Introduction
Cigarette smoke (CS) is a common inhaled oxidant.
Inhalation of CS has been shown to trigger various respiratory
defense reflexes, such as cough and bronchoconstriction in human
and animals (1,2). The activation of lung vagal C-fiber
afferents (LVCAs) is responsible for these defense reflexes
(3,4). The smoke-induced activation of LVCAs
and the triggering reflexes are suppressed by antioxidants
(5), suggesting the importance of
reactive oxygen species (ROS) (5–7).
Several studies suggest that LVCAs are important in detecting the
pulmonary ROS. However, the types of ROS involved in LVCA
activation by CS have not been fully elucidated.
Various types of pharmacological receptors are
located at the nerve endings of LVCAs. These receptors are involved
in the sensory detection of pulmonary ROS (5–9). In
our previous study, transient receptor potential ankyrin 1 (TRPA1)
and ionotropic P2X receptors were demonstrated to be important in
the sensory transduction of ROS-mediated activation of LVCAs
induced by airway CS exposure (5).
Transient receptor potential ankyrin vanilloid 1 (TRPV1) and TRPA1
receptors belong to the TRP superfamily. TRPV1 receptors have been
reported to be involved in the ROS-mediated activation of lung
vagal sensory neurons (6,7,10).
However, the contribution of TRPV1 in the CS-induced activation of
LVCAs remains to be fully elucidated.
CS contains high concentrations of nicotine
(11). Inhalation of CS with a
high concentration of nicotine triggers the activation of LVCAs
(12). Indeed, the application of
nicotine induces inward currents in isolated rat vagal pulmonary
sensory neurons (13). Both
nicotine-induced effects were nearly eliminated by pretreatment
with hexamethanium, suggesting contribution of the nicotinic
acetylcholine (nACh) receptors (12,13).
However, whether the nACh receptors are involved in the activation
of LVCAs by regular smoke remains unknown.
Among ROS, the hydroxyl radical (•OH) is
one of the most deleterious and reactive chemical species known
(14). The present study was
performed in anesthetized rats to determine i) the role of
•OH in the activation of LVCAs by inhaled CS and ii) the
roles of TRPV1, P2X and nACh receptors in •OH-mediated
LVCA activation.
Materials and methods
Animal preparation
The procedures described below were approved by the
Institutional Animal Care and Use Committee of Taipei Medical
University (Taipei, Taiwan). Male Sprague-Dawley rats (~420 g) were
anesthetized with an intraperitioneal injection of α-chloralose
(100 mg/kg) and urethane (500 mg/kg) in a borax solution (2%).
Right femoral artery and right jugular vein were cannulated for
recording the arterial blood pressure and the right-atrial
application of pharmacological agents, respectively. Body
temperature was maintained at ~36°C throughout the experiment by a
heating pad placed under the animal lying in a supine position. At
the end of the experiment, the animals were sacrificed by a
right-atrial injection of KCl (5,7,15).
Recording of LVCA activity
The animals were ventilated by a respirator via a
tracheal cannula inserted just below the larynx. Activity of
single-unit pulmonary afferents was recorded from thin filaments of
the right vagus nerve using the conventional ‘single fiber’
recording technique (5,8,9).
Briefly, a fine afferent filament of the right vagus nerve was
split and placed on a recording electrode for recording afferent
nerve activity. The thin filament was further split until the
afferent activity was electrically isolated. LVCAs were identified
by their intense and short-latency (<1 sec) response to
right-atrial injection of capsaicin (1 μg/kg) and relatively weak
response to hyperinflation of the lung (3–4 tidal volume). At the
end of each experiment, the general locations of fibers were
identified by their responses to pressing of the lungs with a
cotton Q-tip (5,7,15).
Smoke generation and delivery
A cigarette (Marlboro; 0.8 mg of nicotine and 10.0
mg of tar in each cigarette) was connected to a 50-ml syringe. With
a syringe pump, the smoke (20 ml) was then drawn into the syringe
(flow rate, 3 ml/sec) and was defined as 100% smoke. The 50% smoke
was made by the 100% smoke mixed with the same amount of air. A
total amount of 6 ml of smoke at a temperature of ~25°C was
delivered by the respirator within ~3 breaths (5).
Experimental protocols
In this study, 104 rats were classified into 13
groups to conduct 4 series of experiments. Each group contained 8
rats, and only one LVCA was studied in each animal.
In study series 1 (group 1), LVCA responses to air,
50% smoke and 100% CS were compared to assess the
concentration-response relationship. The sequence of these
challenges was alternated to achieve a balanced design. In study
series 2, (groups 2–7), LVCA responses to 100% CS were compared
before and after pretreatment with dimethylthiourea (DMTU, a
•OH scavenger; 1.5 g/kg), capsazepine (CPZ, a TRPV1
receptor antagonist; 3 mg/kg),
iso-pyridoxalphosphate-6-azophenyl-2′,5′-disulphonate (iso-PPADS, a
P2X receptor antagonist; 20 mg/kg) and their vehicles, in order to
study the role of •OH, TRPV1 and P2X receptors. In study
series 3 (groups 8–11), LVCA responses to 100% smoke were compared
before and after pretreatment with CPZ and iso-PPADS in
combination, CPZ, iso-PPADS and DMTU in combination, or their
vehicles, in order to assess the functional relationships of
•OH, TRPV1 and P2X receptors. In study series 4 (groups
12,13), LVCA responses to 100% smoke were compared before and after
pretreatment of hexamethonium (an antagonist of nACh receptor; 15
mg/kg), to assess the role of nACh receptors.
Materials
Solutions of these pharmacological agents at the
working concentrations were prepared daily by dilution with saline.
With the exception of iso-PPADS (Tocris Cookson, Bristol, UK), all
drugs were purchased from Sigma (St. Louis, MO, USA). The
antioxidant and all the receptor antagonists (~0.4 ml) were slowly
injected into the vein for a >20-sec duration.
Data and statistical analysis
LVCA fiber activity (FA) was continuously analyzed
at 1-sec intervals over an interval of ≥20 sec before and 120 sec
after airway challenge. Heart rate and mean arterial blood pressure
were continuously analyzed at 1-sec intervals. The parameters were
analyzed using a computer equipped with an analog-to-digital
converter (Gould DASA 4600, Gould Instrument Systems Inc., Valley
View, OH, USA) and a software (BioCybernetics 1.0). Data from study
series 1 were analyzed with one way ANOVA; data from the remaining
study series were analyzed with a two-way mixed factorial ANOVA.
When the ANOVA showed a significant interaction, pair-wise
comparisons were performed with a post hoc analysis (Fisher’s least
significant difference). A P-value of <0.05 was considered to
indicate a statistically significant difference. All the data are
the means ± standard error (SE).
Results
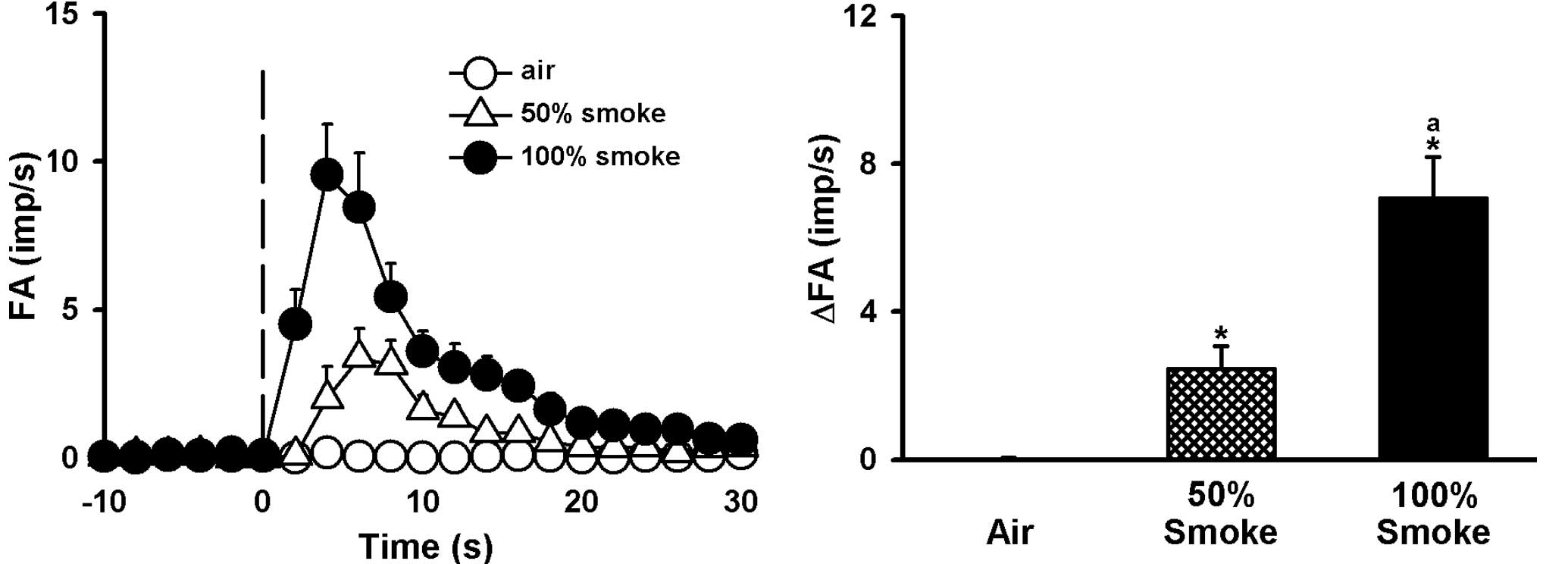
Concentration effect of CS on LVCAs
CS stimulated LVCA in a concentration-dependent
manner (Figs. 1 and 2). Airway exposure to 100% CS activated
all the LVCAs examined [change in fiber activity (ΔFA) =8.47±1.56
impulses/sec)], most of the LVCAs started to discharge within 2
sec, and the activity reached a peak in ~4 sec and returned to
baseline within 15 sec after the injection (Fig. 2). LVCA response to 50% smoke
(ΔFA=2.88±0.57 impulses/sec) was signicantly different from that to
the vehicle. Air challenge did not activate any of the LVCAs
examined (ΔFA=0.02±0.02 impulses/sec; Figs. 1C and 2).
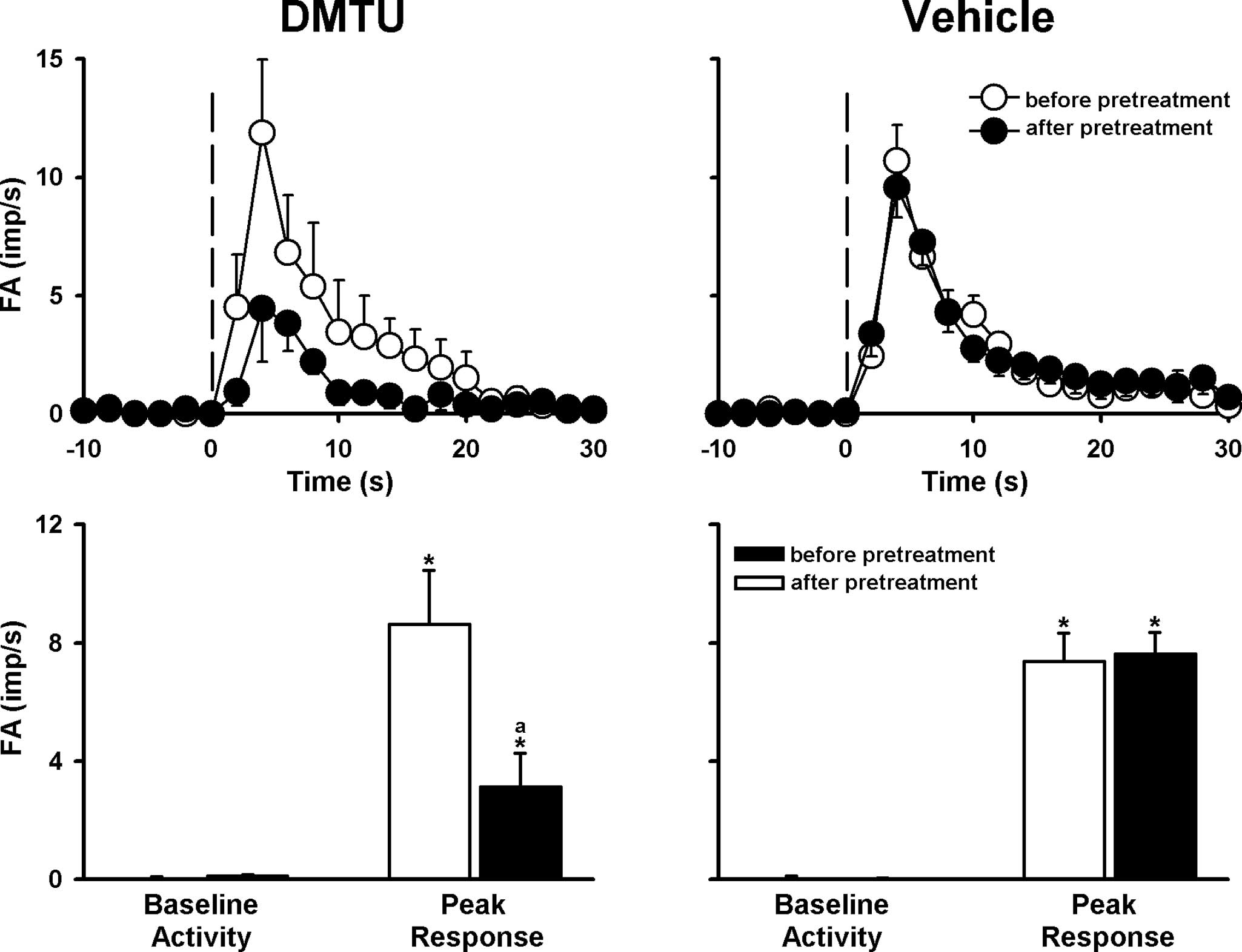
Role of •OH, TRPV1 and P2X
receptors
Airway challenge of CS caused LVCA activation, which
was attenuated by pretreatment with DMTU (ΔFA=−64%; Fig. 3). LVCA responses to 100% CS were
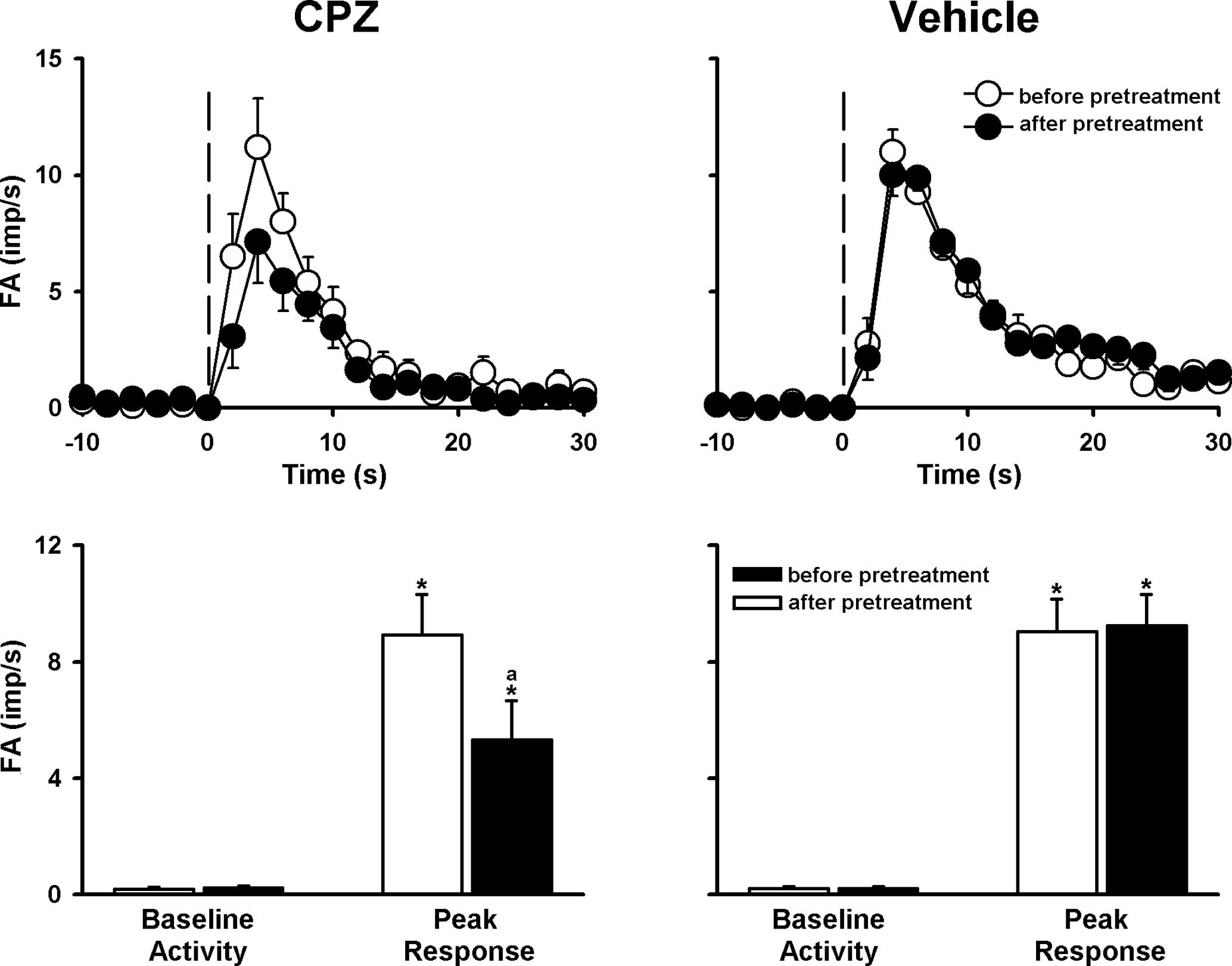
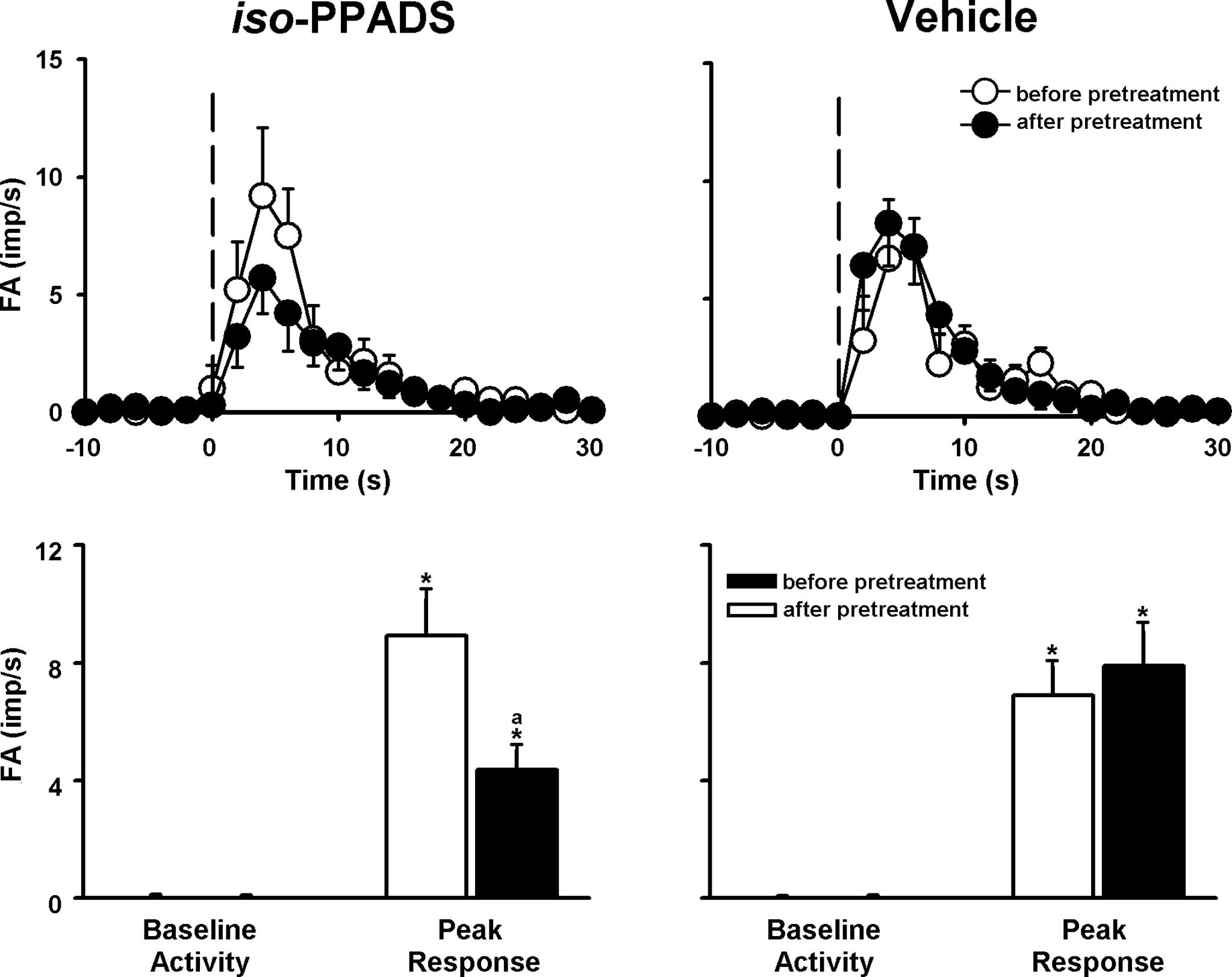
markedly suppressed by either CPZ (ΔFA=−40%; Fig. 4) or by iso-PPADS (ΔFA=−44%;
Fig. 5) and were further reduced
by a combination of these two antagonists (Figs. 6 and 7). Pretreatment with DMTU, CPZ and
iso-PPADS did not further suppress LVCA responses to CS (ΔFA=−67%;
Fig. 8), as compared with
pretreatment with DMTU alone. By contrast, CS-evoked LVCA response
was not significantly affected by pretreatment with any vehicle of
the antagonist or scavenger (Figs.
3–6).
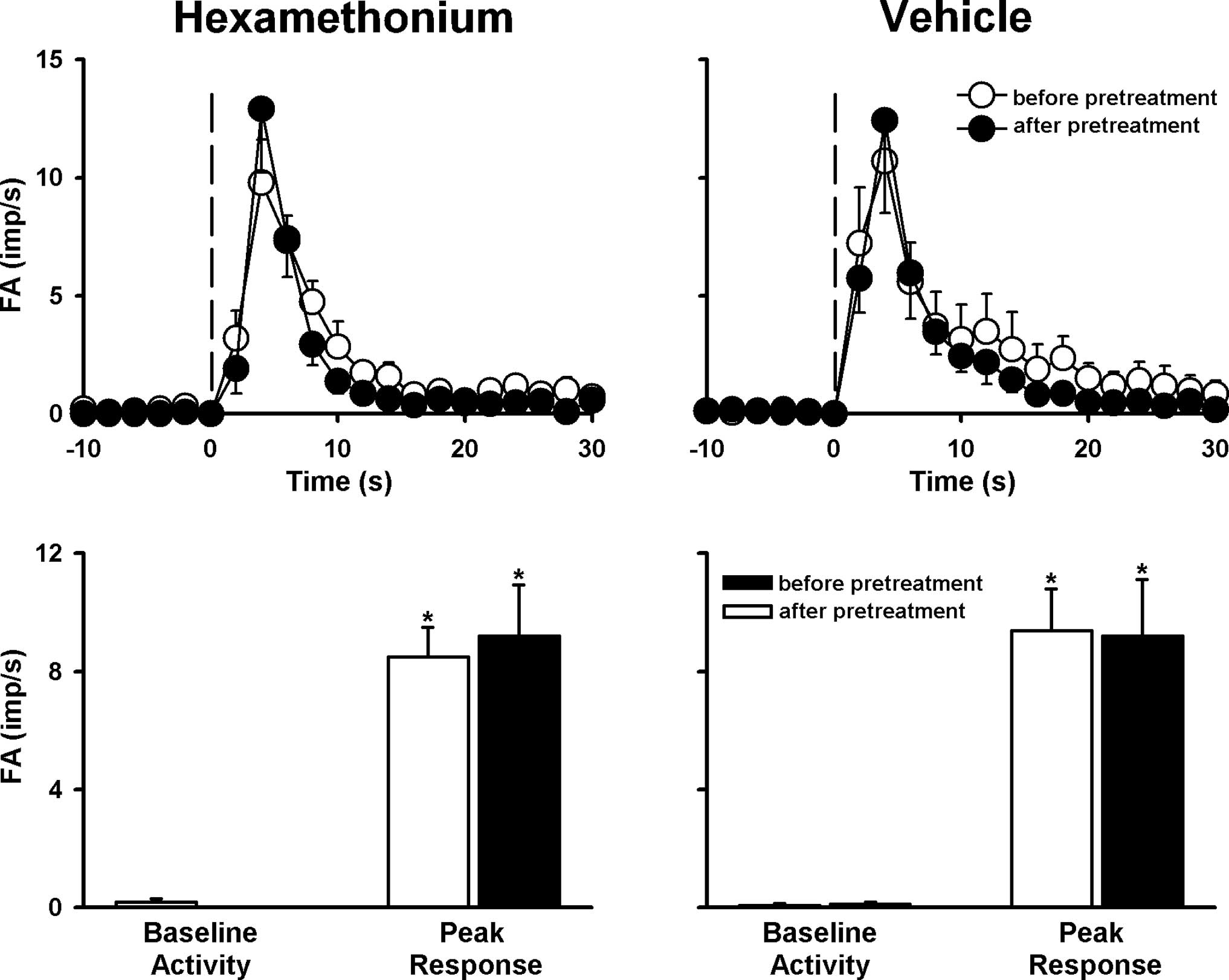
Role of nAChR receptors
Pretreatment with hexamethonium did not influence
LVCA responses to 100% CS, whereas pretreatment with the vehicle of
hexamethonium failed to cause this effect (Fig. 9).
Discussion
In our previous study, it was shown that airway
exposure to CS activated LVCAs which was mediated through TRPA1 and
P2X mechanisms (5). The novel
findings of the present study are that inhaled CS activates LVCAs
in rats through the action of •OH on TRPV1 and P2X
mechanisms, since pretreatment with •OH scavenger, TRPV1
and P2X antagonists in combination did not further suppress LVCA
responses to CS, as compared with pretreatment with •OH
scavenger alone. Furthermore, pretreatment with antagonist of nACh
receptors did not influence LVCA responses to CS, suggesting no
contribution of nACh receptors.
It has been reported that the CS-induced activation
of LVCAs is nearly mediated through the action of ROS (5). The type of ROS responsible for this
action is unclear. The present study demonstrated that pretreatment
with •OH scavenger reduced ~60% of smoke-evoked LVCA
response, indicating that •OH is the major ROS in
eliciting this activation. In the biological system, the three
major ROS are hydrogen peroxide, superoxide anion and
•OH (14). Among them,
•OH is the most reactive ROS produced in the biological
system (14). •OH was
reported to participate in the smoke-related LVCA-medicated
responses, such as plasma extravasation (16) and slowing in respiration (17).
Smoke-evoked activation is dependent on ROS
(5,12,15,16).
It has been shown that airway exposure to ROS triggers the
activation of LVCAs and their consequent reflexes in anesthetized
rats, which is, in part, mediated through the activation of TRPV1
receptor (6,7). Furthermore, application of oxidative
stress by 4-oxononenal evoked Ca2+ transient in cultured
pulmonary sensory neurons (10).
Taken together, these results support our findings that TRPV1
receptor is important to the CS-induced activation of LVCAs.
Nicotine in CS is believed to act as a causative
agent responsible for the stimulation of LVCA in dogs (1,3,12).
In addition, exogenous application of nicotine activates rat vagal
pulmonary sensory neurons (13).
These results are inconsistent with our finding according to which
nicotine is not involved in CS-evoked activation. Although there is
not an exact understanding of the mechanism, a possible explanation
for this inconsistency may be the different animal species used.
Nicotine is mainly present in the particulate phase of CS (18,19).
While CS is inhaled into lungs, the particular of smoke deposits
more in the larger/central airways compared with peripheral
airways. Therefore, nicotine contained in the CS may reach much
less to peripheral/small airways where LVCAs are preferentially
located (18,19). That might explain why nicotine
plays a role in CS-evoked LVCA activation in large animals (dogs)
but not in small animals (rats). This concept is supported by the
fact that, in a rat model (small animal), the activation of LVCAs
by CS was not altered when the smoke particle was eliminated by
glass-fiber Cambridge filter (17), suggesting no contribution of
nicotine in this LVCA activation.
In conclusion, both TRPV1 and P2X receptors, while
not nACh receptors, participate in the activation of LVCAs by
inhaled CS possibly through the action of •OH.
Acknowledgements
This study was supported by National Taipei
University of Technology-Taipei Medical University Joint Research
Program (NTUT-TMU-98-15).
References
|
1
|
Lee LY, Burki NK, Gerhardstein DC, Gu Q,
Kou YR and Xu J: Airway irritation and cough evoked by inhaled
cigarette smoke: role of neuronal nicotinic acetylcholine
receptors. Pulm Pharmacol Ther. 20:355–364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chung KF and Pavord ID: Prevalence,
pathogenesis and causes of chronic cough. Lancet. 371:1364–1374.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee LY and Gu Q: Cough sensors. IV.
Nicotinic membrane receptors on cough sensors. Pharmacology and
Therapeutics of Cough. Chung KF and Widdicombe J: Springer; New
York, NY: pp. 77–98. 2009, View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu J: Airway receptors and their reflex
function-invited article. Adv Exp Med Biol. 648:411–420. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin YS, Hsu CC, Bien MY, Hsu HC, Wing HT
and Kou YR: Activations of TRPA1 and P2X receptors are important in
ROS-mediated stimulation of capsaicin-sensitive lung vagal
afferents by cigarette smoke in rats. J Appl Physiol.
108:1293–1303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruan T, Lin YS, Lin KS and Kou YR:
Mediator mechanisms involved in TRPV1 and P2X receptor-mediated,
ROS-evoked bradypneic reflex in anesthetized rats. J Appl Physiol.
101:644–654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruan T, Lin YS, Lin KS and Kou YR: Sensory
transduction of pulmonary reactive oxygen species by
capsaicin-sensitive vagal lung afferent fibres in rats. J Physiol.
565:563–578. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bessac BF and Jordt SE: Breathtaking TRP
channels: TRPA1 and TRPV1 in airway chemosensation and reflex
control. Physiology (Bethesda). 23:360–370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bessac BF, Sivula M, von Hehn CA, Escalera
J, Cohn L and Jordt SE: TRPA1 is a major oxidant sensor in murine
airway sensory neurons. J Clin Invest. 118:1899–1910. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taylor-Clark TE, McAlexander MA,
Nassenstein C, Sheardown SA, Wilson S, Thornton J, Carr MJ and
Undem BJ: Relative contributions of TRPA1 and TRPV1 channels in the
activation of vagal bronchopulmonary C-fibres by the endogenous
autacoid 4-oxononenal. J Physiol. 586:3447–3459. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borgerding M and Klus H: Analysis of
complex mixtures - cigarette smoke. Exp Toxicol Pathol. 57(Suppl
1): 43–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee LY, Kou YR, Frazier DT, Beck ER,
Pisarri TE, Coleridge HM and Coleridge JC: Stimulation of vagal
pulmonary C-fibers by a single breath of cigarette smoke in dogs. J
Appl Physiol. 66:2032–2038. 1989.PubMed/NCBI
|
|
13
|
Xu J, Yang W, Zhang G, Gu Q and Lee LY:
Calcium transient evoked by nicotine in isolated rat vagal
pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol.
292:L54–L61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pryor WA: Oxy-radicals and related
species: their formation, lifetimes, and reactions. Annu Rev
Physiol. 48:657–667. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin YS and Lee LY: Stimulation of
pulmonary vagal C-fibres by anandamide in anaesthetized rats: role
of vanilloid type 1 receptors. J Physiol. 539:947–955. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lei YH, Barnes PJ and Rogers DF:
Involvement of hydroxyl radicals in neurogenic airway plasma
exudation and bronchoconstriction in guinea-pigs in vivo. Br J
Pharmacol. 117:449–454. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee LY: Inhibitory effect of gas phase
cigarette smoke on breathing: role of hydroxyl radical. Respir
Physiol. 82:227–238. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coggins CRE, Fouillet XL, Lam R and Morgan
KT: Cigarette smoke induced pathology of the rat respiratory tract:
a comparison of the effects of the particulate and vapor phases.
Toxicology. 16:83–101. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pankow JF: A consideration of the role of
gas/particle partitioning in the deposition of nicotine and other
tobacco smoke compounds in the respiratory tract. Chem Res Toxicol.
14:1465–1481. 2001. View Article : Google Scholar : PubMed/NCBI
|