Introduction
The incidence of breast cancer has increased
annually, and is a major threat to women’s health. With the
application of new chemotherapy drugs and molecular-targeted
therapy, the treatment of breast cancer has made marked progress,
but there remains no effective treatment for metastatic breast
cancer, and its mortality has not significantly reduced (1,2).
Gene therapy, considered as a promising alternative
treatment, has received increasing attention and has shown good
application prospects (3). Tumors
are composed of cancer and endothelial cells, which are
interdependent and have a mutually reinforcing vicious cycle
(4,5). Therefore, the best treatment strategy
is to kill cancer cells and destroy the cancer cell
microenvironment, which includes neovascular endothelial cells,
infiltrated immune cells and mesenchymal cells. In this study, we
successfully constructed a bifunctional plasmid expressing
ENDO-VEGI151 and survivin siRNA, and confirmed that it not only
promotes tumor cell apoptosis, but also has anti-angiogenic
effects. On the basis of these bifunctional activities (6), attenuated Salmonella
typhimurium (S. typhimurium) was selected to carry the
plasmid, and a human breast cancer model was established in nude
mice to further evaluate the efficacy of this new approach in
vivo. The purpose of this study was to evaluate comprehensive,
effective, convenient (oral bacteria) and cheaper new approaches
for breast cancer gene therapy.
Materials and methods
Reagents
Plasmids pENDO-VEGI151 (pEV),
pENDO-VEGI151/survivin-shRNA (pEV/si-survivin), pSurvivin-siRNA
(psi-survivin) and pcDNA3.1 were constructed and preserved by our
laboratory. The plasmid pEGFPN1, mouse Salmonella LB5000 and
attenuated S. typhimurium SL7207 were kindly provided by
Professor Zhao Ping from the Department of Microbiology, Second
Military Medical University, China. Human breast cancer cells
MDA-MB-231 were purchased from the cell bank of the Chinese Academy
(Shanghai, China). Rabbit anti-human survivin polyclonal antibody
was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA), rabbit anti-human VEGI151 polyclonal antibody was kindly
provided by Dr Zhang Min (Department of Microbiology, Second
Military Medical University), and the mouse anti-human factor
VIII-related antigen monoclonal antibody was purchased from Dako
(Carpinteria, CA, USA). β-actin antibody was purchased from Sigma
(St. Louis, MO, USA). The apoptosis detection kit was purchased
from GenScript (Piscataway, NJ, USA). An ultra-sensitive, SP
immunohistochemistry kit was purchased from Mai-xin Bio (Fuzhou,
China). Healthy, purebred, SPF, 4–6-week-old, female Balb/c nude
mice were purchased from the Experimental Animal Center of Second
Military Medical University.
Preparation of recombinant bacteria
Plasmids pEV/si-survivin, pEV, psi-survivin,
pcDNA3.1 and pEGFPN1 were transformed into calcium chloride-treated
murine S. typhimurium LB5000 cells, plasmids were extracted
using the Gene Pulser (Bio-Rad, Hercules, CA, USA) electroporation
instrument. We obtained 5 types of recombinant Salmonella
known as SL-pEV/si-survivin, SL-pEV, SL-psi-survivin, SL-pcDNA3.1
and SL-pEGFP. Salmonella SL7207 cells were attenuated with
10% glycerol.
Recombinant bacterial in vitro infection
and identification of therapeutic gene expression
Breast cancer cells MDA-MB-231 were inoculated into
24-well plates, grown to 80% confluence, washed with
phosphate-buffered saline (PBS) four times, and SL-pEV/si-survivin,
SL-pEV, SL-psi-survivin, SL-pcDNA3.1, or untransformed plasmid
empty bacteria SL7207 [1×106 colony forming units (CFU)]
were added into the recombinant bacteria, respectively. One hour
later at room temperature, PBS containing 50 μg/ml kanamycin was
used for washing 3 times, then cells were incubated with culture
medium RPMI-1640 containing the same concentration of kanamycin (to
kill extracellular bacteria) for 4 h. A final concentration of 10
μg/ml of tetracycline (inhibition of intracellular bacteria
proliferation) was then added, cells were incubated for 48 h, then
collected and lysed. Real-time fluorescence quantitative PCR and
western blot analysis were performed in order to detect the
expression of ENDO-VEGI151 and survivin.
Establishment of the human breast cancer
xenograft model and evaluation of bacterial distribution in
animals
Breast cancer cells MDA-MB-231 growing in the
logarithmic phase were collected and inoculated into nude mice
subcutaneously. Tumors were detected after 10 days. Tumor-bearing
nude mice were divided into 2 groups (n=6) and administered with
1×109 colony forming U/ml (cfu/ml−1) of
SL7207 (control group) and 0.1 ml (~108 cfu) SL-pEGFP
(experimental group). Tumor-bearing nude mice were sacrificed after
inoculation at 24 and 48 h, 5, 10, 20 and 35 days, respectively.
Frozen sections (12 μm) obtained from liver, spleen and tumor
tissues were analyzed with confocal microscopy (Leica TCS NT,
Wetzlar, Germany). Green fluorescent protein (GFP) was observed and
calculated to evaluate the distribution and survival time of
SL-pEGFP in vivo. The use of nude mice for this study was
approved by the Institutional Animal Care and Use Committee at the
Second Military Medical University, Shanghai, China.
Oral treatment of the tumor-bearing nude
mice with recombinant bacteria carrying a bifunctional expression
plasmid
Breast cancer cells were transplanted into the nude
mice model until tumors grew to 100 mm3, then the mice
were randomly divided into 6 groups: PBS, SL7207 empty bacteria,
SL-pcDNA3.1, SL-pEV, SL-psi-survivin and SL-pEV/si-survivin (n=5).
Each nude mouse was administered 5% NaHCO3 0.2 ml by
oral gavage, then given the corresponding recombinant S.
typhimurium (dosage 1×108 cfu/0.2 ml) by oral gavage
after 30 min. The control group was administered PBS by gavage.
Mice were inoculated 3 times, at an interval of 7 days. The longest
diameter (a) and shortest diameter (b) of the tumors were measured
weekly with vernier calipers and the tumor volume was calculated
according to the formula: V = πab2/6. Mice were
sacrificed at 8 weeks and the tumor was separated from the normal
tissues in order to calculate the tumor volume. Tumors were weighed
after cleaning with saline. After fixation with 15% neutral
buffered formalin, tumors were evaluated with conventional biopsy
and hematoxylin-eosin staining. FVIII factor, survivin and
ENDO-VEGF151 were detected by immunohistochemical staining and
apoptosis analysis was determined by TUNEL staining. Microvessel
density (MVD) was counted under ×200 objective light microscopy;
factor VIII antibody was used to show the vascular numbers, and the
results of the average number of blood vessels in five fields were
evaluated. The apoptotic index (AI) was evaluated with ×400
objective light microscopy. Five tumor areas were selected randomly
and the proportion of apoptosis-positive cells was calculated in
all cancer cells and the mean value was calculated.
Statistical analysis
SPSS 13.0 statistical software was used for analysis
of variance. P<0.05 was considered to indicate a statistically
significant difference. The mapping was achieved using Excel
(Microsoft).
Results
The efficiency of the recombinant S.
typhimurium infection and evaluation of expression
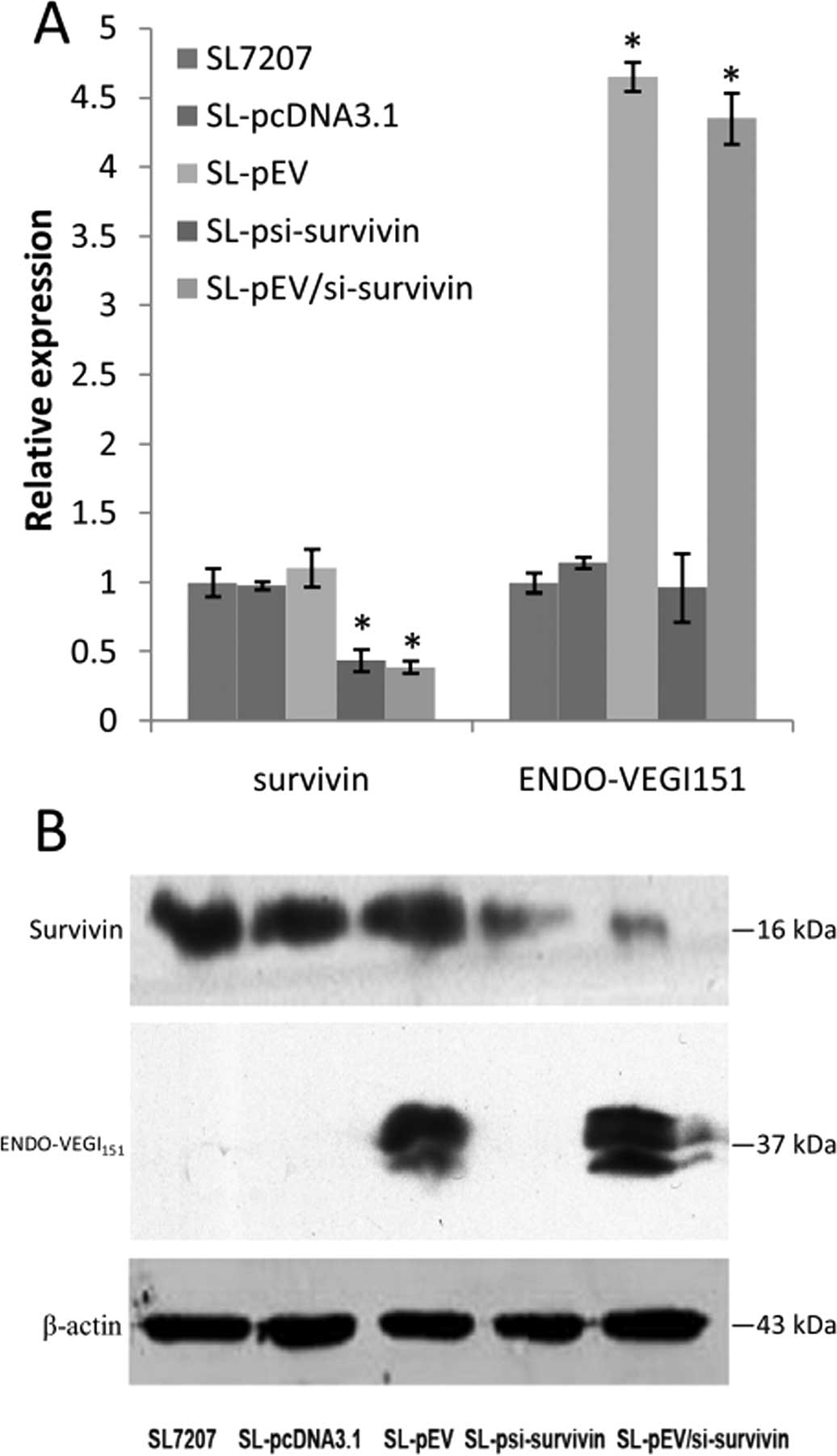
After 48-h infection of MDA-MB-231 cells with the
recombinant bacteria, real-time PCR results revealed that infection
with recombinant strain SL-psi-survivin and SL-pEV/si-survivin
reduced survivin mRNA levels by 2.28 and 2.57 times, respectively,
while the corresponding inhibition rates were 56 and 61%,
respectively (P<0.01). Survivin mRNA levels in the recombinant
strain SL-pEV and SL-pcDNA3.1-infected cells were similar to that
of empty bacteria SL7207-infected cells. Infection with recombinant
strain SL-pEV and SL-pEV/si-survivin increased the ENDO-VEGI151
mRNA level by 4.6 and 4.3 times (P<0.01), while the ENDO-VEGI151
mRNA level in cells infected with the recombinant strain
SL-psi-survivin and SL-pcDNA3.1 was similar to that of empty
bacteria SL7207-infected cells (Fig.
1A). Western blot analysis further confirmed at the protein
level that SL7207-pEV/Si-survivin infection can inhibit the
expression of survivin effectively while expressing ENDO-VEGI151
fusion protein at the same time (Fig.
1B).
Recombinant bacterial distribution and
survival time in nude mice
GFP expression was detected in a few organs at 24 h,
while marked GFP signals were detected in tumors at 48 h. Spotty
and sparse signals (Fig. 2) were
observed in liver and spleen tissues. GFP expression peaked at 5
days, with the expression almost disappearing after 10 days. GFP
expression in tumor tissues was still visible at 35 days, while no
fluorescent signal was detected in the control group.
The effect of recombinant bacterial
treatment on breast cancer cell tumor growth in nude mice
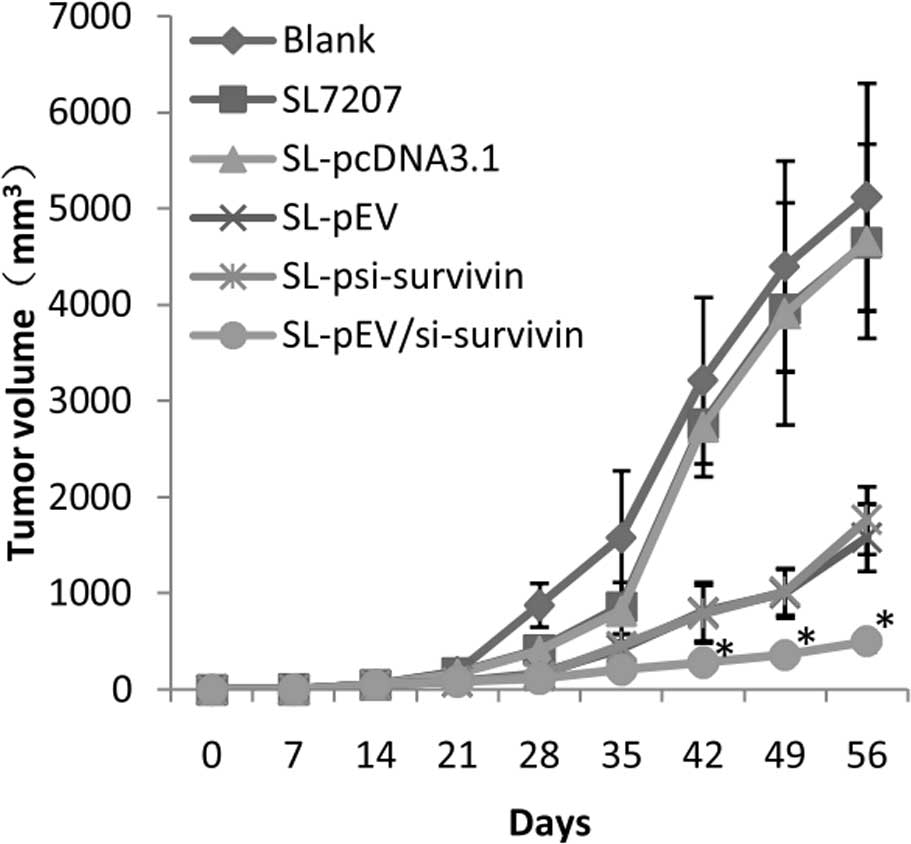
Following inoculation with MDA-MB-231 cells for 7
days, breast tumors were detected in nude mice. The tumor growth
curve in nude mice after breast cancer cell inoculation is shown in
Fig. 3. At day 15 after cancer
cell inoculation, vernier calipers were used to measure tumor
volume in order to monitor the tumor growth. The volume of tumors
from PBS, SL7207 empty bacteria, SL-pcDNA3.1, SL-pEV,
SL-psi-survivin and SL-pEV/si-survivin groups was measured from the
21st day (35 days) of the start of treatment (Fig. 4). The transplanted tumors in the
SL-pEV, SL-psi-survivin and SL-pEV/si-survivin groups grew slower
than the blank group with a statistically significant difference
(P<0.05). From the 35th day, the tumor volume of the
SL-pEV/si-survivin treatment group was significantly lower than
that of the SL-pEV and SL-psi-survivin groups. The difference was
more significant at the end of treatment (P<0.01). Compared with
the PBS group, attenuated Salmonella inhibited tumor growth
at an early stage with no statistically significant difference
(P>0.05), and its inhibitory effect diminished from the 49th
day, at which point the growth rate of the tumor accelerated
suddenly. The results show that SL-pEV/si-survivin significantly
inhibits tumor growth, while SL7207 and SL-pcDNA3.1 groups have
minimal impact on the efficacy (Table
I). This shows that the efficacy is not caused by the bacterial
carrier, but that the therapeutic genes play a role. The antitumor
effects of the SL-pEV/si-survivin, SL-pEV and SL-psi-survivin
groups were significantly better than that of the SL-pcDNA3.1
group. The SL-pEV/si-survivin treatment is significantly better
than the SL-psi-survivin or SL-pEV groups (P<0.01). There was no
significant difference (P>0.05) between the SL7207 and
SL-pcDNA3.1 groups.
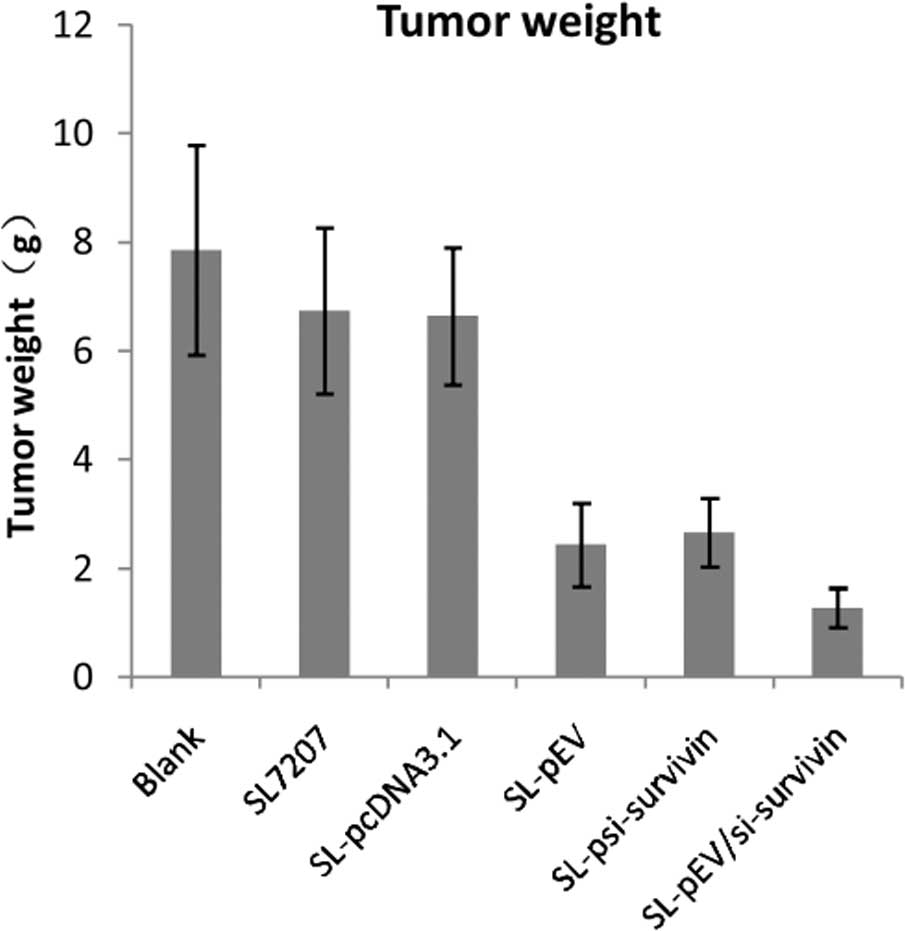
 | Table INude mice tumor volume, weight and
inhibition rate. |
Table I
Nude mice tumor volume, weight and
inhibition rate.
| Groups | Tumor volume
(mm3, mean ± SD) | Tumor weight (g, mean
± SD) | Tumor inhibition
rate |
|---|
|
|---|
| Volume (%) | Weight (%) |
|---|
| Vehicle control | 5119.64±1180.44 | 7.86±1.93 | - | - |
| SL7207 | 4644.69±1007.81 | 6.74±1.53 | 9.27 | 14.25 |
| SL-pcDNA3.1 | 4661.68±805.22 | 6.64±1.26 | 8.95 | 15.52 |
| SL-pEV |
1580.87±349.28a | 2.44±0.77a | 69.12a | 68.96a |
|
SL-psi-survivin |
1760.85±350.71a | 2.68±0.63a | 65.61a | 66.16a |
|
SL-pEV/si-survivin |
497.48±107.37a,b | 1.28±0.36a,b | 90.28a,b | 83.72a,b |
The evaluation of the bifunctional
expression plasmid in xenografts
ENDO-VEGI151 protein expression was detected in
transplanted tumors from the SL-pEV and SL-pEV/si-survivin groups.
The positive particles were distributed in the cytoplasm and
extracellular matrix. Due to the presence of IL3 signal, the fusion
protein is secreted into the extracellular matrix in order to
execute its function. There was no fusion protein expression in the
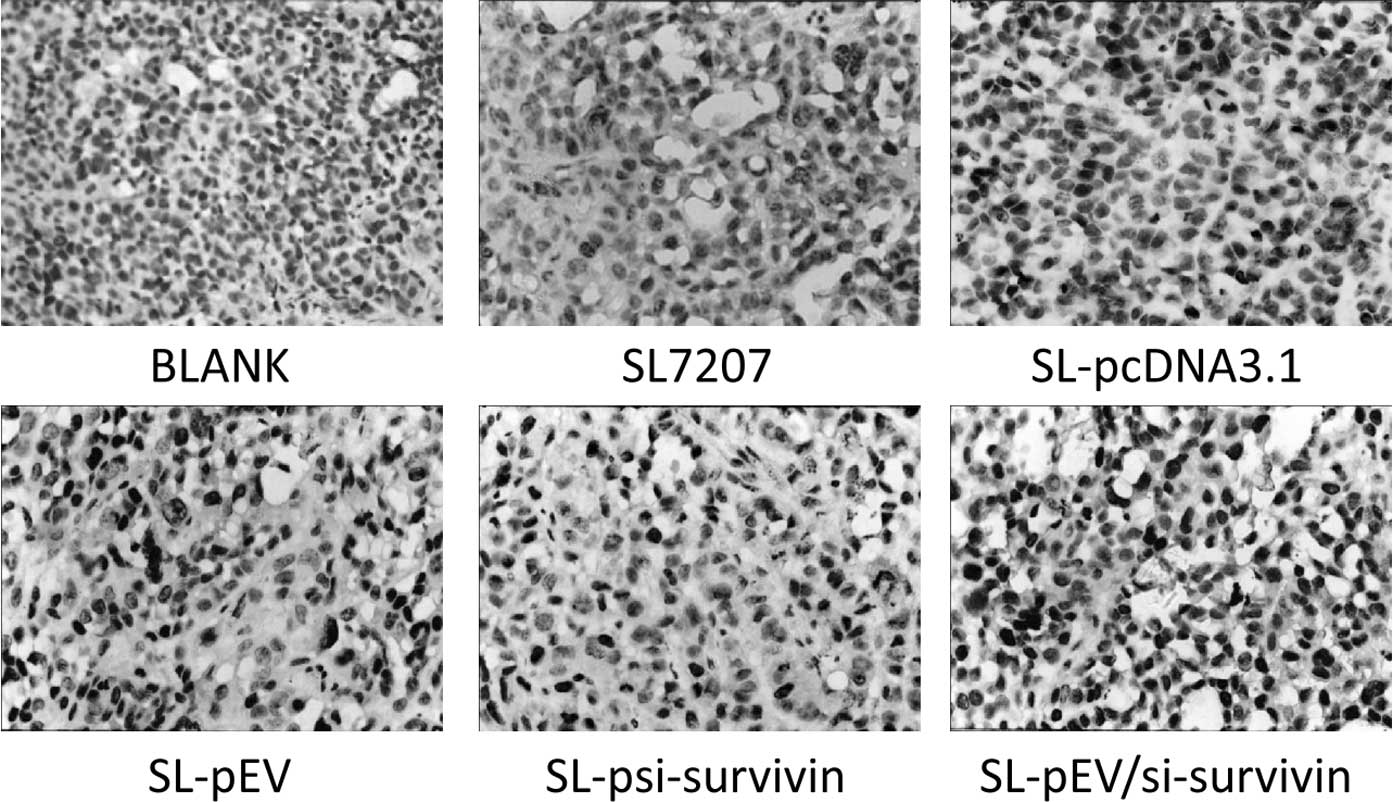
other groups (Fig. 5). Survivin
expression was detected in the tumor tissues of all groups. The
positive signal was mainly detected in the cytoplasm of tumor cells
with some located within the nucleus. The expression of survivin in
transplanted tumors of the SL-psi-survivin and SL-Pev/si-survivin
groups was significantly lower than that of the other treatment
groups (Fig. 6). This indicates
that SL-pEV/si-survivin effectively expressed ENDO-VEGI151 fusion
protein in vivo in transplanted tumor tissue while reducing
the expression of survivin protein.
Effects of bifunctional expression
plasmid treatment on angiogenesis and apoptosis in nude mice
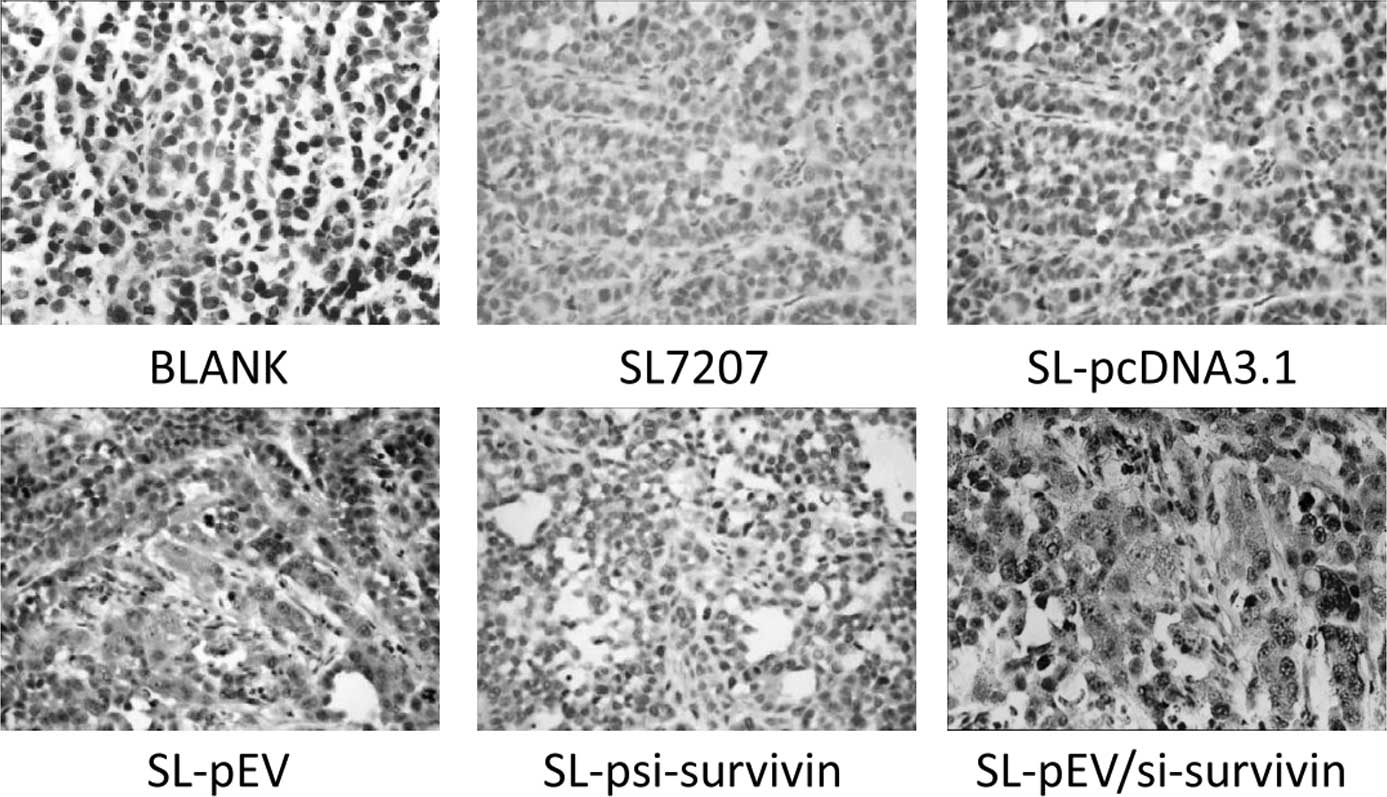
Tumor tissue sections stained with positive FVIII
factor signal were brown. Intensive, positive staining of
endothelial cells could be observed in the tumor stroma of the
control group with uneven microvascular distribution. The dense
area of staining was located at the edge of the foci. The blood
vessels in the group treated with SL-pEV/si-survivin or SL-pEV were
sparse, while the tumor cells grew around the vessels which had 4–6
layers and large nuclei were deeply stained. The tumor cells
outside these layers have apoptotic morphology. Large areas of
coagulative necrosis appeared around the apoptosis cells (Fig. 7). In the SL-pEV/si-survivin, SL-pEV
and SL-psi-survivin treatment groups, tumor cell apoptosis
increased significantly with clustered distribution, while the
SL7207, SL-pcDNA3.1 and control groups had only a scattered
distribution of apoptotic cells (Fig.
8).
MVD in the SL-pEV/si-survivin, SL-pEV and
SL-si-survivin groups was significantly lower than that in the
SL-pcDNA3.1, SL7207 and control groups (P<0.05). AI from the
SL-pEV/si-survivin, SL-pEV and SL-si-survivin treatment groups
increased significantly compared with SL-pcDNA3.1, SL7207 and
control groups (P<0.05). Compared with the two single genes and
the control group, MVD reduced significantly in the
SL-pEV/si-survivin bifunctional plasmid treatment group with a
significant increase in the proportion of apoptotic cells. Survivin
expression was significantly decreased (Table II) with a statistically
significant difference between the various groups (P<0.05).
 | Table IIMVD, AI and survivin expression in
tumors of nude mice. |
Table II
MVD, AI and survivin expression in
tumors of nude mice.
| Groups | MVD | AI | Survivin |
|---|
| Vehicle
control | 52.32±6.48 | 13.52±4.85 | 82.20±4.46 |
| SL7207 | 42.44±3.81 | 11.84±3.35 | 77.59±5.79 |
| SL-pcDNA3.1 | 40.28±4.89 | 11.73±2.92 | 77.64±5.51 |
| SL-pEV | 16.52±6.95a | 49.83±4.71a | 65.59±5.52a |
| SL-si-survivin | 28.44±4.39a | 54.71±7.63a | 38.03±5.44a |
|
SL-pEV/si-survivin | 13.48±4.95a,b | 60.09±5.89a,b | 36.46±5.98a |
Discussion
With a deeper understanding of tumor molecular
pathology, gene therapy is gaining increasing attention as a cancer
treatment, and shows great promise. Currently, cancer gene therapy
includes several treatment types: tumor gene antagonists (tumor
suppressor gene transfection/tumor gene inactivation) and induction
of apoptosis genes (pro-apoptotic gene introduction/inactivation of
anti-apoptotic genes), immune genes (cytokine/modified
antigen-presenting cell/tumor vaccine), oncolytic viral genes,
transduction suicide genes, multidrug resistance genes and
anti-angiogenic genes. Tumor vaccines, drug resistance gene and
anti-angiogenic gene therapies have achieved promising success in
the laboratory and clinical trials have been initiated (7). Tumorigenesis is a complex biological
process involving the activation of a variety of oncogenes and the
inactivation of tumor suppressor genes and other gene mutations. In
addition, tumors are composed of variant cell types; therefore,
gene therapy focusing on a single target achieved limited success
(8). The synergistic effects of
multi-targets using combined gene therapy should in theory be more
effective than a single gene target (9,10),
and be worthy of further investigations. However, the problem is
how to select ideal targets from a large number of candidates.
Abdollahi and Folkman (11) proposed the idea that tumors could
be starved through blood vessel blockade and the anti-angiogenic
doctrine has persisted for almost 40 years of accumulated research.
The central role of angiogenesis in tumor development and its value
as a therapeutic target has been recognized since 1971. A large
number of retrospective studies have shown that breast cancer is a
vascular-dependent disease and angiogenesis is important in breast
cancer invasion and metastasis and is an independent prognostic
factor. We selected tumor angiogenesis as a target for
antiangiogenic therapy and it shows good value and broad
application prospects in a number of large randomized trials
(12,13). Compared to conventional therapy
targeting tumor cells, anti-angiogenic therapy takes the relatively
stable genetic traits of vascular endothelial cells as a
therapeutic target; vascular endothelial cells possess two high
(high targeting specific, high efficiency) and two low ( low
adverse reactions, low resistance) properties. Particularly for the
resistance of micro-metastases and circulating tumor cells in the
patients’ body, anti-angiogenic therapy through neovascularization
inhibition blocks these micrometastases due to lack of oxygen, and
as it maintains the cells at a low mass, it not only notably
reduces the metastatic potential, but also makes the cells more
susceptible to attack by the immune system and prone to apoptosis.
However, antiangiogenic therapy is only a tumor inhibitory
treatment; therefore, it cannot completely remove the remaining
cancer cells in the patients’ body. We hypothesize that combination
with direct anticancer therapy is likely to remove tumors
completely (14,15). The hypoxic environment induced by
anti-angiogenic treatment increases the sensitivity of cancer cells
to apoptosis induction therapy (16,17).
Therefore, a combination of anti-angiogenesis with induction of
apoptosis may achieve the desired therapeutic effect through the
two mutually reinforcing synergistic complementary mechanisms.
Based on above assumptions, we used the
pre-constructed anti-angiogenesis gene ENDO-VEGI151 and survivin
siRNA bifunctional expression plasmid to induce tumor cell
apoptosis and inhibit angiogenesis simultaneously. Survivin is
known as the strongest anti-apoptotic factor (18), and has a correlation with
anti-angiogenesis drug resistance (19). HIF-1 expression is closely related
with survivin (20), and so is the
proliferation of endothelial cells (21). Thus we selected survivin as the
apoptosis induction therapeutic target. In vitro experiments
showed that the dual-function plasmid was capable of expressing the
ENDO-VEGI151 fusion protein and survivin siRNA in the transfected
breast cancer cells MDA-MB-231. In vitro transfection
experiments confirmed that the bifunctional expression plasmid
effectively inhibits the proliferation of breast cancer and
endothelial cells, and also induces apoptosis. It is expected to
establish a mutually reinforcing positive feedback antitumor
mechanism in the body, which can play cooperative complementary
roles.
There are always safety and efficiency concerns
about tumor gene therapy using vector targeting. In addition, the
main limitation of the viral vectors (including adenovirus and
retrovirus) used currently is that hypoxic phenomena weaken the
function of the viral vectors and the expression of target genes in
tumors, and certain cells are unlikely to be transfected by the
viral vector system, which will mean the tumor has the ability to
grow again. Although the solid tumor hypoxia phenomenon is fatal to
viral vectors, Salmonella is suitable for a hypoxic
environment. Hypoxia promotes Salmonella growth and
reproduction (22).
Salmonella is an aggressive intracellular bacterium, and the
response between it and the host is mediated by the type III
secretion mechanism. This mechanism causes the bacterial
performance protein transfer into eukaryotic cells and directly
expresses a variety of therapeutic proteins. These two features
made Salmonella a suitable choice of tumor gene targeting
vector. With genetic engineering techniques, we were able to knock
out the Salmonella pathogenicity gene to obtain attenuated
strains in order to guarantee the safety of the vector (23). The most attractive features of
attenuated Salmonella are that it is capable of infecting
the tumor cells through vaccination by the mucosal route (oral or
nasal) and as a biological carrier its unique remote tumor
targeting effect has been demonstrated in the treatment of a
variety of tumor models. Attenuated S. typhimurium that
carries the therapeutic genes has achieved promising results in
animal experiments, including in mouse melanoma, liver cancer,
renal cell carcinoma, breast cancer and gastric cancer (24,25).
The key problem in RNAi treatment is that RNAi fragments do not
reach the effective site and achieve the effective dose. Ji et
al confirmed in the mouse prostate model that S. typhi
is capable of carrying siRNA fragments deep inside the tumor and
can inhibit tumor growth and metastasis (24). S. typhi has a high degree of
tumor targeting; therefore, it is a good targeting vector for RNA
interference.
In this study, we selected attenuated S.
typhimurium strain SL7207 that has deleted 5-enol acetone
phthalocyanine oxalate-3-phosphate synthase aroA gene as a
dual-function plasmid vector in order to evaluate the activity of
the dual-function plasmid in vivo by oral gavage treatment
of nude mice. SL7207 with the aroA gene defect is only able to
proliferate to a limited level in mammalian cells prior to death,
and then the bacteria release the expression plasmid inside the
bacteria. These plasmids express their encoded protein in the
cytoplasm. This bacterial strain has low toxicity but still retains
the cell invasive property, and is capable of carrying encoded
exogenous gene eukaryotic expression plasmid into the tumor tissue
and ultimately achieving therapeutic effects by the gene expression
product (26). The results show
that SL7207 infects breast cancer cells effectively in vitro
and in vivo. Although SL7207 gathered in the normal organs
of the liver and spleen within a short time, it is capable of
remaining at high concentrations in tumors for long periods of time
and can express the targeting products effectively. As the
expression of ENDO-VEGI151 recombinant protein and its
anti-angiogenic effect could be affected by survivin siRNA-induced
tumor cell apoptosis, the two therapeutic genes have a synergistic
effect in tumor elimination. However, due to the phase difference
of apoptosis, non-apoptotic or not fully apoptotic cells may
express ENDO-VEGI151. ENDO-VEGI151 may be secreted to the
extracellular domain due to the presence of IL3 signal peptide. Our
immunohistochemical results also suggest that interstitial tumor
tissue also has fusion protein expression, therefore we suggest
that the expression of the two target genes did not interfere with
each other. However, for combination therapy, the appropriate ratio
of the two drugs (ENDO-VEGI and survivin small molecule siRNA) is
essential for optimizing their synergistic effect and safety, and
this should be explored further.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 51003078), the Shanghai Municipal
Health Bureau Research Projects (no. 2008133) and the Shanghai
Municipal Science and Technology Commission (No. 12140902302).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Grammatikakis I, Zervoudis S and Kassanos
D: Synopsis of new antiangiogenetic factors, mutation compensation
agents, and monoclonal antibodies in target therapies of breast
cancer. J BUON. 15:639–646. 2010.PubMed/NCBI
|
|
3
|
Stoff-Khalili MA, Dall P and Curiel DT:
Gene therapy for carcinoma of the breast. Cancer Gene Ther.
13:633–647. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Butler JM, Kobayashi H and Rafii S:
Instructive role of the vascular niche in promoting tumour growth
and tissue repair by angiocrine factors. Nat Rev Cancer.
10:138–146. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weigand M, Hantel P, Kreienberg R and
Waltenberger J: Autocrine vascular endothelial growth factor
signalling in breast cancer. Evidence from cell lines and primary
breast cancer cultures in vitro. Angiogenesis. 8:197–204. 2005.
View Article : Google Scholar
|
|
6
|
Li Z, Yang S, Chang T, Cao X, Shi L and
Fang G: Anti-angiogenesis and anticancer effects of a plasmid
expressing both ENDO-VEGI151 and small interfering RNA against
survivin. Int J Mol Med. 29:485–490. 2012.PubMed/NCBI
|
|
7
|
Cao S, Cripps A and Wei MQ: New strategies
for cancer gene therapy: progress and opportunities. Clin Exp
Pharmacol Physiol. 37:108–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jimeno A and Hidalgo M: Multitargeted
therapy: can promiscuity be praised in an era of political
correctness? Crit Rev Oncol Hematol. 59:150–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Qu ZH, Cui M, Guo C, Zhang XM, Ma
CH and Sun WS: Combined endostatin and TRAIL gene transfer
suppresses human hepatocellular carcinoma growth and angiogenesis
in nude mice. Cancer Biol Ther. 8:466–473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu B, Zhang Y, Zhan Y, Zha X, Wu Y, Zhang
X, Dong Q, Kong W and Yu X: Co-expression of herpes simplex virus
thymidine kinase and Escherichia coli nitroreductase by an
hTERT-driven adenovirus vector in breast cancer cells results in
additive anti-tumor effects. Oncol Rep. 26:255–264. 2011.PubMed/NCBI
|
|
11
|
Abdollahi A and Folkman J: Evading tumor
evasion: current concepts and perspectives of anti-angiogenic
cancer therapy. Drug Resist Updat. 13:16–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miles DW, Chan A, Dirix LY, et al: Phase
III study of bevacizumab plus docetaxel compared with placebo plus
docetaxel for the first-line treatment of human epidermal growth
factor receptor 2-negative metastatic breast cancer. J Clin Oncol.
28:3239–3247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Robert NJ, Diéras V, Glaspy J, et al:
RIBBON-1: randomized, double-blind, placebo-controlled, phase III
trial of chemotherapy with or without bevacizumab for first-line
treatment of human epidermal growth factor receptor 2-negative,
locally recurrent or metastatic breast cancer. J Clin Oncol.
29:1252–1260. 2011. View Article : Google Scholar
|
|
14
|
Kerbel RS: Issues regarding improving the
impact of antiangiogenic drugs for the treatment of breast cancer.
Breast. 18(Suppl 3): S41–S47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leite de Oliveira R, Hamm A and Mazzone M:
Growing tumor vessels: more than one way to skin a cat
-implications for angiogenesis targeted cancer therapies. Mol
Aspects Med. 32:71–87. 2011.PubMed/NCBI
|
|
16
|
Pang RW and Poon RT: Clinical implications
of angiogenesis in cancers. Vasc Health Risk Manag. 2:97–108. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eichhorn ME, Kleespies A, Angele MK, Jauch
KW and Bruns CJ: Angiogenesis in cancer: molecular mechanisms,
clinical impact. Langenbecks Arch Surg. 392:371–379. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carrasco RA, Stamm NB, Marcusson E,
Sandusky G, Iversen P and Patel BK: Antisense inhibition of
survivin expression as a cancer therapeutic. Mol Cancer Ther.
10:221–232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rice C and Huang LE: From antiangiogenesis
to hypoxia: current research and future directions. Cancer Manag
Res. 3:9–16. 2010.PubMed/NCBI
|
|
20
|
Peng XH, Karna P, Cao Z, Jiang BH, Zhou M
and Yang L: Cross-talk between epidermal growth factor receptor and
hypoxia-inducible factor-1alpha signal pathways increases
resistance to apoptosis by up-regulating survivin gene expression.
J Biol Chem. 281:25903–25914. 2006. View Article : Google Scholar
|
|
21
|
Liu C, Liang B, Wang Q, Wu J and Zou MH:
Activation of AMP-activated protein kinase alpha1 alleviates
endothelial cell apoptosis by increasing the expression of
anti-apoptotic proteins Bcl-2 and survivin. J Biol Chem.
285:15346–15355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gardlik R and Fruehauf JH: Bacterial
vectors and delivery systems in cancer therapy. IDrugs. 13:701–706.
2010.PubMed/NCBI
|
|
23
|
Gardlik R, Behuliak M, Palffy R, Celec P
and Li CJ: Gene therapy for cancer: bacteria-mediated
anti-angiogenesis therapy. Gene Ther. 18:425–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ji K, Wang B, Shao YT, et al: Synergistic
suppression of prostatic cancer cells by coexpression of both
murine double minute 2 small interfering RNA and wild-type p53 gene
in vitro and in vivo. J Pharmacol Exp Ther. 338:173–183. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu X, Zhou P, Cai J, Yang G, Liang S and
Ren D: Tumor antigen delivered by Salmonella III secretion protein
fused with heat shock protein 70 induces protection and eradication
against murine melanoma. Cancer Sci. 101:2621–2628. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu XL, Jiang XB, Liu RE and Zhang SM: The
enhanced anti-angiogenic and antitumor effects of combining
flk1-based DNA vaccine and IP-10. Vaccine. 26:5352–5357. 2008.
View Article : Google Scholar : PubMed/NCBI
|