Introduction
Intracerebral hemorrhage (ICH) is a common subtype
of stroke and produces severe neurological deficits in survivors
(1), with a poor prognosis that is
much worse than that for ischemic strokes of a similar size.
Currently, medical therapy for patients with ICH is generally
limited to supportive care or surgical evacuation of the hematoma,
with variable and controversial efficacy.
One important response of the brain to various
injuries is an increased production of endogenous neural cells,
which are differentiated from resident neural stem cells (NSCs) in
discrete brain regions. NSCs are induced to proliferate in response
to brain injury and migrate into areas of brain damage (2). NSCs retain the capacity for
proliferation and lesion-directed migration in adult patients with
cerebral ischemia (3). This
provides the possibility of functional neuronal transplantation
through endogenous neurogenesis.
ICH and ischemic strokes share similar responses to
NSCs in the adult human brain. Increased neurogenesis has been
reported following ICH in rats and humans (4,5),
which resulted from the activation of NSCs in the adult human brain
following subarachnoid hemorrhage (SAH), thus contributing to the
promotion of spontaneous recovery (6). Significant improvements in behavioral
tests have been reported in animals with ICH following NSC
transplantation (7), indicating a
potentially therapeutic effect in humans. The difficulty in
obtaining NSCs limits their clinical applications; other sources of
stem cells are required for replacement therapy in ICH. Among the
various types of stem cells, one of the most promising stem cell
sources are bone marrow-derived mesenchymal stem cells (MSCs), as
they are capable of differentiating into neurons (8) and can be obtained from the patient’s
own bone marrow (9). Previously,
it was reported that MSCs are able to differentiate into neural
cells in the rat brain following ICH, with significantly improved
neural function (10). In this
study, we investigated the therapeutic effect of intravenous
transplantation of MSCs following ICH in rats. Our data suggest
that transplantation of MSCs induces neural differentiation and
proliferation of neural cells in the rat brain following ICH.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats (12 weeks old) were
used for the experiment. The experiments and procedures were
approved by the local animal care committee of Dalian Central
Hospital.
Isolation and culture of bone
marrow-derived MSCs
Rats were sacrificed and placed in 70% alcohol for
disinfection. Both femurs were dissected and the attached muscle
and soft tissue were stripped. A 21-gauge needle was applied to
aspirate the bone marrow, followed by flushes through the bone
using a 1 ml syringe filled with 2 ml of DMEM medium (Gibco,
Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS).
Approximately 10×106 bone marrow cells were diluted with
an equal volume of Ficoll separation medium for centrifugation at
400 × g for 30 min. The white film-like layer of mononuclear cells
was removed and seeded at a density of 1×105/ml in a 150
cm2 tissue culture flask coated with collagen I (Becton
Dickinson Labware, Oxford, UK), and cultured at 37°C in a
humidified incubator with 5% CO2. Every 3–4 days, the
cultured cells were digested by 0.25% trypsin with a passage ratio
of 1:2. After the third passages reached 90% confluence, the cells
were collected and preserved for further study.
Characterization and labeling of
MSCs
MSCs were collected from the tissue culture flasks
following the third passage and centrifuged at 200 × g for 5 min. A
single cell suspension of 0.5–1×106 cells (50 μl PBS)
was incubated with primary antibodies against CD90, CD29, CD45 or
CD11bc for 40 min and then FITC-labeled secondary antibody for 30
min. Flow cytometry was applied to evaluate cell fluorescence
(Becton Dickinson). Prior to injection, MSCs were labeled with
5-bromo 2′deoxy-uridine (BrdU, 5 μM) (Sigma Chemical, St. Louis,
MO, USA) for 12 h.
Rat ICH model generation
Adult male SD rats (n=12) weighing 270–320 g were
used in the study. The study used collagenase to induce ICH
(11). Briefly, after preoperative
fasting for 12 h and water deprivation for 4 h, intraperitoneal
anesthesia was performed with 10% chloral hydrate. Animals were
anesthetized with their head fixed on the stereotaxic instrument. A
midline scalp incision was made, and the injection sites of the
exposed skull were 0.5 mm anterior and 3.5 mm lateral to bregma
(caudate nucleus). Using a syringe pump (Harvard Instruments), 0.4
U type VII bacterial collagenase (Sigma C0773) in 2 μl normal
saline, or saline alone (vehicle) was infused slowly (0.5 μl/min)
into the centre of the striatum. The needle was left in place for 2
min and withdrawn slowly, with the hole sealed by bone wax. At 24 h
post-surgery, animals were anesthetized with overdose chloral
hydrate and sacrificed by decapitation. The brain was rapidly
extracted and slices of cortex and basal ganglia were made. The
brain tissue was fixed with 10% formaldehyde or homogenized for use
in subsequent experiments.
Experimental groups
One hour following ICH, the rats were randomly
divided into two groups, which were injected with l ml PBS (ICH
group) or l ml PBS with cell suspension (1×106 MSCs)
(ICH-MSCs group), respectively. Briefly, 1×106 MSCs
suspended in l ml sterile PBS were injected into each animal in the
ICH-MSCs group using a 27-G needle via the tail vein. Animals in
the ICH group received an equal volume of PBS vehicle.
Behavioral testing of neurological
deficits
To examine the effects of the transplanted MSCs on
the neurological deficits of rats following ICH, two investigators
who were blinded to the treatment status performed the behavioral
tests, with the modified neurological severity score (mNSS). The
mNSS is a composite index of motor, sensory, beam balance and
reflex tests (12). Injury in the
right hemisphere striatum of ICH rats causes sensory and motor
functional deficits, leading to elevated scores on motor, sensory
and beam balance tests. The animals were monitored at 1, 3, 7, 14,
21 and 28 days following ICH (n=6/group).
Hemorrhage volume detection
Hemorrhage volume was quantified at 24 or 72 h via a
spectrophotometric assay conducted as described previously
(13). Measurements from perfused
brains subjected to ICH were compared with the standard curve to
obtain data in terms of hemorrhage volume (ml; n=6/group). Three
duplicate experiments were performed in each group.
Tissue preparation and
immunohistochemistry
On day 3, the anesthetized animals were sacrificed
by decapitation (n=6/group). The brains were rapidly removed and
placed in 4% paraformaldehyde at 4°C for 48 h. Samples were
incubated at 4°C overnight with primary antibodies against Brdu,
caspase 3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), NF200,
GFAP (Cell Signaling Technology Inc., Beverly, MA, USA) at
concentrations of 1:100 (GFAP) and 1:200 (caspase 3, NF200). As the
secondary antibody, immunoglobulin G conjugated with horseradish
peroxidase (HRP) (Promega Biotechnology Co., Ltd., Beijing, China)
was used for 30 min at 37°C. Prior to dehydration and mounting,
slides were counterstained with hematoxylin. The marker-specific
cells were counted throughout the entire section (three sections
per antibody staining) and then the total counts in these sections
were converted into cell densities for quantitation.
Western blotting
Twenty-four hours following the induction of ICH,
the rats were sacrificed to extract the brain (n=3/group).
Homogenates of the brain tissues in perihematomal areas were
serially processed for western blotting. Cytosolic extracts (50 μg)
were resolved by 15% SDS-polyacrylamide gels (SDS-PAGE) and
electrotransferred onto a nitrocellulose membrane. The extracts
were then incubated with the primary antibodies anti-phospho-Akt
(serine-473) (1:200) or anti-bcl-2 (1:200) for 1 h, followed by
secondary antibodies conjugated with HRP. Visualization was
performed using the chemiluminescence method. β-actin was used as
an internal reference.
Sandwich ELISA method for G-CSF and BDNF
detection
Levels of granulocyte colony-stimulating factor
(G-CSF) and brain-derived neurotrophic factor (BDNF) protein in the
cytosolic extracts of brain tissue were assayed using the ChemiKine
ELISA Kit (Chemicon, Temecula, CA, USA), according to the
manufacturer’s instructions. Briefly, the cytosolic extracts and
standards were incubated for 2 h, followed by washing with buffer
and incubation with anti-G-CSF or anti-BDNF polyclonal antibody at
room temperature for 2 h. After washing, the plates were incubated
with anti-IgG antibody conjugated to HRP for 1 h. The plates were
then incubated in peroxidase substrate and tetramethylbenzidine
solution to produce a color reaction, followed by the addition of 1
M HCl to stop the reaction. A microplate reader was applied to
measure absorbance at 450 nm. Values are expressed as percentages
of the controls.
Statistical analysis
Data in this study were presented as the means ± SD.
Data were analyzed by Student’s t-test if the data were normally
distributed (Kolmogorov-Smirnov test; P>0.05). When data were
not normally distributed, the Mann-Whitney U test was used.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of bone marrow-derived
MSCs and expression of surface markers
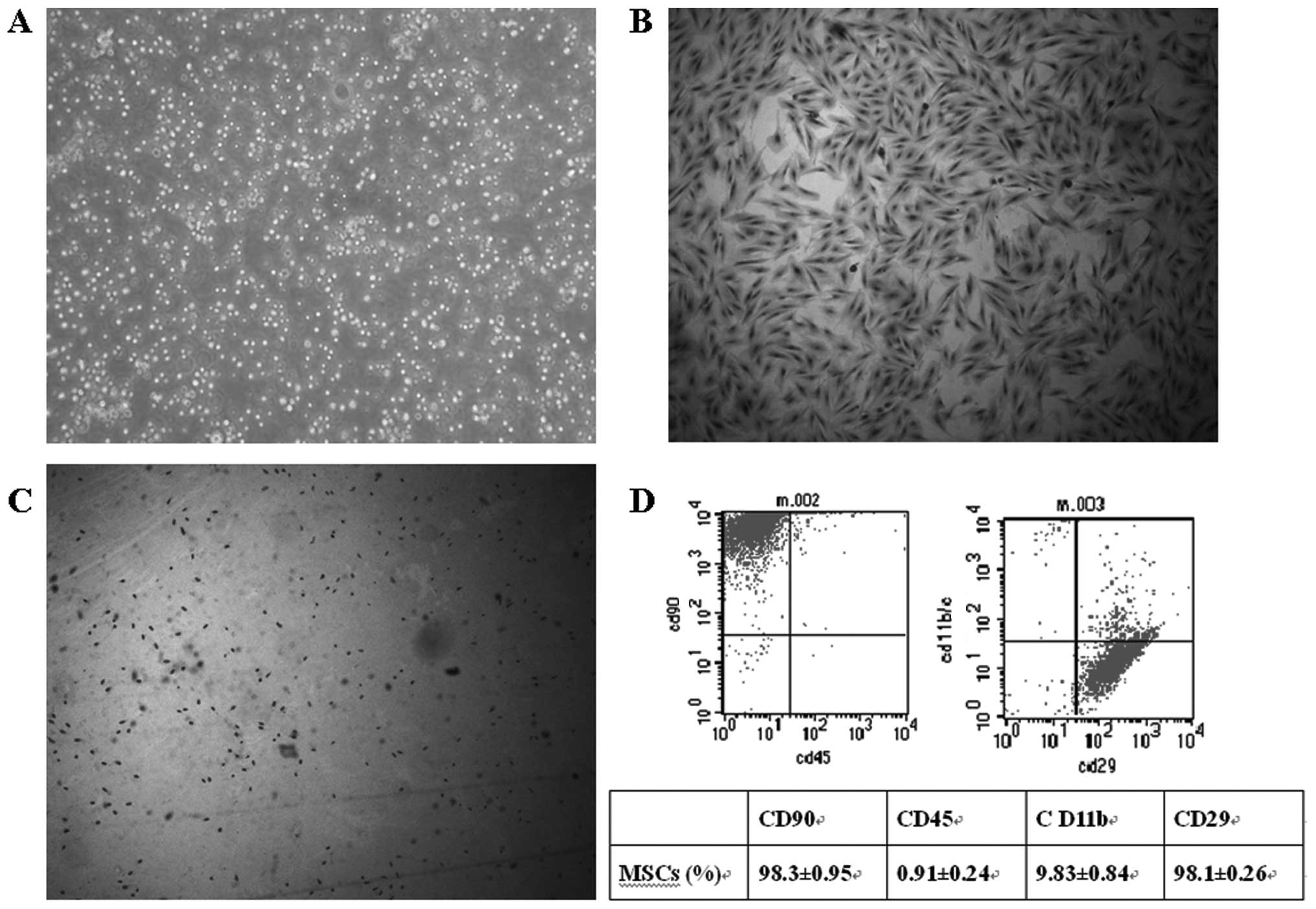
The bone marrow mononuclear cells were isolated
using the Ficoll method to form a single cell suspension (Fig. 1A). The cells were seeded in tissue
culture flasks. After 24 h, some cells were adhered, extended and
showed a short spindle appearance. The cells exhibited clone-like
growth, with the majority of clones composed of the spindle,
multi-shaped fibroblast-like cells. After 10–15 days, cells reached
confluence in the bottom of the flasks. At passage 3, cells showed
relatively uniform bipolar spindle-like morphology and bundles of
fibers were arranged in parallel under the inverted microscope,
with no hematopoietic cell hybrid clones (Fig. 1B). At passage 3, Brdu was labeled
to demonstrate the proliferation of MSCs and the cells with
positive Brdu labeling indicated dividing cells (Fig. 1C).
MSCs were analyzed for cell surface
antigens at passage 3
The results showed that MSCs were highly positive
for CD90 (98.1±0.26%) and CD29 (98.3±0.95%). The positive rates for
CD11bc (9.83±0.84%) and CD45 (0.91±0.24%) were low (Fig. 1D). Therefore, a high purity of MSCs
could be reached.
MSC transplantation promotes functional
recovery and reduces hemorrhage volume in rats with ICH
ICH is usually characterized by behavioral deficits.
The rats in the ICH-MSC group exhibited less profound neurological
deficits and more neurological improvement on mNSS than the
ICH-only group at 1 day and 3 days following the induction of ICH,
with an insignificant difference (P>0.05). The recovery of
neurological functions was faster in the ICH-MSC group than in the
ICH group, and the difference was statistically significant at 7,
14, 21 and 28 days (P<0.05; t-test) (Fig. 2A). Compared with the ICH group,
transplantation of MSCs attenuated the hemorrhage volume by 52% at
24 h following ICH, and by 61% at 72 h following ICH (Fig. 2B).
MSCs increase proliferation and reduce
apoptosis of perihematomal cells
Brdu is a synthetic nucleoside and is commonly used
in the detection of proliferating cells in living tissues. Brdu
staining showed a high density of positively stained cells within
the hemorrhage lesions (Fig. 3A).
Quantitative analysis revealed differences in BrdU positively
stained cells between the two groups. The ICH-MSCs group exhibited
a significantly higher number of Brdu+ cells (261.5±34.3
cells/mm2) than the ICH group (1.9±0.75
cells/mm2; P<0.01; Fig.
3B). The ICH-MSCs group possessed significantly lower numbers
of caspase 3+ cells (118.7±34.8 cells/mm2) than were
observed in the ICH group (264.9±31.5 cells/mm2) at 1
day following ICH (P<0.01; Fig.
3B).
Neural protection and regeneration by
MSCs
To determine the survival and differentiation of
neural cells, immunohistochemistry was performed to detect the
protein expression of NF200 and GFAP. The ICH-MSCs group
demonstrated a higher expression of NF200 and GFAP than the ICH
group (Fig. 4A). Quantitative
analysis revealed differences between the two groups. The ICH-MSCs
group exhibited a significantly higher number of NF200+ and GFAP+
cells (NF200+: 295.7±28.5 cells/mm2; GFAP+: 178.8±32.6
cells/mm2) than the ICH group (NF200+: 133.7±23.6
cells/mm2; GFAP+: 62.6±16.0 cells/mm2)
(P<0.01; Fig. 4B).
MSCs upregulated anti-apoptotic
molecules, combined with the upregulation of G-CSF and BDNF
Western blotting demonstrated the upregulation of
Akt and bcl-2 expression induced by MSC transplantation (Fig. 5A). Analysis of the protein band
corresponding to Akt demonstrated a 3.6±0.6-fold increase in the
cytosolic extracts of hemorrhagic brain tissue at 24 h after the
onset of ICH (Fig. 5B). The bcl-2
band showed a 5.9±0.8-fold increase (Fig. 5C). The protein expression levels of
Akt and bcl-2 were significantly increased in the ICH-MSC group
compared to the ICH group (P<0.01, t-test). The sandwich ELISA
method was performed to detect the G-CSF and BDNF protein content
in the cytosolic extracts of brain tissue. Significantly higher
G-CSF and BDNF protein content was found in the ICH-MSC group than
in the ICH group (P<0.01, t-test) (Fig. 5D).
Discussion
In this study, we undertook to characterize the
therapeutic effects of MSCs and the mechanisms involved.
Intravenous MSC transplantation during the hyperacute stage (at 1 h
after ICH) was able to reduce initial neurological deterioration,
reduce hemorrhage volume and enhance functional recovery. MSCs
provided neuroprotection, which was confirmed by the increased
proliferation and reduced apoptosis of perihematomal cells. The
transplanted MSCs also differentiated into neural cells, with an
increased expression of anti-apoptotic proteins Akt and bcl-2 and
trophic factors G-CSF and BDNF.
Our results suggest that earlier intravenous MSC
administration is capable of reducing cerebral oedema following
hemorrhagic stroke and improving neural function. ICH results in
the mechanical disruption of brain tissue, including the neural and
glial cells. Therefore, it was formerly thought that neural
transplantation would be less likely to benefit this disorder.
However, a previous report showed that intravenously transplanted
NSCs are capable of entering the brain of mice with ICH, and are
able to survive, migrate and consequently improve functional
recovery (14), indicating
transplanted human NSCs are capable of restoring neurological
deficits in experimental ICH. The scarce sources of NSCs limit
their clinical application in ICH. MSCs were shown to possess stem
cell features similar to NSCs, such as the self-renewal potential
and multipotency, thus acting as potential candidate stem cells for
replacement therapy in ICH. Our in vivo study shows that
MSCs exhibit neuroprotective effects and promote functional
recovery following CNS injury.
Early hematoma enlargement occurs in approximately
35% of patients with ICH within 3 h after onset, and the hemorrhage
volume had been thought to be an independent predictor of poor
outcome (15). Moreover, hematoma
growth was also found to be a predictor of early neurological
deterioration (16). Therefore, it
has substantial clinical implications for early phase therapeutic
interventions, particularly within 3 h of onset (17). In this study, we measured the
hemorrhage volume at 24 and 72 h, and found a significantly reduced
hemorrhage volume at 24 and 72 h after ICH by MSCs. We also found
that the hemorrhage volume was smaller at 72 h than at 24 h,
indicating its spontaneous absorption over time. Notably,
transplanted MSCs are capable of accelerating this process.
In ICH, apoptosis is a prominent feature of
neurotoxicity in the perihematomal region of brains with ICH
(18). To investigate whether
transplanted MSCs were able to reduce neurotoxicity and protect
cells from apoptosis, immunohistochemistry was performed and a
significantly reduced caspase 3 protein expression was found in
perihematomal regions with MSC transplantation. Since transplanted
MSCs attenuated the activities of caspase-3, which is an executive
molecule in the common apoptotic pathway, we speculate that MSCs
are capable of alleviating the intrinsic and extrinsic apoptotic
pathways, which are activated in ICH. This suggests that the
neuroprotective functions of MSCs may partly result from the
elevated survival of neural cells in the perihematomal region of
brains with ICH.
To explore whether the elevated survival of neural
cells are caused by transplanted MSCs, we labeled MSCs with Brdu to
track these cells in the brains of ICH rats. A large number of
Brdu-labeled MSCs were found in perihematomal regions, which
indicates that the transplanted MSCs are capable of entering the
damaged brain tissue and initiating proliferation. Therefore, the
stem cell properties of MSCs make them replicate or differentiate
into neural cells in the rat brain following ICH, which could
explain the improved neural function after MSC transplantation
(10).
The NSCs and neural progenitor cells (NPCs) reside
in the subventricular zone (SVZ) in the adult mammalian brain.
Neurogenesis occurs in response to brain injury induced by ICH
(4,5), and indicates possible spontaneous
improvement following ICH, which is partly confirmed by a declining
curve over time in the mNSS test (Fig.
2A). In addition, transplanted MSCs promoted this process and
improved neural function at late time points following ICH. To
confirm the neurogenesis effect by transplanted MSCs, we detected
the expression of phenotypic markers of mature neural cells in
perihematomal regions. The results showed that the expression of
NF200 and GFAP markers were increased following MSC
transplantation. The high molecular weight neurofilament marker
NF200 is exclusively expressed on myelinated neurons (19), and GFAP was expressed on glial
cells. Our result indicates enhanced neural differentiation after
MSC transplantation in rats with ICH. The increased neural cells
may come from endogenous neurogenesis or differentiation of MSCs.
Previous reports showed that MSCs which were placed in the
neurogenic area (hippocampus) generated neurons positive for NF200
(20), and GFAP+ glial cells were
derived from transplanted MSCs in rats with experimental SAH
(21). Those studies indicated
that at least a substantial portion of increased neural cells
originate from transplanted MSCs.
To explore the molecular mechanisms underlying
improved survival and differentiation of neural cells, we measured
the protein content of Akt, bcl-2, G-CSF and BDNF in the brain
tissues of perihematomal regions, and found their expression was
increased following MSC transplantation compared with the ICH
group. Akt is a serine/threonine protein kinase and is crucial in
cellular survival pathways by inhibiting apoptotic processes.
Phosphorylation of Akt was reported to be enhanced after SAH
(22), and this suggests that Akt
may be involved in the survival pathway of injured brains after
SAH. Moreover, overexpressed Akt1 in human NSCs provide
neuroprotection and functional improvement in a mouse stroke model
(23). In this study, we found
that Akt was activated in rat brain with ICH by MSCs, indicating a
similar survival pathway between NSCs and MSCs. Whether Akt
overexpression is capable of promoting neuronal survival requires
further investigation. The increased expression of the bcl-2
protein in this study was also confirmed by another report. It
showed that the expression of bcl-2 was upregulated by Ginsenoside
Rbeta1 treatment, which reduced neurological damage and apoptosis
in the rat brain following SAH (24). This indicates that bcl-2 may be a
common survival pathway in intracerebral hemorrhage induced by
different therapeutics, and it therefore also plays a key role in
the increased survival of neural cells by transplanted MSCs.
Our study found an increased G-CSF and BDNF protein
content following MSC transplantation. Notably, the therapeutic
effect of MSC transplantation may be mediated through increased
G-CSF. It induces long-term sensorimotor recovery following ICH and
is involved in reduced brain edema, inflammation, proliferation of
NSCs and perihematomal cell death (25). We used bone marrow-derived MSCs in
this study, which were reported to secrete more cytokines than
umbilical cord blood-derived MSCs, indicating a higher possible
therapeutic effect by MSCs from bone marrow than umbilical cord
blood. BDNF is a type of growth factor that plays a key role in the
growth and differentiation of central and peripheral nervous
systems. BDNF is synthesized and expressed in neuronal and glial
cells (26). Decreased BDNF
concentrations are associated with intraventricular hemorrhage
(27). Brain transplantation of
BDNF-overexpressed human NSCs provided the differentiation and
survival of NSCs and functional recovery of ICH animals (28). In addition to the BDNF expressed by
neural cells, the increased BDNF in our study may also be secreted
from grafted bone marrow-derived MSCs (29). Moreover, BDNF treatment also
exerted an enforcing effect on MSCs and increased the BDNF
production by MSCs, thus forming an autocrine regulation of MSCs
(30).
In conclusion, we have provided convincing evidence
to suggest that MSC transplantation exerts therapeutic effects in
experimental ICH by improving neural function and reducing
hemorrhage volume. The underlying mechanisms involve enhanced
survival, the differentiation of neural cells and an elevated
expression of anti-apoptotic proteins and trophic factors.
Increased understanding of the mechanisms underlying
neuroprotection by MSCs may lead to new treatments for ICH.
References
|
1
|
Qureshi AI, Mendelow AD and Hanley DF:
Intracerebral haemorrhage. Lancet. 373:1632–1644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakatomi H, Kuriu T, Okabe S, Yamamoto S,
Hatano O, Kawahara N, et al: Regeneration of hippocampal pyramidal
neurons after ischemic brain injury by recruitment of endogenous
neural progenitors. Cell. 110:429–441. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang
Y, et al: Evidence for stroke-induced neurogenesis in the human
brain. Proc Natl Acad Sci USA. 103:13198–13202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Masuda T, Isobe Y, Aihara N, Furuyama F,
Misumi S, Kim TS, et al: Increase in neurogenesis and neuroblast
migration after a small intracerebral hemorrhage in rats. Neurosci
Lett. 425:114–119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen J, Xie L, Mao X, Zhou Y, Zhan R,
Greenberg DA, et al: Neurogenesis after primary intracerebral
hemorrhage in adult human brain. J Cereb Blood Flow Metab.
28:1460–1468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sgubin D, Aztiria E, Perin A, Longatti P
and Leanza G: Activation of endogenous neural stem cells in the
adult human brain following subarachnoid hemorrhage. J Neurosci
Res. 85:1647–1655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li F, Liu Y, Zhu S, Wang X, Yang H, Liu C,
et al: Therapeutic time window and effect of intracarotid neural
stem cells transplantation for intracerebral hemorrhage.
Neuroreport. 18:1019–1023. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deng W, Obrocka M, Fischer I and Prockop
DJ: In vitro differentiation of human marrow stromal cells into
early progenitors of neural cells by conditions that increase
intracellular cyclic AMP. Biochem Biophys Res Commun. 282:148–152.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dezawa M, Kanno H, Hoshino M, Cho H,
Matsumoto N, Itokazu Y, et al: Specific induction of neuronal cells
from bone marrow stromal cells and application for autologous
transplantation. J Clin Invest. 113:1701–1710. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Huang Z, Xu Y and Zhang S:
Differentiation and neurological benefit of the mesenchymal stem
cells transplanted into the rat brain following intracerebral
hemorrhage. Neurol Res. 28:104–112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosenberg GA, Mun-Bryce S, Wesley M and
Kornfeld M: Collagenase-induced intracerebral haemorrhage in rats.
Stroke. 21:801–807. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Schallert T, Zhang ZG, Jiang Q,
Arniego P, Li Q, et al: A test for detecting long-term sensorimotor
dysfunction in the mouse after focal cerebral ischemia. J Neurosci
Methods. 117:207–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee ST, Chu K, Sinn DI, Jung KH, Kim EH,
Kim SJ, et al: Erythropoietin reduces perihematomal inflammation
and cell death with eNOS and STAT3 activations in experimental
intracerebral hemorrhage. J Neurochem. 96:1728–1739. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH,
Park IH, et al: Brain transplantation of immortalized human neural
stem cells promotes functional recovery in mouse intracerebral
hemorrhage stroke model. Stem Cells. 25:1204–1212. 2007. View Article : Google Scholar
|
|
15
|
Davis SM, Broderick J, Hennerici M, Brun
NC, Diringer MN, Mayer SA, et al: Hematoma growth is a determinant
of mortality and poor outcome after intracerebral hemorrhage.
Neurology. 66:1175–1181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leira R, Davalos A, Silva Y, Gil-Peralta
A, Tejada J, Garcia M, et al: Early neurologic deterioration in
intracerebral hemorrhage: predictors and associated factors.
Neurology. 63:461–467. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mayer SA: Ultra-early hemostatic therapy
for intracerebral hemorrhage. Stroke. 34:224–229. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qureshi AI, Suri MF, Ostrow PT, Kim SH,
Ali Z, Shatla AA, et al: Apoptosis as a form of cell death in
intracerebral hemorrhage. Neurosurgery. 52:1041–1047. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lawson SN and Waddell PJ: Soma
neurofilament immunoreactivity is related to cell size and fibre
conduction velocity in rat primary sensory neurons. J Physiol.
435:41–63. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lepski G, Jannes CE, Strauss B, Marie SK
and Nikkhah G: Survival and neuronal differentiation of mesenchymal
stem cells transplanted into the rodent brain are dependent upon
microenvironment. Tissue Eng Part A. 16:2769–2782. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ali Khalili M, Anvari M, Hekmati-Moghadam
SH, Sadeghian-Nodoushan F, Fesahat F and Miresmaeili SM:
Therapeutic benefit of intravenous transplantation of mesenchymal
stem cells after experimental subarachnoid hemorrhage in rats. J
Stroke Cerebrovasc Dis. Jan 29–2011.[Epub ahead of print].
|
|
22
|
Endo H, Nito C, Kamada H, Yu F and Chan
PH: Akt/GSK3beta survival signaling is involved in acute brain
injury after subarachnoid hemorrhage in rats. Stroke. 37:2140–2146.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee HJ, Kim MK, Kim HJ and Kim SU: Human
neural stem cells genetically modified to overexpress Akt1 provide
neuroprotection and functional improvement in mouse stroke model.
PLoS One. 4:e55862009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Tang J, Khatibi NH, Zhu M, Chen D
and Zheng W: Ginsenoside Rbeta1 reduces neurologic damage, is
anti-apoptotic, and down-regulates p53 and BAX in subarachnoid
hemorrhage. Curr Neurovasc Res. 7:85–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Shu XJ, Zhou HY, Liu W, Chen Y,
Wang CL, et al: Protective effect of granulocyte colony-stimulating
factor on intracerebral hemorrhage in rat. Neurochem Res.
34:1317–1323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Radka SF, Holst PA, Fritsche M and Altar
CA: Presence of brain-derived neurotrophic factor in brain and
human and rat but not mouse serum detected by a sensitive and
specific immunoassay. Brain Res. 709:122–301. 1996. View Article : Google Scholar
|
|
27
|
Chouthai NS, Sampers J, Desai N and Smith
GM: Changes in neurotrophin levels in umbilical cord blood from
infants with different gestational ages and clinical conditions.
Pediatr Res. 53:965–969. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee HJ, Lim IJ, Lee MC and Kim SU: Human
neural stem cells genetically modified to overexpress brain-derived
neurotrophic factor promote functional recovery and neuroprotection
in a mouse stroke model. J Neurosci Res. 88:3282–3294. 2010.
View Article : Google Scholar
|
|
29
|
Wilkins A, Kemp K, Ginty M, Hares K,
Mallam E and Scolding N: Human bone marrow-derived mesenchymal stem
cells secrete brain-derived neurotrophic factor which promotes
neuronal survival in vitro. Stem Cell Res. Mar 27–2009.[Epub ahead
of print].
|
|
30
|
Choi YJ, Li WY, Moon GJ, Lee PH, Ahn YH,
Lee G, et al: Enhancing trophic support of mesenchymal stem cells
by ex vivo treatment with trophic factors. J Neurol Sci. 298:28–34.
2010. View Article : Google Scholar : PubMed/NCBI
|