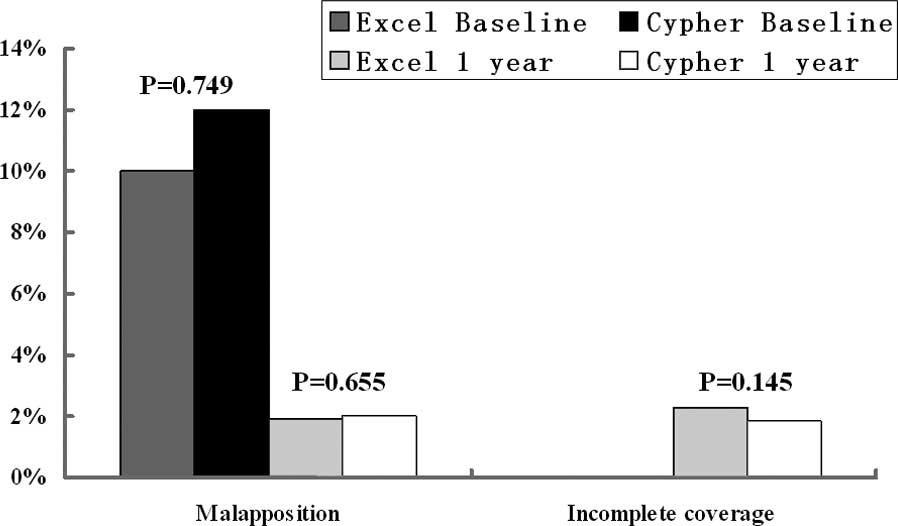
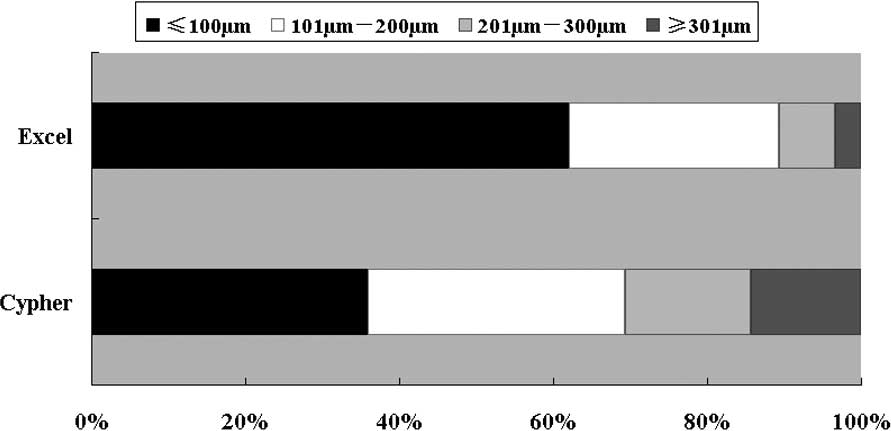
|
1
|
Iakovou I, Schmidt T, Bonizzoni E, et al:
Incidence, predictors, and outcome of thrombosis after successful
implantation of drug-eluting stents. JAMA. 293:2126–2130. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuchulakanti PK, Chu WW, Torguson R, et
al: Correlates and long-term outcomes of angiographically proven
stent thrombosis with sirolimus- and paclitaxel-eluting stents.
Circulation. 113:1108–1113. 2006. View Article : Google Scholar
|
|
3
|
McFadden EP, Stabile E, Regar E, et al:
Late thrombosis in drug-eluting coronary stents after
discontinuation of antiplatelet therapy. Lancet. 364:1519–1521.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guagliumi G, Farb A, Musumeci G, Valsecchi
O, Tespili M, Motta T and Virmani R: Images in cardiovascular
medicine. Sirolimus-eluting stent implanted in human coronary
artery for 16 months: pathological findings. Circulation.
107:1340–1341. 2003. View Article : Google Scholar
|
|
5
|
Byrne RA, Joner M and Kastrati A: Polymer
coatings and delayed arterial healing following drug-eluting stent
implantation. Minerva Cardioangiol. 57:567–584. 2009.PubMed/NCBI
|
|
6
|
Takano M, Inami S, Jang IK, Yamamoto M,
Murakami D, Seimiya K, Ohba T and Mizuno K: Evaluation by optical
coherence tomography of neointimal coverage of sirolimus-eluting
stent three months after implantation. Am J Cardiol. 99:1033–1038.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsumoto D, Shite J, Shinke T, Otake H,
Tanino Y, Ogasawara D, Sawada T, Paredes OL, Hirata K and Yokoyama
M: Neointimal coverage of sirolimus-eluting stents at 6-month
follow-up: evaluated by optical coherence tomography. Eur Heart J.
28:961–967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yabushita H, Bouma BE, Houser SL, Aretz
HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Kang DH,
Halpern EF and Tearney GJ: Characterization of human
atherosclerosis by optical coherence tomography. Circulation.
106:1640–1645. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jang IK, Tearney GJ, MacNeill B, Takano M,
Moselewski F, Iftima N, Shishkov M, Houser S, Aretz HT, Halpern EF
and Bouma BE: In vivo characterization of coronary atherosclerotic
plaque by use of optical coherence tomography. Circulation.
111:1551–1555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manfrini O, Mont E, Leone O, Arbustini E,
Eusebi V, Virmani R and Bugiardini R: Sources of error and
interpretation of plaque morphology by optical coherence
tomography. Am J Cardiol. 98:156–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kume T, Akasaka T, Kawamoto T, Ogasawara
Y, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y and
Yoshida K: Assessment of coronary arterial thrombus by optical
coherence tomography. Am J Cardiol. 97:1713–1717. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kotani J, Awata M, Nanto S, Uematsu M,
Oshima F, Minamiguchi H, Mintz GS and Nagata S: Incomplete
neointimal coverage of sirolimus-eluting stents: angioscopic
findings. J Am Coll Cardiol. 47:2108–2111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joner M, Finn AV, Farb A, Mont EK,
Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK and Virmani R:
Pathology of drug-eluting stents in humans: delayed healing and
late thrombotic risk. J Am Coll Cardiol. 48:193–202. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Onuma Y, Serruys P, den Heijer P, et al:
MAHOROBA, first-in-man study: 6-month results of a biodegradable
polymer sustained release tacrolimus-eluting stent in de novo
coronary stenoses. Eur Heart J. 30:1477–1485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Byrne RA, Kastrati A, Massberg S, et al:
Biodegradable polymer versus permanent polymer drug-eluting stents
and everolimus- versus sirolimus-eluting stents in patients with
coronary artery disease: 3-year outcomes from a randomized clinical
trial. J Am Coll Cardiol. 58:1325–1331. 2011.
|
|
16
|
Windecker S, Serruys PW, Wandel S, et al:
Biolimus-eluting stent with biodegradable polymer versus
sirolimus-eluting stent with durable polymer for coronary
revascularisation (LEADERS): a randomised non-inferiority trial.
Lancet. 372:1163–1173. 2008. View Article : Google Scholar
|
|
17
|
Barlis P, Regar E, Serruys PW, et al: An
optical coherence tomography study of a biodegradable vs. durable
polymer-coated limus-eluting stent: a LEADERS trial sub-study. Eur
Heart J. 31:165–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guagliumi G, Sirbu V, Musumeci G, et al:
Strut coverage and vessel wall response to a new-generation
paclitaxel-eluting stent with an ultrathin biodegradable abluminal
polymer: Optical Coherence Tomography Drug-Eluting Stent
Investigation (OCTDESI). Circ Cardiovasc Interv. 3:367–375. 2010.
View Article : Google Scholar
|
|
19
|
Ahmed TA, Bergheanu SC, Stijnen T, Plevier
JW, Quax PH and Jukema JW: Clinical performance of drug-eluting
stents with biodegradable polymeric coating: a meta-analysis and
systematic review. EuroIntervention. 7:505–516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JS, Jang IK, Kim JS, Kim TH, Takano M,
Kume T, Hur NW, Ko YG, Choi D, Hong MK and Jang Y: Optical
coherence tomography evaluation of zotarolimus-eluting stents at
9-month follow-up: comparison with sirolimus-eluting stents. Heart.
95:1907–1912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han Y, Jing Q, Xu B, et al: Safety and
efficacy of biodegradable polymer-coated sirolimus-eluting stents
in ‘real-world’ practice: 18-month clinical and 9-month
angiographic outcomes. JACC Cardiovasc Interv. 2:303–309. 2009.
|
|
22
|
Gonzalo N, Barlis P, Serruys PW,
Garcia-Garcia HM, Onuma Y, Ligthart J and Regar E: Incomplete stent
apposition and delayed tissue coverage are more frequent in
drug-eluting stents implanted during primary percutaneous coronary
intervention for ST-segment elevation myocardial infarction than in
drug-eluting stents implanted for stable/unstable angina: insights
from optical coherence tomography. JACC Cardiovasc Interv.
2:445–452. 2009.
|
|
23
|
Guagliumi G, Costa MA, Sirbu V, et al:
Strut coverage and late malapposition with paclitaxel-eluting
stents compared with bare metal stents in acute myocardial
infarction: optical coherence tomography substudy of the
Harmonizing Outcomes with Revascularization and Stents in Acute
Myocardial Infarction (HORIZONS-AMI) Trial. Circulation.
123:274–281. 2011.
|
|
24
|
Finn AV, Nakazawa G, Kolodgie FD and
Virmani R: Temporal course of neointimal formation after
drug-eluting stent placement: is our understanding of restenosis
changing? JACC Cardiovasc Interv. 2:300–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Finn AV, Kolodgie FD, Harnek J, Guerrero
LJ, Acampado E, Tefera K, Skorija K, Weber DK, Gold HK and Virmani
R: Differential response of delayed healing and persistent
inflammation at sites of overlapping sirolimus- or
paclitaxel-eluting stents. Circulation. 112:270–278. 2005.
View Article : Google Scholar : PubMed/NCBI
|