Introduction
Irritable bowel syndrome (IBS) is a chronic
gastrointestinal disorder characterized by abdominal discomfort or
pain associated with altered bowel habits, bloating and abdominal
distension. In total, 5–20% of the world population has been
reported to suffer from IBS (1).
The degree and frequency of symptoms vary between patients, ranging
from tolerable to severe and from daily symptoms to intermittent
symptoms at intervals of weeks/months (2–14).
IBS is not known to be associated with the
development of serious disease or with high mortality (15,16).
However, IBS considerably reduces quality of life to the same
degree of impairment as major chronic diseases, including diabetes,
congestive heart failure, renal insufficiency and hepatic cirrhosis
(17–21). Besides the increased morbidity
caused by IBS, the syndrome is an economic burden to society in
varying forms, for example by increasing the incidence of sick
leave and the over-consumption of healthcare resources (1).
The pathogenesis of IBS is unknown. Intestinal
low-grade inflammation has been proposed as one of the factors
contributing to the development of IBS (1). Support of this assumption is observed
in histopathological examinations of mucosal biopsies from the
ileum, caecum, colon and rectum, mostly from IBS patients with
diarrhoea as the predominant symptom (IBS-D) but even from patients
with constipation as the predominant symptom (IBS-C), which
revealed a mucosal infiltration of mast cells and lymphocytes
(22–28). Low-grade mucosal inflammation
appears to be evident in a subset of IBS, i.e., post-infectious IBS
(PI-IBS) (1,24,26),
but it is not clear, however, whether low-grade inflammation also
occurs in sporadic IBS. The present study was therefore undertaken
to examine the possible occurrence of low-grade mucosal
inflammation in the rectum as a representative of the large
intestine of patients with sporadic IBS.
Patients and methods
Patients and controls
In total, 50 patients with IBS that fulfilled the
Rome III Criteria (http://www.romecriteria.org) using the IBS module were
included in the study (29). These
patients consisted of 42 females and 8 males with an average age of
34 years (range 18–62 years). Of these, 30 patients had IBS-D and
20 patients had IBS-C. All patients had their symptoms for numerous
years and were not able to connect the onset of the IBS symptoms to
any particular events, including gastrointestinal or other
infections. All patients underwent a complete physical examination
and had the following investigative blood tests: full blood count,
electrolytes, calcium, inflammatory markers, liver and thyroid
function tests. They underwent a gastroscopy with duodenal biopsies
and coeliac disease was excluded.
The controls used in this study consisted of 27
subjects, 19 of which were female and 8 of which were male, with an
average age of 53 years (range 20–65 years) that underwent
colonoscopy with rectal biopsies. Of these subjects, 20 underwent
colonoscopy due to gastrointestinal bleeding, where the source of
the bleeding was identified as haemorrhoids (18) or angiodysplasia (2) and 7 subjects were examined due to
health worries caused by a diagnosis of colon carcinoma in a
relative. All control subjects had no other gastrointestinal
complaints or systemic diseases.
The present study was performed in accordance with
the Declaration of Helsinki and was approved by the local Committee
for Medical Research Ethics. All subjects provided oral and written
consent.
Colonoscopy
A standard colonoscopy was performed in the patients
and controls, and biopsies were taken from the rectum ~15 cm from
the anus. The biopsies were fixed in 4% buffered paraformaldehyde
overnight, embedded in paraffin and cut into 5-μm-thick
sections.
Histopathology and
immunohistochemistry
The sections were stained with haematoxylin and
eosin and then immunostained with the avidin-biotin complex (ABC)
method using the Vectastain ABC and the 3,3′-diaminobenzidine (DAB)
Peroxidase Substrate kits (Vector Laboratories, Burlingame, CA,
USA). The primary antibodies used were monoclonal mouse anti-human
CD45 (Dako, Carpinteria, CA, USA; code no. M0701), monoclonal mouse
anti-human CD47 (Dako; code no. I5647), monoclonal mouse anti-human
CD68 (Dako; code no. M0814) and monoclonal mouse anti-human mast
cell tryptase (Dako; code no. M7052). CD45 is considered as a
leucocyte common antigen and is expressed exclusively on cells of
the haematopoietic system and their progenitors. CD57 is expressed
by subsets of NK cells and CD8+ lymphocytes and by a
small percentage of CD4+/CD45R0+ T
lymphocytes. CD68 labels human monocytes, macrophages and myeloid
cells. Human mast cell tryptases comprise a family of trypsin-like
neutral serine proteases that are predominantly expressed in mast
cells.
Computerized image analysis
A computerised image analysis was performed using
Olympus software: Cell D. When using x40 objectives, the frame
(field) on the monitor represented an area of 0.14 mm2
of the tissue. The number of intraepithelial leucocytes cells and
the area of the epithelial cells were measured in each field. The
number of leucocytes, lymphocytes, macrophages and mast cells in
the lamina propria were counted per microscopic field. All
measurements were performed in 10 randomly chosen fields for each
individual. The immunostained sections from the IBS patients and
the controls were coded and mixed and the measurements were taken
without the knowledge of the section’s identity.
Statistical analysis
A Mann-Whitney U test was performed and P<0.05
was considered to indicate a statistically significant result.
Results
Colonoscopy, histopathology and
immunohistochemistry
The colon and rectum of the patients and control
subjects were macroscopically normal. Histopathological examination
of the colon and rectum biopsies from the patients and controls
revealed a normal histology.
Computerised image analysis
Leucocytes
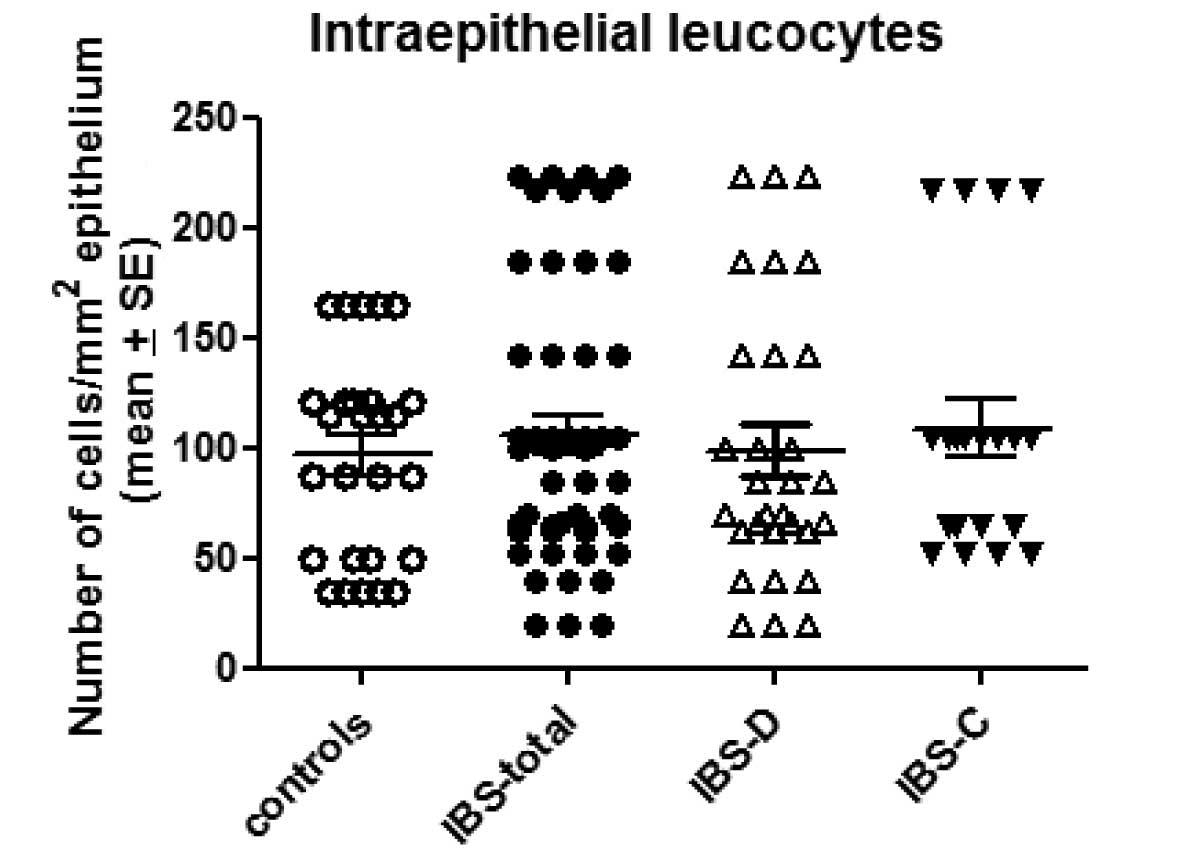
The number of intraepithelial leucocytes observed in
the controls, total IBS patients and the IBS-D and IBS-C patients
was 95.2±48.4, 102.1±16.2, 98.8±20.6 and 108±29/mm2
epithelium (mean ± SE), respectively (Figs. 1 and 2). There was no statistically significant
difference between the controls and the total IBS, IBS-D or IBS-C
patients (P=0.97, 0.96 and 0.77, respectively).
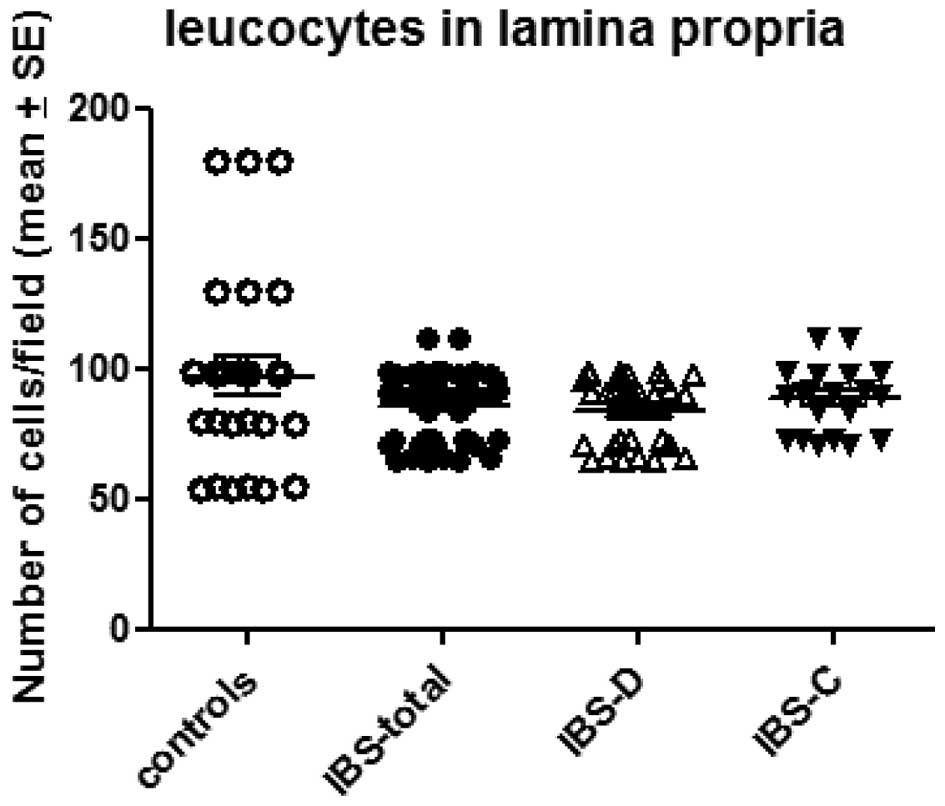
The number of leucocytes in the lamina propria was
97.1±7.2/field in the controls and 85.8±1.9/field in the total IBS
patients. The corresponding figures in the IBS-D and IBS-C patients
were 84.1±2.4 and 88.3±2.9/field, respectively (Figs. 2 and 3). There was no significant difference
between the controls and the total IBS, IBS-D or IBS-C patients
(P=0.17, 0.13 and 0.48, respectively).
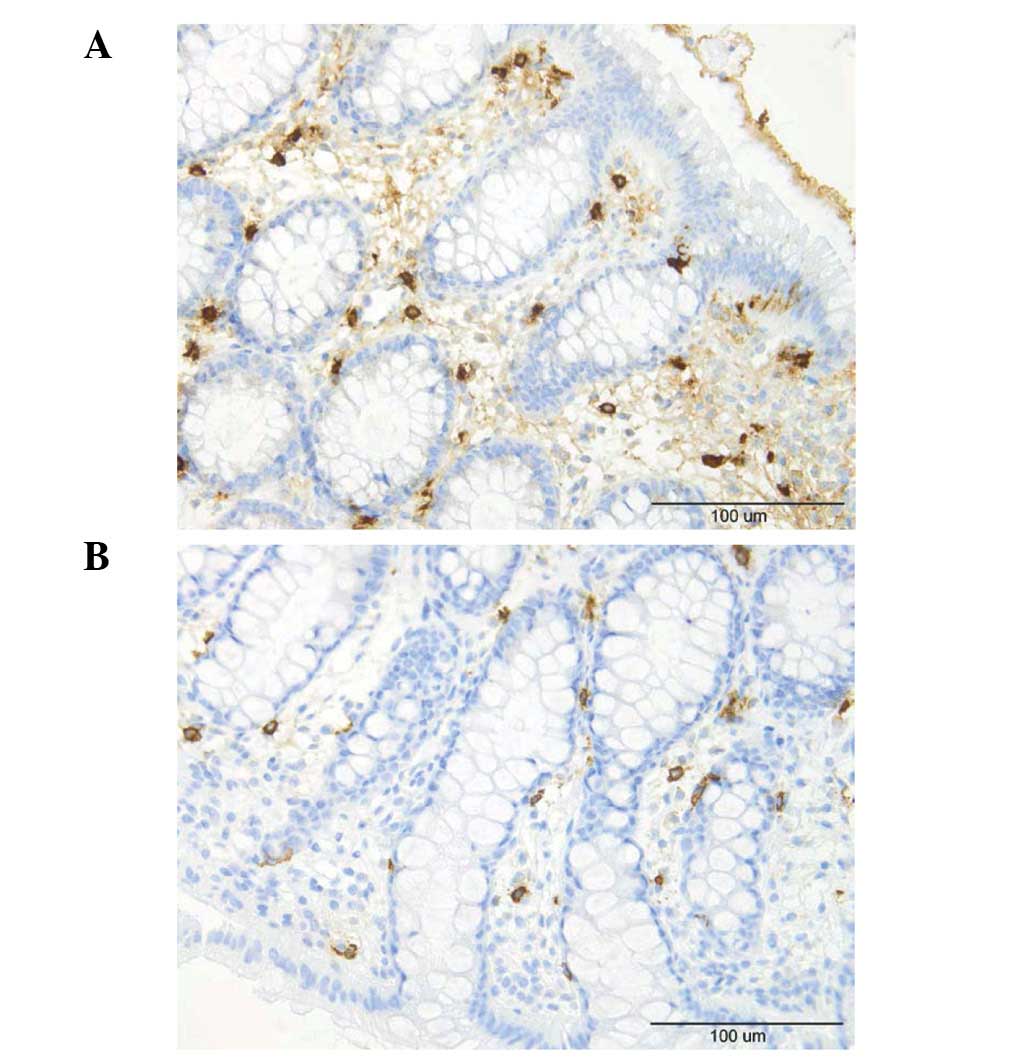
Mast cells
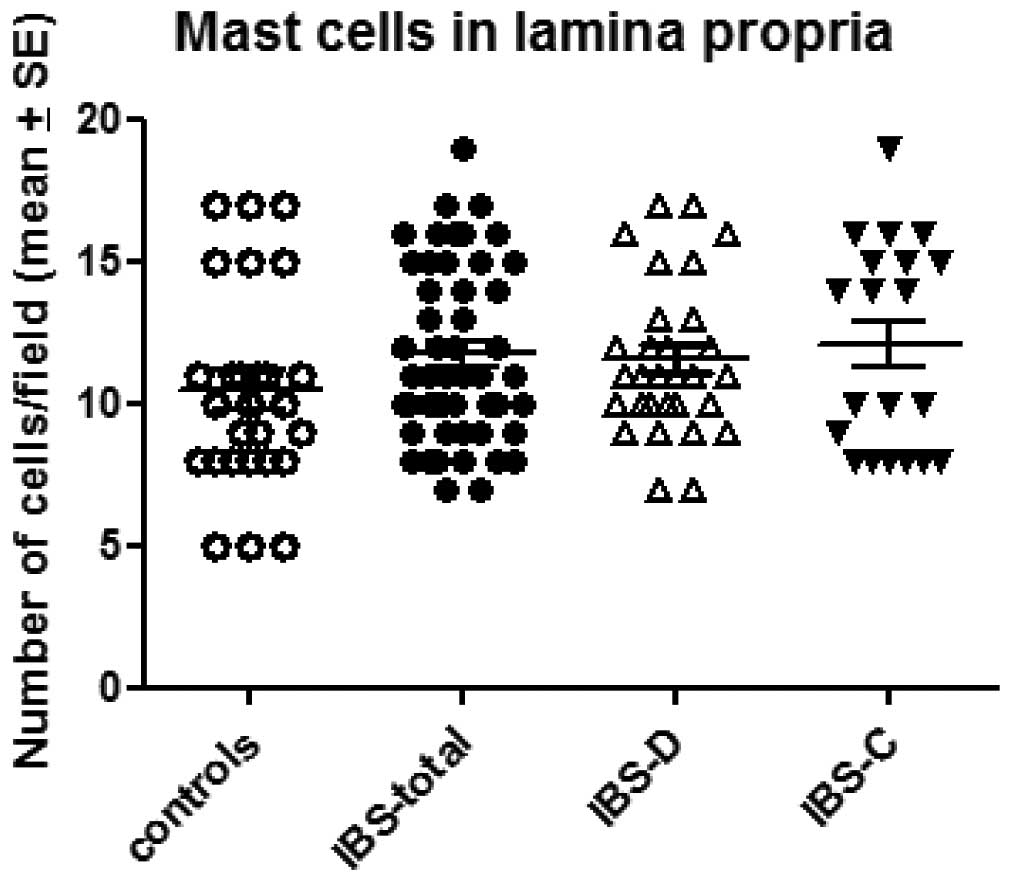
The number of mast cells in the lamina propria of
the controls, total IBS patients and the IBS-D and IBS-C patients
was 10.4±0.7, 11.7±0.4, 11.5±0.5 and 12.1±0.8/field, respectively
(Figs. 4 and 5). There was no sigificant difference
between the controls and the total IBS patients (P=0.11). Nor was
any significant difference observed between the controls and the
IBS-D or IBS-C patients (P=0.01 and 0.32).
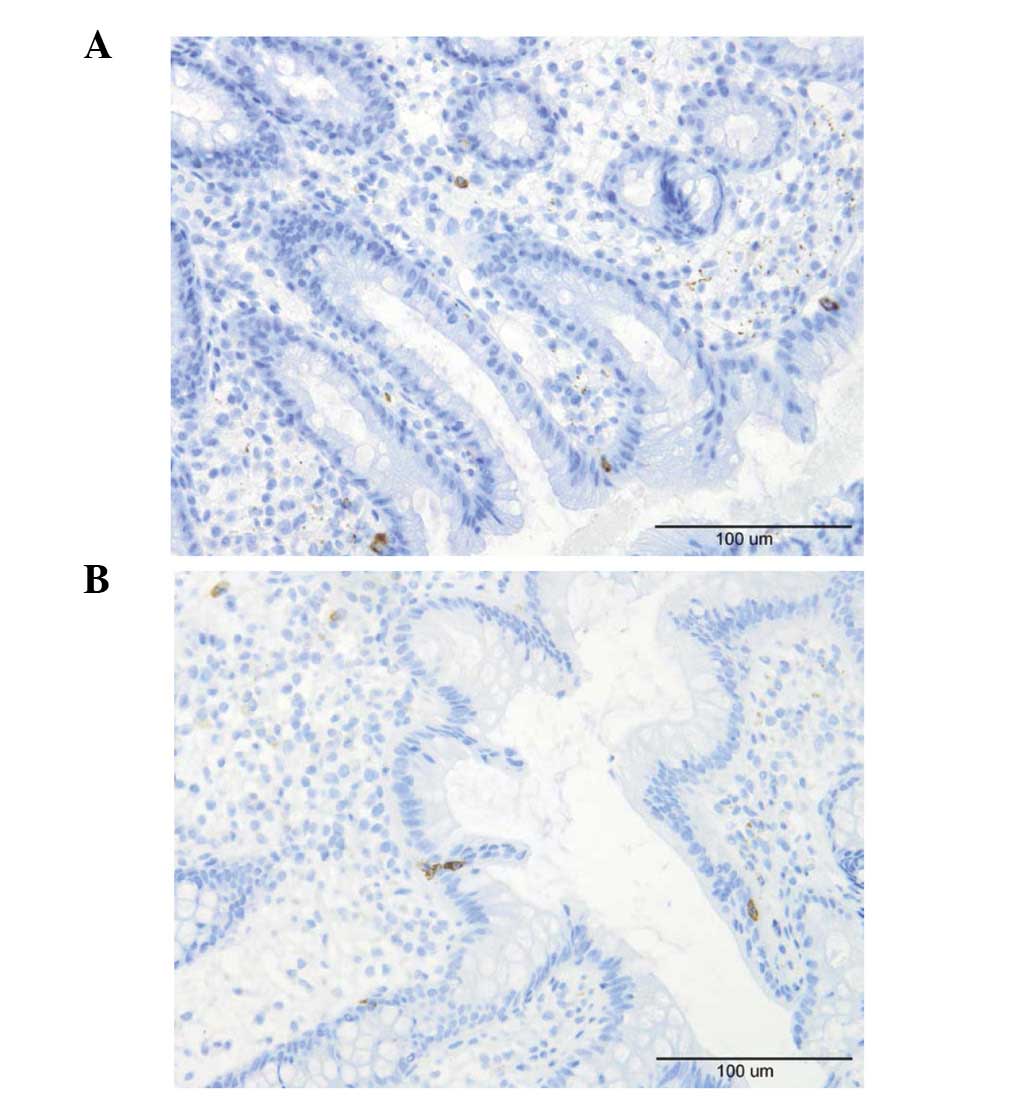
Lymphocytes, macrophages and
monocytes
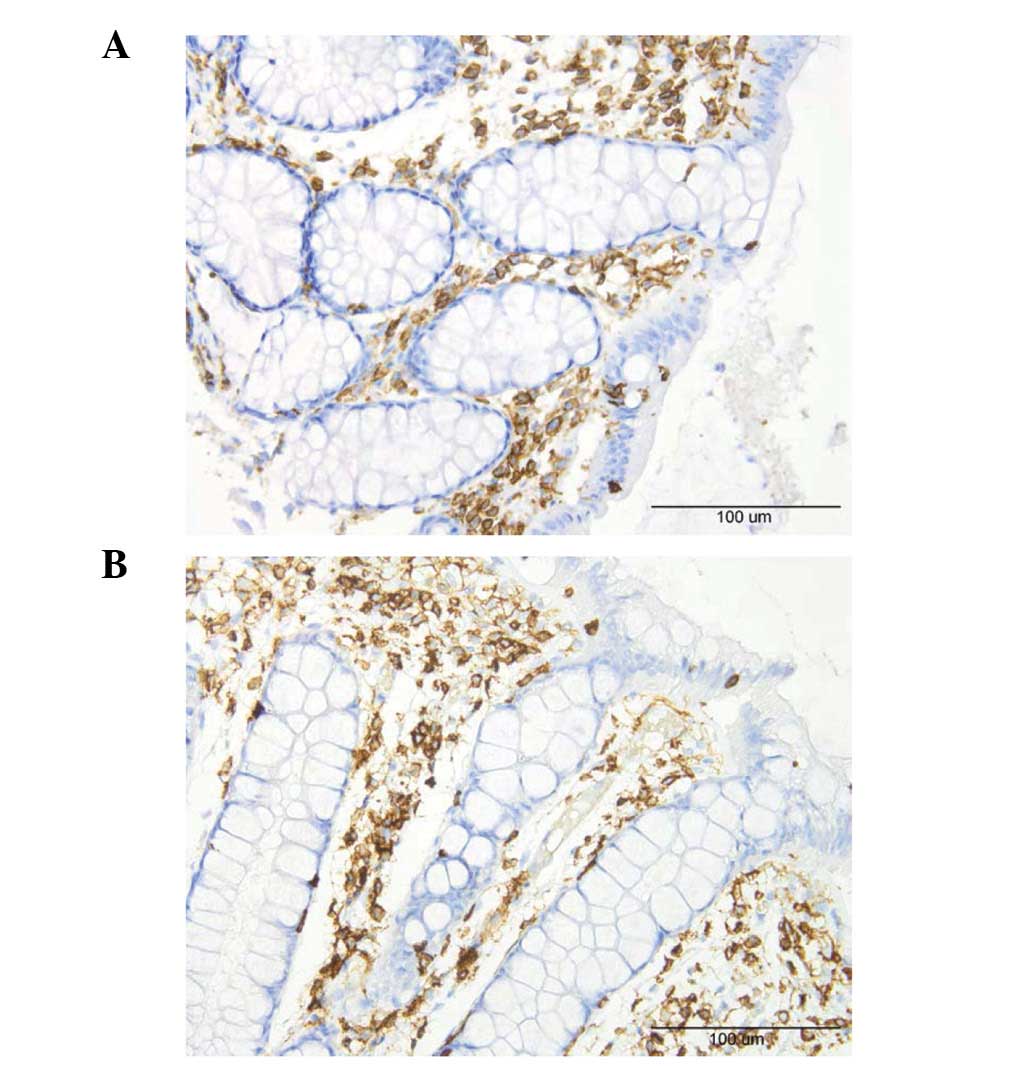
A few lymphocytes were identified intraepithelially
and in the lamina propria of the controls and the patients
(Fig. 6). Macrophages and
monocytes were seldomly encountered in the lamina propria of the
controls or patients. These three cell types were sparse in the
biopsy material examined which made it difficult to perform a
reliable quantification.
Discussion
In unselected cohorts of IBS patients, an increased
mucosal cell density of mast cells was observed in the ileum,
caecum and colon (22,23,25,27),
however, this increase did not occur in all IBS patients that were
examined (22,25,27).
A previous study reported that numbers of plasma cells,
lymphocytes, eosinophils, neutrophils and macrophages were
unchanged in a cohort of unselected IBS patients (23). However, a second study of another
cohort of unselected IBS patients identified an increase of
lymphocytes in 50% of the patients (27). It is conceivable to conclude,
therefore, that the increased infiltration of immune cells in the
terminal ileum, colon and rectum occurs only in a subset of IBS
patients.
PI-IBS is a subset of IBS that is defined as a
sudden onset of IBS symptoms following gastroenteritis in
individuals who previously have not had any gastrointestinal
complaints (30). PI-IBS however,
has also been reported following non-gastrointestinal infections,
including respiratory, urinary tract and skin infections (31). Sporadic IBS, however, may be
defined as a long duration of IBS symptoms in individuals without
any connection to a previous gastrointestinal infection. In total,
6–17% of patients with IBS believe that their symptoms began with
an infective illness (32).
Furthermore, 7–31% of patients who suffer an acute episode of
infectious gastroenteritis develop PI-IBS despite clearance of the
inciting pathogen (33).
The intestinal mucosa of patients with PI-IBS, as
well as of the animal models for PI-IBS, show a low-grade
inflammation. Thus, in patients with chronic giardiasis (patients
with Giardia infection despite antibiotic treatment), as
well as in patients with PI-IBS following Giardia infection,
an increased intraepithelial infiltration of lymphocytes has been
observed in the duodenal mucosa. The lymphocyte infiltration in
chronic giardiasis was reported to be much more prominent than in
PI-IBS (28). Similarly, an
increased infiltration of T lymphocytes, as well as mast cells, has
been reported in the duodenal and jejunal mucosa of an animal model
for PI-IBS (34). In the terminal
ileum of PI-IBS patients following Shigella infection, an
increase in the number of mast cells was also observed (35). The density of T lymphocytes and
mast cells was increased in the lamina propria of the rectum in
patients with PI-IBS (24,34,35).
Similarly, rectal biopsies taken from patients following
Campylobacter enteritis showed an increase in the density of
CD3, CD4 and CD8 lymphocytes in the intraepithelium and in the
lamina propria, which persisted for >1 year subsequent to
infection (26,36).
The present study showed that the mucosal density of
leucocytes as a whole and lymphocytes, monocytes, macrophages and
mast cells in the rectum in the sporadic IBS patients did not
differ from that of the controls. These findings oppose low-grade
inflammation as a pathogenic factor in sporadic IBS. From the data
presented above, low-grade inflammation may play a role in the
pathogenesis of a subset of IBS, namely PI-IBS.
Acknowledgements
This study was supported by a grant from
Helse-Fonna.
References
|
1
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Irritable Bowel Syndrome. Nova Scientific Publishers;
New York, NY: 2012
|
|
2
|
Agréus L, Svärsudd K, Nygrén O and Tibblin
G: Irritable bowel syndrome and dyspepsia in the general
population: overlap and lack of stability over time.
Gastroenterology. 109:671–680. 1995.PubMed/NCBI
|
|
3
|
Thompson WG and Heaton KW: Functional
bowel disorders in apparently healthy people. Gastorenterology.
79:283–288. 1980.PubMed/NCBI
|
|
4
|
Kennedy TM, Jones RH, Hungin AP,
O’Flanagan H and Kelly P: Irritable bowel syndrome,
gastro-oesophageal reflux, and bronchial hyper-responsiveness in
the general population. Gut. 43:770–774. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Drossman DA, Li Z, Andruzzi E, Temple RD,
Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P,
Corazziari E, et al: U.S. householder survey of functional
gastrointestinal disorders. Prevalence, sociodemography, and health
impact. Dig Dis Sci. 38:1569–1580. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Talley NJ, Gabriel SE, Harmsen WS,
Zinsmeister AR and Evans RW: Medical costs in community subjects
with irritable bowel syndrome. Gastroenterology. 109:1736–1741.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hungin AP, Whorwell PJ, Tack J and Mearin
F: The prevalence, patterns and impact of irritable bowel syndrome:
an international survey of 40,000 subjects. Aliment Pharmacol Ther.
17:643–650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jones R and Lydeard S: Irritable bowel
syndrome in the general population. BMJ. 304:87–90. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bordie AK: Functional disorders of the
colon. J Indian Med Assoc. 58:451–456. 1972.PubMed/NCBI
|
|
10
|
O’Keefe EA, Talley NJ, Zinsmeister AR and
Jacobsen SJ: Bowel disorders impair functional status and quality
of life in the elderly: a population-based study. J Gerontol A Biol
Sci Med Sci. 50:M184–M189. 1995.PubMed/NCBI
|
|
11
|
Everhart JE and Renault PF: Irritable
bowel syndrome in office-based practice in the United States.
Gastroenterology. 100:998–1005. 1991.PubMed/NCBI
|
|
12
|
Wilson S, Roberts L, Roalfe A, Bridge P
and Singh S: Prevalence of irritable bowel syndrome: a community
survey. Br J Gen Pract. 54:495–502. 2004.PubMed/NCBI
|
|
13
|
Harvey RF, Salih SY and Read AE: Organic
and functional disorders in 2000 gastroenterology outpatients.
Lancet. 1:632–634. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spiegel BM: The burden of IBS: looking at
metrics. Curr Gastroenterol Rep. 11:265–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thompson WG: A world view of IBS.
Irritable Bowel Syndrome: Diagnosis and Treatment. Camilleri M and
Spiller R: Saunders Ltd; Philadelphia and London: pp. 17–26.
2002
|
|
16
|
Quigley EM, Locke GR, Mueller-Lissner S,
Paulo LG, Tytgat GN, Helfrich I and Schaefer E: Prevalence and
management of abdominal cramping and pain: a multinational survey.
Aliment Pharmacol Ther. 24:411–419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miller V, Whitaker K, Morris JA and
Whorwell PJ: Gender and irritable bowel syndrome: the male
connection. J Clin Gastroenterol. 38:558–560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Whitehead WE, Burnett CK, Cook EW III and
Taub E: Impact of irritable bowel syndrome on quality of life. Dig
Dis Sci. 41:2248–2253. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gralnek IM, Hays RD, Kilbourne A, Naliboff
B and Mayer EA: The impact of irritable bowel syndrome on health
related quality of life. Gastroenterology. 119:654–660. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huerta I, Hinojosa C, Santa Maria A and
Schmulson M: Diferencias en la calidad de vida (CV) entre pacientes
con sindrome de Intestino irritable (SII) y la poblacon mexicana
evaluadas mediante el SF-36. Rev Mex Gastroenterol. 66(Suppl 2):
145–146. 2001.(In Spanish).
|
|
21
|
Schmulson M, Robles G, Kershenobich,
Lopez-Ridaura R, Hinojosa C and Durate A: Los pacientes con
trastornos funcionales digestivos (TFD) tienen major compromiso de
la calidad de vida (CV) evaluadas por el SF-36 comparados con
pacientes con hepatitis C y pancreatitis cronica. Rev Mex
Gastroenterol. 65(Suppl-Resumenes): 50–51. 2000.(In Spanish).
|
|
22
|
Weston AP, Biddle WL, Bhatia PS and Miner
PB Jr: Terminal ileal mucosal mast cells in irritable bowel
syndrome. Dig Dis Sci. 38:1590–1595. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O’Sullivan M, Clayton N, Breslin NP,
Herman I, Bountra C, McLaren A and O’Morain CA: Increased mast
cells in the irritable bowel syndrome. Neurogastroenterol Motil.
12:449–457. 2000.
|
|
24
|
Dunlop SP, Jenkins D, Neal KR and Spiller
RC: Relative importance of enterochromaffin cell hyperplasia,
anxiety and depression in post-infectious IBS. Gastroenterology.
125:1651–1659. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barbara G, Stanghellini V, De Giorgio R,
Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate
AM, Grady EF, Bunnett NW, Collins SM and Corinaldesi R: Activated
mast cells in proximity to colonic nerves correlate with abdominal
pain in irritable bowel syndrome. Gastroenterology. 126:693–702.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spiller RC, Jenkins D, Thornley JP, Hebden
JM, Wright T, Skinner M and Neal KR: Increased rectal mucosal
enteroendocrine cells, T lymphocytes, and increased gut
permeability following acute Campylobacter enteritis and in
post-dysenteric irritable bowel syndrome. Gut. 47:804–811. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cremon C, Gargano L, Morselli-Labate AM,
Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi
R and Barbara G: Mucosal immune activation in irritable bowel
syndrome: gender-dependence and association with digestive
symptoms. Am J Gastroenterol. 104:392–400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dizdar V, Hanevik K, Lærum OD, Gilja OH,
Langeland N and Hausken T: Duodenal mucosal lymphocytes in
Giardia-induced functional gastrointestinal disorder. In:
Presented at the 19th United European Gastroenterology Week (UEGW);
(abstract P0996). 2011
|
|
29
|
Longstreth GF, Thompson WG, Chey WD,
Houghton LA, Mearin F and Spiller RC: Functional bowel disorders.
Gastroenterology. 130:1480–1491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghoshal UC, Park H and Gwee KA: Bugs and
irritable bowel syndrome: The good, the bad and the ugly. J
Gastroenterol Hepatol. 25:244–251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mckeown ES, Parry D, Stansfield R, Barton
JR and Welfare MR: Postinfectious irritable bowel syndrome may
occur after non-gastrointestinal and intestinal infection.
Neurogastroenterol Motil. 18:839–843. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Longstreth CF, Hawkey CJ, Mayer EA, Jones
RH, Naesdal J, Wilson IK, Peacock RA and Wiklund IK:
Characteristics of patients with irritable bowel syndrome recruited
from three sources: implications for clinical trials. Aliment
Pharmacol Ther. 15:959–964. 2001.PubMed/NCBI
|
|
33
|
Spiller R and Garsed K: Infection,
inflammation and the irritable bowel syndrome. Dig Liver Dis.
41:844–849. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wheatcroft J, Wakelin D, Smith A, Mahoney
CR, Mawe G and Spiller R: Enterochromaffin cell hyperplasia and
decreased serotonin transporter in a mouse model of postinfectious
bowel dysfunction. Neurogastroenterol Motil. 17:863–870. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang LH, Fang XC and Pan GZ: Bacillary
dysentery as a causative factor for irritable bowel syndrome and
its pathogenesis. Gut. 53:1096–1101. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK
and Cho SW: The alteration of enterochromaffin cell, mast cell, and
lamina propria T lymphocyte numbers in irritable bowel syndrome and
its relationship with psychological factors. J Gastroenterol
Hepatol. 23:1689–1694. 2008. View Article : Google Scholar : PubMed/NCBI
|