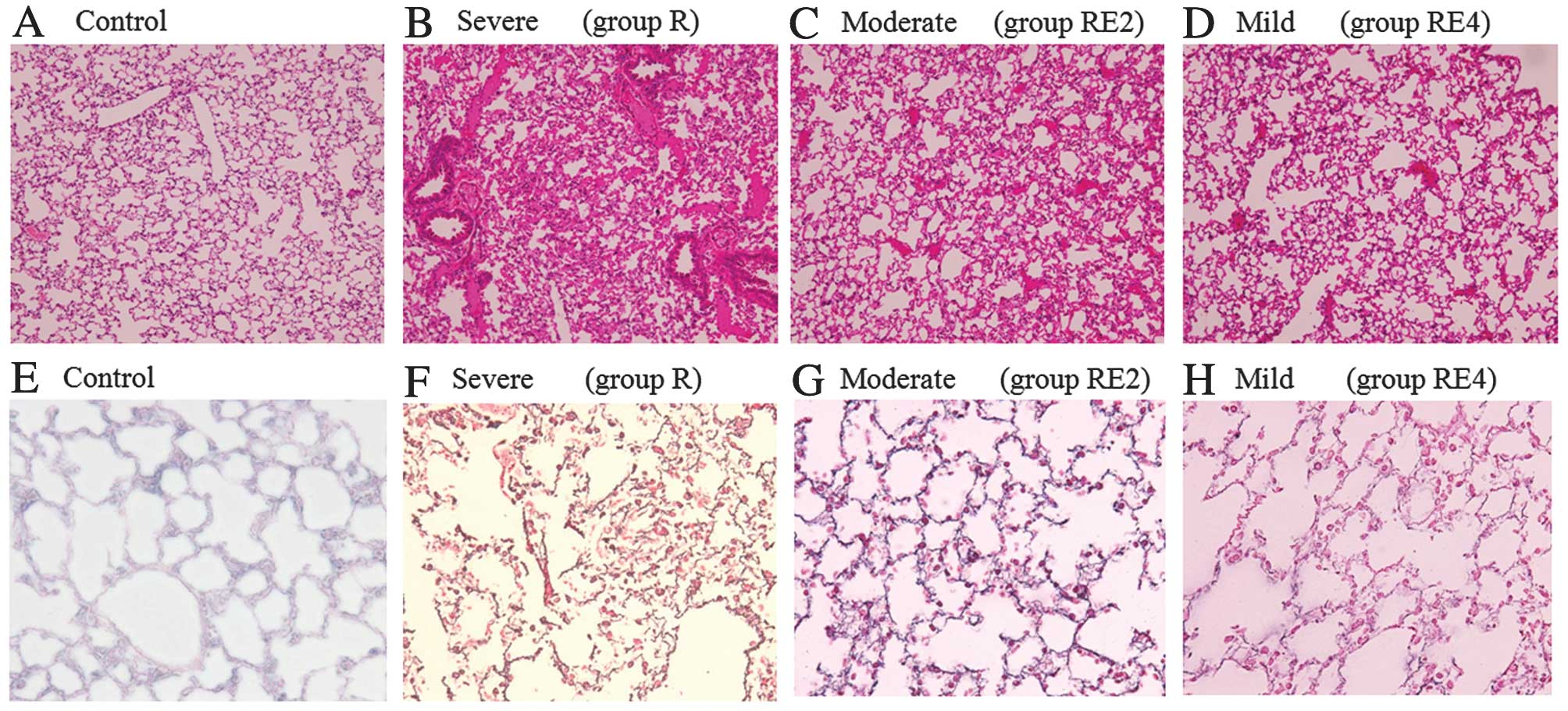
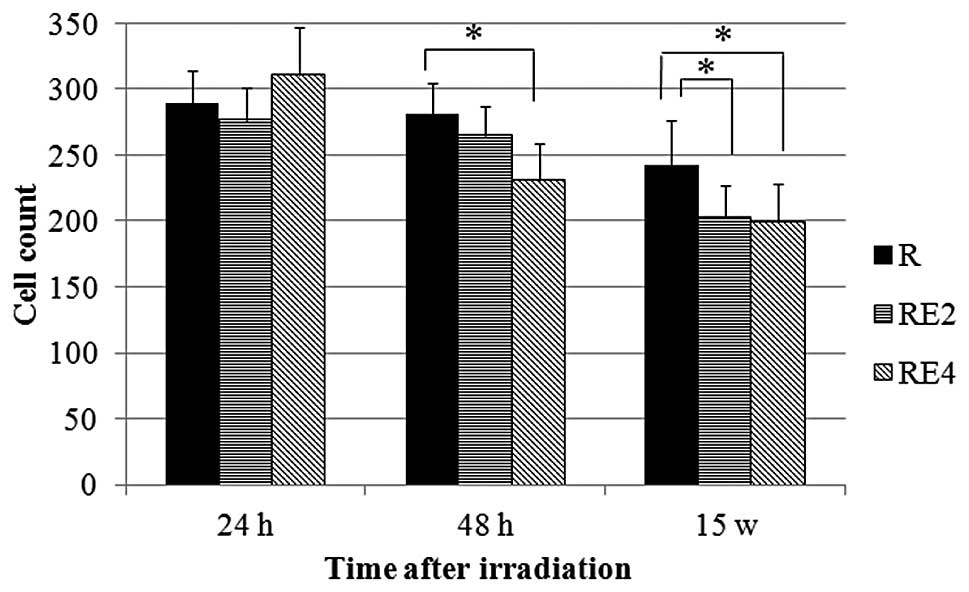
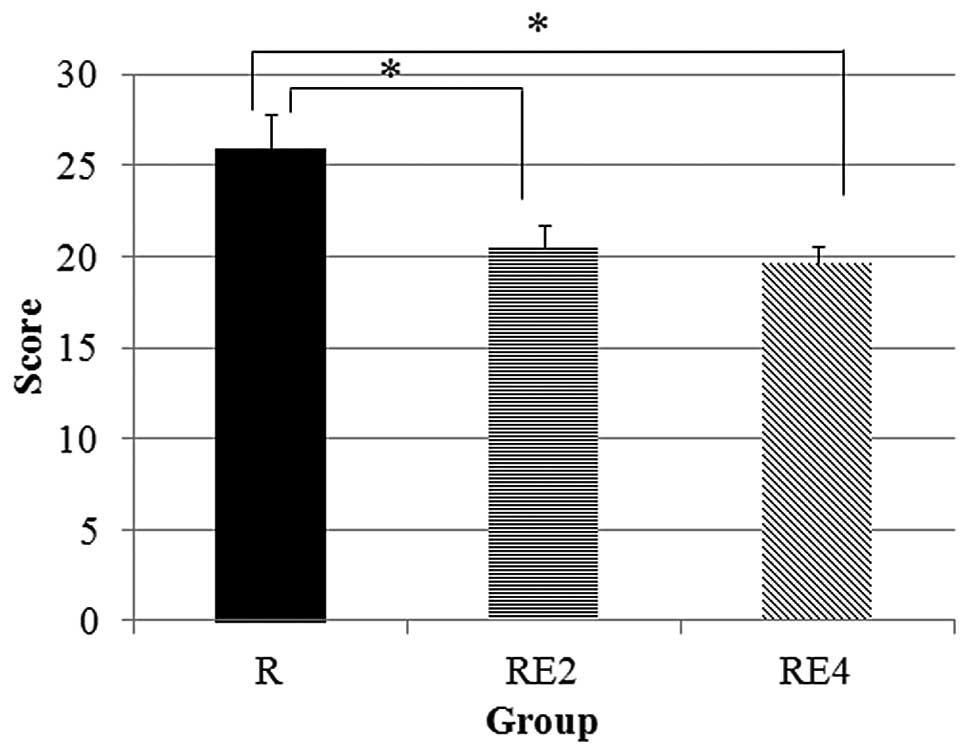
|
1
|
Sekine I, Sumi M, Ito Y, et al:
Retrospective analysis of steroid therapy for radiation-induced
lung injury in lung cancer patients. Radiother Oncol. 80:93–97.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Havemman K and Gramse M: Physiology and
pathophysiology of neutral proteinases of human granulocytes. Adv
Exp Med Biol. 84:1–20. 1984. View Article : Google Scholar
|
|
3
|
Kawabata K, Hagio T and Matsuoka S: The
role of neutrophil elastase in acute lung injury. Eur J Pharmacol.
451:1–10. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakashima H, Akimoto A, Kitagawa T, et al:
General pharmacological studies of sodium
N-[2-[4-(2,2-dimethethyl-propionyloxy) phenylsulfonylamino]
benzoyl] aminoacetate tetrahydrate (ONO-5046 Na). Pharmacometrics.
54:267–277. 1997.(In Japanese).
|
|
5
|
Shimbo T, Inomata T, Takahashi M, Tatsumi
T, Uesugi Y, Narabayashi I and Sonobe H: Effects of sivelestat
sodium hydrate on the reduction of radiation pneumonitis. Int J Mol
Med. 20:817–822. 2007.PubMed/NCBI
|
|
6
|
Kawabata K, Hagio T, Matsumoto S, Nakao S,
Orita Y, Aze Y and Ohno H: Delayed neutrophil elastase inhibition
prevents subsequent progression of acute lung injury induced by
endotoxin inhalation in hamster. Am J Respir Crit Care Med.
161:2013–2018. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Movsas B, Raffin TA, Epstein AH and Link
CJ Jr: Pulmonary radiation injury. Chest. 111:1061–1076. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsoutsou PG and Koukourakis MI: Radiation
pneumonitis and fibrosis: mechanisms underlying its pathogenesis
and implications for future research. Int J Radiat Oncol Biol Phys.
66:1281–1293. 2006. View Article : Google Scholar
|
|
9
|
Rubin P, Johnston CJ, Williams JP,
McDonald S and Finkelstein JN: A perpetual cascade of cytokines
postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol
Biol Phys. 33:99–109. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnston CJ, Piedboeuf B, Rubin P,
Williams JP, Baggs R and Finkelstein JN: Early and persistant
alterations in the expression of interleukin-1α, interleukin-1β and
tumor necrosis factor α mRNA levels in fibrosis-resistant and
sensitive mice after thoracic irradiation. Radiat Res. 145:762–767.
1996.
|
|
11
|
Rübe CE, Uthe D, Wilfert F, et al: The
bronchiolar epithelium as a prominent source of pro-inflammatory
cytokines after lung irradiation. Int J Radiat Oncol Biol Phys.
61:1482–1492. 2005.PubMed/NCBI
|
|
12
|
Mukaida N: Pathophysiological roles of
interleukin-8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell
Mol Physiol. 284:L566–L577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Araya J, Maruyama M, Sassa K, et al:
Ionizing radiation enhances matrix metalloproteinase-2 production
in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol.
280:L30–L38. 2001.PubMed/NCBI
|
|
14
|
Yang K, Palm J, König J, et al:
Matrix-metallo-proteinases and their tissue inhibitors in
radiation-induced lung injury. Int J Radiat Biol. 83:665–676. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rube CE, Uthe D, Schmid KW, et al:
Dose-dependent induction of transforming growth factor β (TGF-β) in
the lung tissue of fibrosis-prone mice after thoracic irradiation.
Int J Radiat Oncol Biol Phys. 47:1033–1042. 2000.
|
|
16
|
Martin M, Lefaix J and Delanian S:
TGF-beta1 and radiation fibrosis: a master switch and a specific
therapeutic target? Int J Radiat Oncol Biol Phys. 47:277–290. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishioka A, Ogawa Y, Mima T, et al:
Histopathologic amelioration of fibroproliferative change in rat
irradiated lung using soluble transforming growth factor-beta
(TGF-beta) receptor mediated by adenoviral vector. Int J Radiat
Oncol Biol Phys. 58:1235–1241. 2004. View Article : Google Scholar
|
|
18
|
Trott KR, Herrmann T and Kasper M: Target
cells in radiation pneumopathy. Int J Radiat Oncol Biol Phys.
58:463–469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takemasa A, Ishii Y and Fukuda T: A
neutrophil elastase inhibitor prevents bleomycin-induced pulmonary
fibrosis in mice. Eur Respir J. 40:1475–1482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hagio T, Matsumoto S, Nakao S, Abiru T,
Ohno H and Kawabata K: Elastase inhibition reduced death associated
with acid aspiration-induced lung injury in hamsters. Eur J
Pharmacol. 488:173–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taooka Y, Maeda A, Hiyama K, Ishioka S and
Yamakido M: Effect of neutrophil elastase inhibitor on
bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care
Med. 156:260–265. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hagio T, Nakao S, Matsuoka H, Matsumoto S,
Kawabata K and Ohno H: Inhibition of neutrophil elastase activity
attenuates complement-mediated lung injury in the hamster. Eur J
Pharmacol. 426:131–138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang T, Zhang J, Sun L, et al: Combined
effects of a neutrophil elastase inhibitor (sivelestat sodium) and
a free radical scavenger (edaravone) on lipopolysaccharide-induced
acute lung injury in rats. Inflamm Res. 61:563–569. 2012.
View Article : Google Scholar
|
|
24
|
Tamakuma S, Ogawa M, Aikawa N, et al:
Relationship between neutrophil elastase and acute lung injury in
humans. Pulm Pharmacol Ther. 17:271–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hayakawa M, Katabami K, Wada T, Sugano M,
Hoshino H, Sawamura A and Gando S: Sivelestat (selective neutrophil
elastase inhibitor) improves the mortality rate of sepsis
associated with both acute distress syndrome and disseminated
intravascular coagulation patients. Shock. 33:14–18. 2010.
View Article : Google Scholar
|