Introduction
Among the elderly, osteoarthritis is the most common
joint disease and a significant cause of physical illness (1). Symptoms include joint pain,
stiffness, limited movement, joint deformity and varying degrees of
joint inflammation (2,3). Current therapeutic strategies for
osteoarthritis focus on alleviating symptoms, particularly pain and
inflammation. Non-steroidal anti-inflammatory drugs (NSAIDs) and
corticosteroids are the mainstream treatments for osteoarthritis
(4). Although NSAIDs are
recommended as an initial drug therapy to reduce joint inflammation
and pain, their chronic use is limited by gastrointestinal-related
toxicities, including nausea, dyspepsia, upper gastrointestinal
bleeding and ulcer perforation (5). To minimize these toxicities, a new
generation of NSAIDs, the cyclooxygenase (COX)-2 selective
inhibitors (celecoxib, rofecoxib and valdecoxib), have been
developed in an attempt to improve gastrointestinal tolerance.
However, reported cardiovascular risks, including myocardial
infarction and stroke, have led to the removal of rofecoxib from
the market (6–8). Although other COX-2 inhibitors
provide effective symptomatic relief, their substantial toxicities
limit long-term use. Additionally, this therapeutic approach is not
curative, but relieves clinical signs and symptoms of the disease;
thus, a more effective and safe drug is necessary for the curative
treatment of osteoarthritis.
Joins™, a herbal drug combining the extracts of
Clematis mandshurica, Trichosanthes kirilowii and
Prunella vulgaris, is commonly used for the curative
treatment of osteoarthritis in Korea. Clinical studies have
demonstrated that Joins relieves joint pain and improves
functionality in osteoarthritis patients. Its efficacy may be
attributed to cartilage protection and anti-inflammation (9); however, its lack of an immediate
analgesic effect is a major drawback. The screening of herbs and
natural products for a more efficient compound may lead to the
development of a superior therapeutic drug, particularly one with
more immediate analgesic effects.
Tabebuia avellanedae Lorentz ex Griseb, a
Bignoniaceae, is a tree found in tropical rain forests in
northeastern Brazil. Taheebo, a product obtained from the purple
bark of the tree, has been traditionally used for over 1,500 years
in South America to treat a variety of diseases (10). Its various fractions have been
previously reported to exhibit anti-inflammatory, anti-bacterial,
anti-fungal, diuretic, anti-coagulant and laxative properties in
addition to an anticancer effect (11–14).
In particular, Taheebo demonstrated anti-nociceptive and
anti-edematogenic effects in formalin- or acetic acid-induced
nociceptive experimental models in mice and in the rat paw edema
model (15). Compared with the
anti-inflammatory effect of Tabebuia avellanedae, the
analgesic effects of Taheebo have not been extensively studied to
date (16). For the development as
a therapeutic drug against osteoarthritis, the anti-inflammatory
and analgesic effects of Taheebo ethanolic extract require further
evaluation with various nociceptive and inflammatory experimental
animal models. The current study investigated the anti-inflammatory
and anti-nociceptive effects of the ethanolic extract of
Tabebuia avellanedae on various animal models.
Materials and methods
Chemicals
Arachidonic acid,
12-O-tetradecanoylphorbol-13-acetate (TPA), acetic acid,
carboxymethyl cellulose (CMC), Evans blue, diclofenac,
indomethacine and carrageenan were purchased from Sigma-Aldrich
(St. Louis, MO, USA).
Animals
Male 6- to 8-week-old imprinting control region
(ICR) mice and Sprague Dawley (SD) rats were obtained from
OrientBio Co., Ltd. (Sungnam, Korea). Male ICR mice were used in
the following tests: TPA- or arachidonic acid-induced ear edema,
hot plate, acetic acid-induced vascular permeability and acetic
acid-induced writhing response. SD rats were used in
carrageenan-induced paw edema tests. The animals were maintained in
plastic cages at 21–24°C under a 12-h light/dark cycle and were
provided with free access to pellet food and water. The animals
were adapted for at least 1 week prior to the start of the
experiment. The subjects were habituated to the behavioral test
chambers and handled with special care to minimize stress. All
methods were approved by the Animal Care and Use Committee of Kyung
Hee University and all procedures were conducted in accordance with
the Guide for the Care and Use of Laboratory Animals, published by
the Korean National Institute of Health.
Preparation of ethanolic extract of
Taheebo
An ethanolic extract from Taheebo, the inner bark of
Tabebuia avellanedae Lorentz ex Griseb, was prepared as
previously reported (17).
Briefly, the inner bark of the plant was purchased from GIPI Korea
Co. Ltd. (Seoul, Korea), who authenticated the product as Taheebo.
Dried inner bark (1 kg) was extracted three times with 70% ethanol
(12 l) at room temperature for 3 days. The combined extract was
filtered and then concentrated under reduced pressure at 40°C with
an end result of ~13% yield (based on the weight of the dried inner
bark). The extract was suspended in 0.5% sodium CMC (CMC-Na)
solution immediately prior to the start of the experiments. The
extract was administered orally to the animals at doses of 100, 200
or 400 mg/kg. Control experiments were performed with 0.5% CMC-Na
solution. A combination of diclofenac (25 mg/kg) and indomethacin
(1 mg/kg) was used as the positive control.
Hot plate test
The hot plate test was conducted at a fixed
temperature of 55±1°C according to a previously reported method
(18). Animals were placed into a
50 cm-diameter glass beaker on the heated surface and the time
between animal placement and shaking or licking of the paws or
jumping was recorded as the index of response latency. Mice
exhibiting latency time of 10–30 sec were selected to receive
vehicle (0.5% CMC in distilled water), Taheebo extract (100, 200 or
400 mg/kg) or diclofenac (25 mg/kg); 1 h later, the latency time
was re-determined.
Acetic acid-induced writhing
response
The anti-nociceptive activities of the Taheebo
extract were assessed by measuring the response to an
intraperitoneal injection of acetic acid solution, which causes
contraction of the abdominal muscles and stretching of the hind
limbs (19). Each experimental
group was administered orally with the vehicle (0.5% CMC in
distilled water), Taheebo extract (100, 200 or 400 mg/kg), or
diclofenac (25 mg/kg). After 1 h, 1% acetic acid was administered
at a dose of 10 ml/kg body weight; measurement of the number of
writhes began 10 min later and continued for 20 min.
Acetic acid-induced vascular
permeability
The acetic acid-induced vascular permeability test
was carried out with a modification of a previously described
method (20). Evans blue (10 ml/kg
of 2% solution) was injected intravenously into each mouse tail 30
min following oral administration of vehicle (0.5% CMC in distilled
water), Taheebo extract (100, 200 or 400 mg/kg) or diclofenac (25
mg/kg). After 20 min, 10 ml/kg of 1% acetic acid in saline was
injected intraperitoneally and the mice were sacrificed by cervical
dislocation 20 min later. Saline (5 ml) was injected into the
abdominal cavity, the washings were collected into test tubes and
subsequently centrifuged at 2,000 rpm for 10 min. The absorbance of
the supernatant was read at 650 nm with a SpectraMax 190
spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) and the
amount of Evans blue leakage in the abdominal cavity (the vascular
permeability) was determined from the absorbance measurement of the
supernatant.
TPA-induced ear edema formation in
mice
Skin inflammation was induced in the right ear of
each mouse by topical application of TPA as previously described
(21). Each experimental group
orally received the vehicle (0.5% CMC in distilled water), Taheebo
extract (100, 200 or 400 mg/kg) or indomethacin (1 mg/kg). After 1
h, TPA solution (1.0 μg dissolved in 20 μl acetone) was applied to
the inner and outer surfaces of the ears and 4 h later the mice
were sacrificed by cervical dislocation. A mouse ear punch was
obtained with a 5-mm dermal biopsy punch and then weighed. The
thickness of the punch was measured with calipers (Mitutoyo
Corporation, Kawasaki, Japan). The degree of ear swelling was
expressed as the increase in ear thickness (mm).
Arachidonic acid-induced mouse ear edema
assay
The vehicle (0.5% CMC in distilled water), Taheebo
extract (100, 200 or 400 mg/kg) or indomethacin (1 mg/kg) were
orally administered 1 h prior to the topical application of 2%
arachidonic acid dissolved in acetone (20 μl/ear) to the right ear
of the mice (22). After 4 h, the
mice were sacrificed by cervical dislocation. A mouse ear punch was
obtained with a 5-mm dermal biopsy punch and then weighed. The
thickness of the punch was measured with calipers and the degree of
ear swelling was expressed as the increase in ear thickness
(mm).
Carrageenan-induced paw edema
The anti-inflammatory activity of Taheebo was
determined by the carrageenan-induced edema test. Taheebo extract
(100, 200 or 400 mg/kg), diclofenac (25 mg/kg) or the vehicle (0.5%
CMC in distilled water) was administered orally 1 h prior to the
injection of 100 μl of 1% carrageenan in saline into the plantar
side of the left hind paws of the rats. Paw volume was measured
prior to the carrageenan injection and 1, 2, 3 and 4 h following
the administration of the edematogenic agent using a plethysmometer
(Ugo Basile, Comerio, Italy). The degree of swelling was determined
by the ratio a/b, where a and b are the volumes of the left hind
paws following and prior to the carrageenan treatment,
respectively. The increase in paw volume (%) was calculated as
follows: [(a-b)/b] × 100.
Statistical analysis
Data were presented as the mean ± standard error of
the mean (SEM). Comparisons between the experimental and control
groups were performed by one-way analysis of variance (ANOVA)
followed by Dunnett's post hoc test. P<0.05 was considered to
indicate a statistically significant result. The program used for
the statistical analysis was GraphPad Prism software 5 (San Diego,
CA, USA).
Results
Analgesic effect of the ethanolic Taheebo
extract in animal models
Oral administration of the Taheebo extract
significantly increased the pain threshold of the mice compared
with control treatment, as assessed by the hot plate test (Fig. 1). Notably, treatment with Taheebo
led to a significant analgesic effect even at 200 mg/kg (~30%
greater than control), an effect that was similar to treatment with
diclofenac (25 mg/kg). The acetic acid-induced writhing response
also revealed the analgesic effect of Taheebo. The cumulative
amount of abdominal stretching was associated with the level of
acetic acid-induced pain. Thus, Taheebo treatment (100–400 mg/kg)
significantly inhibited the number of writhes in comparison to
control treatment (Fig. 2). The
inhibition of writhes was ~30 and 40% higher than the control at
Taheebo doses of 100 and 400 mg/kg, respectively, although this
inhibition did not achieve statistical significance at 100
mg/kg.
Anti-inflammatory effect of ethanolic
Taheebo extract in animal models
The anti-inflammatory effects of Taheebo extract
were assessed through the use of various animal models, including
acetic acid-induced vascular permeability, TPA-induced ear edema,
arachidonic acid-induced mouse ear edema and carrageenan-induced
paw edema. First, in the acetic acid-induced vascular permeability
test, the oral administration of Taheebo extract at 100 and 400
mg/kg resulted in the significant inhibition of dye leakage by 30
and 35%, respectively (Fig. 3).
The inhibition degree was similar to 100–400 mg/kg, although only
the inhibition at 400 mg/kg achieved statistical significance. In
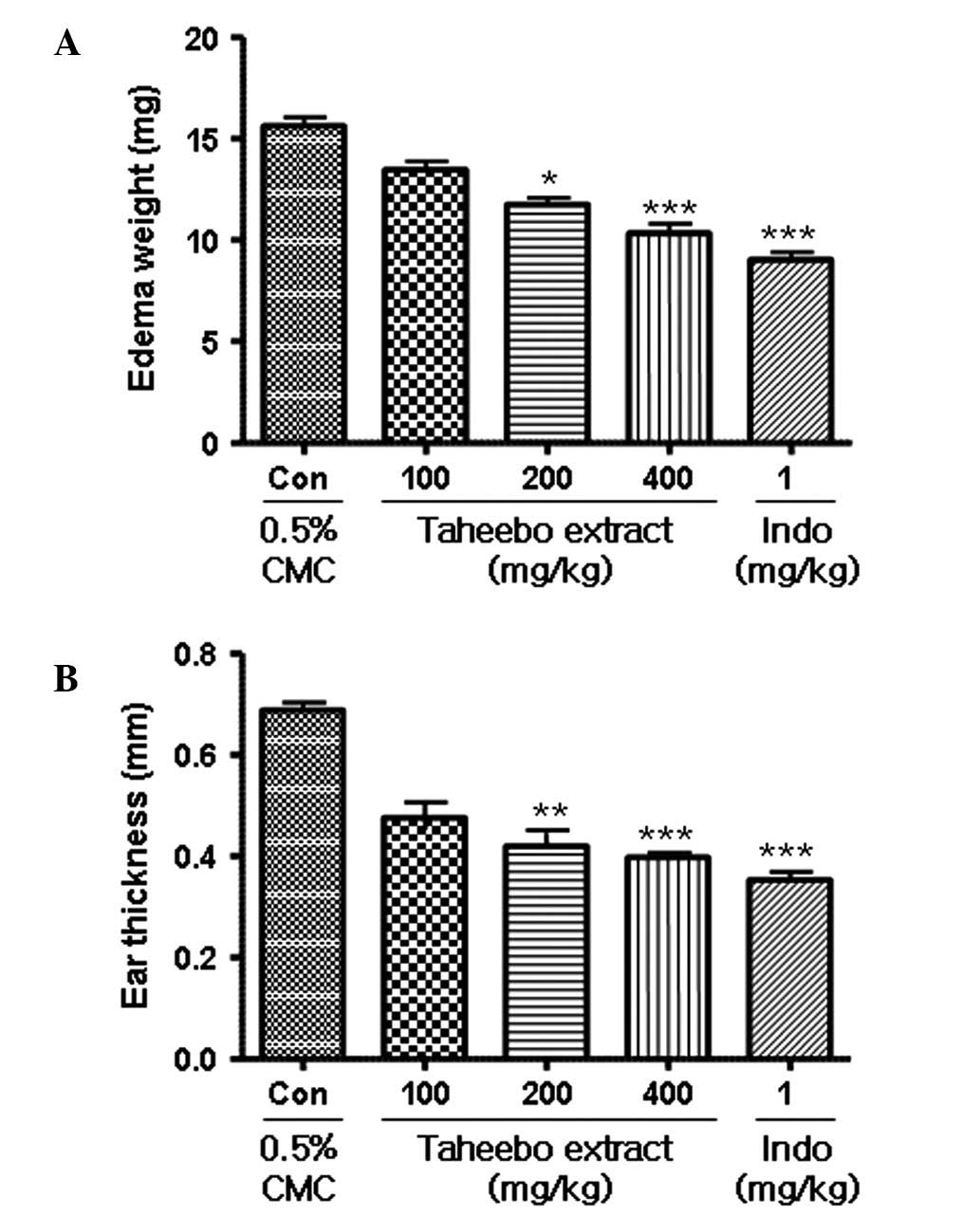
the TPA-induced ear edema test, the Taheebo extract led to an
inhibition of ear weight and thickness in a dose-dependent manner
(Fig. 4). In the arachidonic
acid-induced mouse ear edema model, Taheebo extract also exhibited
a dose-dependent anti-inflammatory effect (Fig. 5). In the two models, indomethacin
(1 mg/kg) inhibited ear inflammation by 50% compared with the
control, while the Taheebo extract at 100 mg/kg inhibited ear
inflammation by >25% compared with the control. In the
carrageenan-induced hind paw edema test, 200 mg/kg Taheebo extract
significantly inhibited ~30% of paw edema 3 h following carrageenan
injection, although 100 mg/kg Taheebo extract was unable to
significantly decrease edema volume compared with the control at
all time points (Table I).
 | Table IAnti-inflammatory effect of Taheebo
extract on carrageenan-induced paw edema in rats. |
Table I
Anti-inflammatory effect of Taheebo
extract on carrageenan-induced paw edema in rats.
| Percentage increase
of paw edema (mean ± SEM) |
|---|
|
|
|---|
| Dose (mg/kg) | 1 h | 2 h | 3 h | 4 h |
|---|
| CON | 29.36±2.66 | 59.57±3.10 | 76.28±2.24 | 76.14±2.52 |
| Taheebo (100) | 29.47±1.89 | 56.24±2.31 | 62.23±3.95 | 64.04±2.77 |
| Taheebo (200) | 30.73±2.12 | 50.58±2.60a | 57.86±2.43b | 60.78±2.22b |
| Taheebo (400) | 30.60±2.11 | 47.10±2.27b | 51.32±2.72b | 52.19±2.30b |
| Diclofenac
(25) | 27.83±2.82 | 37.98±2.37b | 41.18±2.93b | 35.23±2.69b |
Discussion
Numerous studies have elucidated the pharmacological
activities of the Taheebo extract, the inner bark of Tabebuia
avellanedae (11–14). However, the few demonstrations of
the in vivo anti-nociceptive effects were performed in
limited animal models (15). Those
studies focused on the aqueous and methanolic extracts of
Tabebuia avellanedae; to the best of our knowledge, the
ethanolic extracts have not undergone much study. This
investigation has demonstrated that the ethanolic extract of
Taheebo significantly attenuated acetic acid-induced writhing
(Fig. 2) and the nociception
produced by hot-plate thermal stimulation (Fig. 1). Treatment with the extract also
decreased the inflammation induced by acetic acid (Fig. 3), TPA (Fig. 4), arachidonic acid (Fig. 5) and carrageenan (Table I) in animal models.
For nociception induction in animals, acetic acid
causes inflammatory pain by inducing capillary permeability
(23), while hot-plate-induced
pain indicates narcotic involvement (24). Although formalin is known to induce
neurogenic and inflammatory pain (25), the formalin test was not performed
in this study. However, the demonstration that Taheboo aqueous
extract also exerts an analgesic effect on formalin-induced pain
(15) suggests that the Taheebo
extract has an inhibitory effect on three types of pain induction.
These analgesic effects may be partly related to Taheebo's
anti-inflammatory neurogenic and narcotic properties. Oral
administration of 200–400 mg/kg of the ethanolic Taheebo extract
inhibited pain with statistical significance in the two animal
models and a dose of 100 mg/kg was extremely effective in the
majority of tested animal models. This inhibitory concentration is
similar to that of the aqueous extract of Tabebuia
avellanedae (15). Notably,
the inhibitory effect of this aqueous extract at 200 mg/kg was not
detected following the 400 mg/kg dose, as the aqueous extract may
contain compounds that undercut its own inhibitory action. By
contrast, in this study the ethanolic extract caused significant
anti-nociceptive effects at doses of 200–400 mg/kg in various
animal models, suggesting that the ethanolic extracts contain
constituents to relieve pain that differ from the aqueous
extracts.
The molecular mechanism by which the extract
attenuated the pain level in the animal models has not been
defined. Collier et al postulated that acetic acid acts
indirectly by inducing the release of endogenous mediators that
stimulate nociceptive neurons sensitive to NSAIDs and narcotics
(26). It has also been proposed
that acetic acid induces pain by increasing the amount of
PGE2 and PGF2α at the peritoneal receptors
(27,28). On the basis of these studies, the
Taheebo extract is thought to regulate the production of
PGE2 and PGF2α in acetic acid-induced animal
models of pain. By contrast, as the hot plate test is commonly used
for assays of narcotic analgesics, our observations suggest that
the extract exerts a central anti-nociceptive effect that may be
associated with a reduction in Ca2+ influx at the axon
termini of the afferent nerves. This reduction may induce a
decrease in adenylate cyclase activity, thereby decreasing the
levels of cyclic AMP and K+ efflux and leading to nerve
hyperpolarization and an apparent anti-nociceptive effect (29).
Carrageenan, arachidonic acid, TPA and acetic acid
were used to induce inflammation in animal models, since edema
induced by phlogistic agents is a widely accepted model for
inflammation. For example, carrageenan-induced paw edema is a
classical model of acute inflammation involving various chemical
mediators, including histamine, serotonin, bradykinin and
prostgladins (30), in which the
involvement of the COX products of arachidonic acid metabolism and
the production of reactive oxygen species are well-established
(31). Oral administration of the
Taheebo extract inhibited the inflammation in these animal models
and thus the extract may be presumed to be involved in the
downregulation of the production of various chemical mediators of
inflammation.
The ethanolic extract of Taheebo exhibited
anti-nociceptive and anti-inflammatory effects in animal models,
emphasizing its potential for development as a novel therapeutic
drug against osteoarthritis. However, studies using other pain and
inflammation animal models are required, particularly since the
animal models used in this investigation were short-term. The
effects of lower doses of Taheebo should be demonstrated in
long-term animal models to support its development as a therapeutic
drug.
The following major active compounds have been
identified in Tabebuia avellandae: flavonoids, cyclopentene
dialdehydes, benzoic acid and benzaldehyde derivatives, quinones,
furanonaphthoquinones, naphthoquinones and anthraquinones (32). Of the 18 most relevant quinines,
lapachol and β-lapachone appear to have clinical importance, since
they have been associated with anticancer pharmacological activity.
Thus, HPLC analysis was conducted to determine the content of
lapachol in the ethanol extract of Taheebo (data not shown).
Lapachol was detected at 6.642 min retention time. The extract (1
g) contained approximately 0.03 mg of lapachol, although we did not
confirm the content of all the compounds. Toxicity was observed
with no therapeutic response following oral administration of
lapachol and phase I clinical trials of lapachol as an anticancer
drug were closed in 1970. The anticancer chemotherapeutic
β-lapachone succeeded as the research molecule of note from
Tabebuia avellandae and the molecular mechanisms associated
with its anticancer activity, including topoisomerase inhibition,
have been well defined (32).
Other compounds, including the furonaphthoquinones and
anthraquinones, have been the focus of investigations for various
therapeutic roles in several experimental systems. Other molecules
considered to be significant in disease treatment should be studied
to further understand the molecular mechanisms by which Taheebo
extracts relieve pain and inflammation in animal models.
In conclusion, the Taheebo ethanolic extracts
demonstrated anti-nociceptive and anti-inflammatory effects in
various animal models. These anti-nociceptive effects and the
extract constituents related to pain relief require clarification
for the development of a safe drug that produces immediate
analgesia without adverse effects.
References
|
1
|
Ameye LG and Chee WS: Osteoarthritis and
nutrition. From nutraceuticals to functional foods: a systematic
review of the scientific evidence. Arthritis Res Ther. 8:R1272006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lark MW, Bayne EK, Flanagan J, et al:
Aggrecan degradation in human cartilage. Evidence for both matrix
metalloproteinase and aggrecanase activity in normal,
osteoarthritic and rheumatoid joints. J Clin Invest. 100:93–106.
1997. View Article : Google Scholar
|
|
3
|
Poole AR: An introduction to the
pathophysiology of osteoarthritis. Front Biosci. 4:D662–D670. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kidd BL: Osteoarthritis and joint pain.
Pain. 123:6–9. 2006. View Article : Google Scholar
|
|
5
|
Wolfe F and Cathey MA: The assessment and
prediction of functional disability in rheumatoid arthritis. J
Rheumatol. 18:1298–1306. 1991.PubMed/NCBI
|
|
6
|
Hippisley-Cox J and Coupland C: Risk of
myocardial infarction in patients taking cyclo-oxygenase-2
inhibitors or conventional non-steroidal anti-inflammatory drugs:
population based nested case-control analysis. BMJ. 330:13662005.
View Article : Google Scholar
|
|
7
|
Gottlieb S: COX 2 inhibitors may increase
risk of heart attack. BMJ. 323:4712001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lenzer J: FDA advisers warn: COX 2
inhibitors increase risk of heart attack and stroke. BMJ.
330:4402005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hartog A, Hougee S, Faber J, et al: The
multicomponent phytopharmaceutical SKI306X inhibits in vitro
cartilage degradation and the production of inflammatory mediators.
Phytomedicine. 15:313–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hashimoto G: Illustrated Encyclopedia of
Brazilian Medicinal Plants. Kamakura: Aboc-sha; 1996
|
|
11
|
Choi BT, Cheong J and Choi YH:
Beta-Lapachone-induced apoptosis is associated with activation of
caspase-3 and inactivation of NF-κB in human colon cancer HCT-116
cells. Anticancer Drugs. 14:845–850. 2003.PubMed/NCBI
|
|
12
|
Machado TB, Pinto AV, Pinto MC, et al: In
vitro activity of Brazilian medicinal plants, naturally occurring
naphthoquinones and their analogues, against methicillin-resistant
Staphylococcus aureus. Int J Antimicrob Agents. 21:279–284.
2003. View Article : Google Scholar
|
|
13
|
Awale S, Kawakami T, Tezuka Y, et al:
Nitric oxide (NO) production inhibitory constituents of Tabebuia
avellanedae from Brazil. Chem Pharm Bull (Tokyo). 53:710–713.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Böhler T, Nolting J, Gurragchaa P, et al:
Tabebuia avellanedae extracts inhibit IL-2-independent
T-lymphocyte activation and proliferation. Transpl Immunol.
18:319–323. 2008.
|
|
15
|
de Miranda FG, Vilar JC, Alves IA, et al:
Antinociceptive and antiedematogenic properties and acute toxicity
of Tabebuia avellanedae Lor. ex Griseb inner bark aqueous
extract. BMC Pharmacol. 1:62001.PubMed/NCBI
|
|
16
|
Byeon SE, Chung JY, Lee YG, et al: In
vitro and in vivo anti-inflammatory effects of taheebo, a water
extract from the inner bark of Tabebuia avellanedae. J
Ethnopharmacol. 119:145–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee KH and Choi EM: Analgesic and
anti-inflammatory effects of Ligularia fischeri leaves in
experimental animals. J Ethnopharmacol. 120:103–107. 2008.
View Article : Google Scholar
|
|
18
|
Hu X, Jin H, Xu W, et al:
Anti-inflammatory and analgesic effects of Daphne retusa
Hemsl. J Ethnopharmacol. 120:118–122. 2008. View Article : Google Scholar
|
|
19
|
Fischer LG, Santos D, Serafin C, et al:
Further antinociceptive properties of extracts and phenolic
compounds from Plinia glomerata (Myrtaceae) leaves.
Biol Pharm Bull. 31:235–239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whittle BA: The use of changes in
capillary permeability in mice to distinguish between narcotic and
nonnarcotic analgesics. Br J Pharmacol Chemother. 22:246–253. 1964.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song HY, Lee JA, Ju SM, et al: Topical
transduction of superoxide dismutase mediated by HIV-1 Tat protein
transduction domain ameliorates
12-O-tetradecanoylphorbol-13-acetate (TPA)-induced
inflammation in mice. Biochem Pharmacol. 75:1348–1357. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koshihara Y, Fujimoto Y and Inoue H: A new
5-lipoxygenase selective inhibitor derived from Artocarpus
communis strongly inhibits arachidonic acid-induced ear edema.
Biochem Pharmacol. 37:2161–2165. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amico-Roxas M, Caruso A, Trombadore S, et
al: Gangliosides antinociceptive effects in rodents. Arch Int
Pharmacodyn Ther. 272:103–117. 1984.PubMed/NCBI
|
|
24
|
Besra SE, Sharma RM and Gomes A:
Antiinflammatory effect of petroleum ether extract of leaves of
Litchi chinensis Gaertn. (Sapindaceae). J Ethnopharmacol.
54:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vaz ZR, Filho VC, Yunes RA and Calixto JB:
Antinociceptive action of 2-(4-bromobenzoyl)-3-methyl-4,6-dimethoxy
benzofuran, a novel xanthoxyline derivative on chemical and thermal
models of nociception in mice. J Pharmacol Exp Ther. 278:304–312.
1996.PubMed/NCBI
|
|
26
|
Collier HO, Dinneen LC, Johnson CA and
Schneider C: The abdominal constriction response and its
suppression by analgesic drugs in the mouse. Br J Pharmacol
Chemother. 32:295–310. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deraedt R, Jouquey S, Delevallée F and
Flahaut M: Release of prostaglandins E and F in an algogenic
reaction and its inhibition. Eur J Pharmacol. 61:17–24. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bentley GA, Newton SH and Starr J: Studies
on the antinociceptive action of alpha-agonist drugs and their
interactions with opioid mechanisms. Br J Pharmacol. 79:125–134.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yaksh TL: Spinal systems and pain
processing: development of novel analgesic drugs with
mechanistically defined models. Trends Pharmacol Sci. 20:329–337.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vinegar R, Truax JF, Selph JL, et al:
Pathway to carrageenan-induced inflammation in the hind limb of the
rat. Fed Proc. 46:118–126. 1987.PubMed/NCBI
|
|
31
|
Smith MJ, Ford-Hutchinson AW, Elliott PN
and Bolam JP: Prostaglandins and the anti-inflammatory activity of
a human plasma fraction in carrageenan-induced paw oedema in the
rat. J Pharm Pharmacol. 26:692–698. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gomez Castellanos JR, Prieto JM and
Heinrich M: Red Lapacho (Tabebuia impetiginosa) - a global
ethnopharmacological commodity? J Ethnopharmacol. 121:1–13.
2009.
|