shelton KL: Discriminative stimulus
effects of inhaled 1,1,1-trichloroethane in mice: comparison to
other hydrocarbon vapors and volatile anesthetics.
Psychopharmacology (Berl). 203:431–440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishikawa K and Harrison NL: The actions
of sevoflurane and desflurane on the gamma-aminobutyric acid
receptor type A: effects of TM2 mutations in the alpha and beta
subunits. Anesthesiology. 99:678–684. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kvolik S, Dobrosevic B, Marczi S, Prlic L
and Glavas-Obrovac L: Different apoptosis ratios and gene
expressions in two human cell lines after sevoflurane anaesthesia.
Acta Anaesthesiol Scand. 53:1192–1199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong Y, Zhang G, Zhang B, Moir RD, Xia W,
Marcantonio ER, Culley DJ, Crosby G, Tanzi RE and Xie Z: The common
inhalational anesthetic sevoflurane induces apoptosis and increases
beta-amyloid protein levels. Arch Neurol. 66:620–631.
2009.PubMed/NCBI
|
|
10
|
Porro A, Chrochemore C, Cambuli F, Iraci
N, Contestabile A and Perini G: Nitric oxide control of MYCN
expression and multi drug resistance genes in tumours of neural
origin. Curr Pharm Des. 16:431–439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu YY, Liu CM, Tsai HH, Jong YJ, Chen IJ
and Lo YC: KMUP-1 attenuates serum deprivation-induced
neurotoxicity in SH-SY5Y cells: roles of PKG, PI3K/Akt and
Bcl-2/Bax pathways. Toxicology. 268:46–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ciani E, Guidi S, Della Valle G, Perini G,
Bartesaghi R and Contestabile A: Nitric oxide protects
neuroblastoma cells from apoptosis induced by serum deprivation
through cAMP-response element-binding protein (CREB) activation. J
Biol Chem. 277:49896–49902. 2002. View Article : Google Scholar
|
|
13
|
Iwase K, Takemura M, Shimada T, Wakisaka
S, Nokubi T and Shigenaga Y: Ontogeny of NADPH-diaphorase in rat
forebrain and midbrain. Anat Embryol (Berl). 197:229–247. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Porter AG and Janicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Woo M, Hakem R, Soengas MS, Duncan GS,
Shahinian A, Kägi D, Hakem A, McCurrach M, Khoo W, Kaufman SA, et
al: Essential contribution of caspase 3/CPP32 to apoptosis and its
associated nuclear changes. Genes Dev. 12:806–819. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hirata H, Takahashi A, Kobayashi S,
Yonehara S, Sawai H, Okazaki T, Yamamoto K and Sasada M: Caspases
are activated in a branched protease cascade and control distinct
downstream processes in Fas-induced apoptosis. J Exp Med.
187:587–600. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Janicke RU, Sprengart ML, Wati MR and
Porter AG: Caspase-3 is required for DNA fragmentation and
morphological changes associated with apoptosis. J Biol Chem.
273:9357–9360. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Satomoto M, Satoh Y, Terui K, Miyao H,
Takishima K, Ito M and Imaki J: Neonatal exposure to sevoflurane
induces abnormal social behaviors and deficits in fear conditioning
in mice. Anesthesiology. 110:628–637. 2009. View Article : Google Scholar
|
|
19
|
Win-Shwe TT, Yoshida Y, Kunugita N,
Tsukahara S and Fujimaki H: Does early life toluene exposure alter
the expression of NMDA receptor subunits and signal transduction
pathway in infant mouse hippocampus? Neurotoxicology. 31:647–653.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Young C, Roth KA, Klocke BJ, West T,
Holtzman DM, Labruyere J, Qin YQ, Dikranian K and Olney JW: Role of
caspase-3 in ethanol-induced developmental neurodegeneration.
Neurobiol Dis. 20:608–614. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Knott AB and Bossy-Wetzel E: Nitric oxide
in health and disease of the nervous system. Antioxid Redox Signal.
11:541–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garthwaite J: Concepts of neural nitric
oxide-mediated transmission. Eur J Neurosci. 27:2783–2802. 2008.
View Article : Google Scholar : PubMed/NCBI
|















