Introduction
Gastroesophageal reflux disorder (GERD) is a common
disease worldwide, whose prevalence has been on the increase in
Eastern Asia in addition to Western countries (1). Accordingly, the prescription of
proton pump inhibitors (PPIs) is increasing rapidly (2–5).
Long-term PPI therapy has been established as a standard treatment
strategy to manage refractory GERD, which has not responded to the
standard dose, or recurrent GERD after the withdrawal of PPI
treatment (6,7). PPIs have been known to be safe drugs
without any major complications with long-term use (8). However, a large-scale epidemiologic
study reported that high-dose, long-term therapy with PPIs
significantly increased the risk of osteoporosis and pathologic hip
fracture (9). A previous nested
case-control study revealed that more than one year of PPI therapy
is associated with increased risk of hip or femur fracture
(10). However, there have been
few studies undertaken to clarify the exact mechanism of the PPI
effect. It has been established that PPIs reduce the production and
secretion of hydrochloric acid in the human stomach, increase the
pH in the stomach or small intestine and inhibit absorption of
insoluble calcium in the small intestine, thus leading to
pathologic fractures (11,12). Alternatively, several in
vitro studies have reported that PPIs inhibit the
vacuolar-ATPase (V-ATPase) of osteoclasts and reduce their activity
(13,14). Clinical studies have supported the
theory that the short-term use of PPI in children or adults does
not alter or reduce bone resorption markers (15,16).
There are conflicting data regarding the effect of PPI on bone
metabolism, however, little is known about the long-term effect of
PPI on osteoclast or bone resorption in human or animal models.
Receptor activator of NF-κB ligand (RANKL), its
receptor RANK, and its decoy receptor osteoprotegerin (OPG) are
novel concepts in bone metabolism (17). RANKL, a member of the tumor
necrosis factor (TNF) family, is a membrane-bound cytokine, and is
bound to RANK, a homotrimeric transmembrane protein member of the
TNF receptor superfamily, and is expressed on the surface of the
osteoclast precursor (OCP). RANKL/RANK binding differentiates the
OCP, induces formation, differentiation and activation of
osteoclasts, and acts as an essential stimulator for bone
resorption. By contrast, OPG is known to inhibit differentiation
and activation of osteoclasts effectively under in vivo or
in vitro conditions (18,19).
As a result, regulation of RANKL or OPG expression is crucial in
bone metabolism, and the RANKL/OPG ratio changes in favor of
abnormal osteoclastogenesis and is known as a predictive risk
factor for pathologic fractures (20,21).
Furthermore, sequential activation of c-Fos and the nuclear factor
of activated T cell c1 (NFATc1) by the RANKL/RANK/NF-κB signaling
system is essential for the completion of the differentiation
process of the OCP and osteoclasts (22–24).
We performed a pilot animal study using
ovariectomized ICR mice, into which we injected pantoprazole via an
intraperitoneal route, and observed that messenger RNA (mRNA)
expression levels of IL-1β were inhibited significantly in the
pantoprazole group compared with the control group. No significant
difference was observed in the bone marrow density (BMD) between
the two groups, from which we hypothesized that the ingestion of
PPI can affect bone resorption and bone metabolism (25). In the present study, we aimed to
evaluate the long-term effect of PPIs on bone resorption and
remodeling in an ovariectomized rat model by analyzing the
RANKL/OPG ratio, c-Fos, NFATc1 and osteocalcin, a well-known bone
formation biochemical marker, by using bone marrow cells and serum
C-terminal cross-linking telopeptide of type I (CTX-1), as a bone
resorption marker.
Materials and methods
Experimental animals
Five-week-old female Sprague-Dawley (S-D) rats were
housed for one week for preparatory adaptation to the new
environment, ovariectomized at 6 weeks old and allowed to adapt to
the inflammatory process for 2 weeks. All the rats were maintained
in a laboratory at a temperature of 23±1°C and humidity of 55±5%
under a 12-h light-dark cycle (light 7:00–19:00). At 8 weeks old,
the animals were subdivided equally into 4 groups. The rats were
fed on a regular diet with placebo (group A), low calcium diet with
placebo (group B), regular diet with PPI (group C) and low calcium
diet with PPI (group D). Solid feed was used for the rats including
calcium 1.25%, phosphorus 0.95%, vitamin D 340 IU/100 mg (Samyang
Co., Ltd., Seoul, Korea) as the regular diet, and another solid rat
feed including calcium 0.01%, phosphorus 0.95%, vitamin D 340
IU/100 mg (Oriental Yeast Co., Ltd., Tokyo, Japan) for the low
calcium diet. Omeprazole was administered orally at a dose of 30
mg/kg/day. The above diets and drugs were fed throughout the 8
weeks. At 16 weeks old, the rats were euthanized under general
anesthesia. This study was approved by the Korea University
Institute of Animal Care and Use Committee.
Reverse transcriptase-polymerase chain
reaction
Bone marrow cells were collected from the femurs of
the S-D rats. After washing with PBS three times, the cells were
diluted with a 1 ml solution including 50 mM HEPES (pH 7.0), 150 mM
NaCl, 1.5 mM MgCl, 1% Triton, 10 mM sodium pyrophosphate, 1 mM PMSF
(Sigma-Aldrich, St. Louis, MO, USA) and 10 μg/ml leupeptin
(Sigma-Aldrich). After mixing and centrifugation with 500 μl TRIzol
solution (Invitrogen, Grand Island, NY, USA) and 100 μl chloroform,
supernatants from the acquired cell extracts were used for the
reverse transcriptase-polymerase chain reaction (RT-PCR). After
washing the supernatants with 75% ethanol, the total RNA was
extracted, and the reaction mixture composed of 10X reverse
transcription buffer, RNase inhibitor, dNTP, M-MLV reverse
transcriptase and 1 μl total RNA was incubated at 42°C for 15 min
to synthesize cDNA. The cDNA produced was amplified into PCR using
sense/antisense primers (Table I).
The resulting product was dissolved in 2% agarose gel, dyed with
ethidium bromide and developed under UV light. To qualify the
reactants, the band was measured using densitometry and each ratio
to GAPDH was calculated.
 | Table IPrimer sequence for RT-PCR. |
Table I
Primer sequence for RT-PCR.
| Gene | | Primer sequence | Size (bp) |
|---|
| RANKL | Sense |
5′-AGCCGAGACTACGGCAAGTA-3′ | |
| Antisense |
5′-GCGCTCGAAAGTACAGGAAC-3′ | 208 |
| OPG | Sense |
5′-CTGCCTGGGAAGAAGATCAG-3′ | |
| Antisense |
5′-TTGTGAAGCTGTGCAGGAAC-3′ | 226 |
| c-Fos | Sense |
5′-CCAGTCAAGAGCATCAGCAA-3′ | |
| Antisense |
5′-AAGTAGTGCAGCCCGGAGTA-3′ | 247 |
| NFATc1 | Sense |
5′-GGGTCAGTGTGACCGAAGAT-3′ | |
| Antisense |
5′-GGAAGTCAGAAGTGGGTGGA-3′ | 225 |
| Osteocalcin | Sense |
5′-AAGCAGGAGGGCAATAAGGT-3′ | |
| Antisense |
5′-TTTGTAGGCGGTCTTCAAGC-3′ | 157 |
Measurement of serum CTX-1
After sacrificing the experimental S-D rats, their
blood was sampled from the right atrium. Serum was produced by
centrifugation of the collected blood. An immunoassay kit
(RatLaps™, ImmunoDiagnostic Systems, Herlev, Denmark) was used for
the measurement of CTX-1. In brief, 100 μl of biotinylated RatLaps
antigen (Ag biotin) was added to each well, incubated for 30±5 min
at room temperature, the immuno strips were washed manually five
times using 300 μl of washing solution (wash buffer) and 20 μl
serum samples were added from each group into the appropriate wells
followed by 100 μl of primary antibody. Samples were incubated
overnight (18±3 h) at 2–8°C and washed a further five times.
Following the addition of 100 μl of peroxidase-conjugated goat
anti-rabbit IgG antibody (enzyme conjugate) to each well, samples
were incubated for 60±5 min at room temperature,100 μl of substrate
solution was added to each well and incubated for 15±2 min at room
temperature in the dark and 100 μl of stopping solution was then
added to each well. Finally, the absorbance was measured within 2 h
at 450 nm, with a reading at 650 nm acting as a reference.
Measurement of serum calcium and alkaline
phosphatase levels
Whole blood was collected and serum was obtained
using the same method as that mentioned previously. Serum levels of
calcium and alkaline phosphatase (ALP) were measured by activity
analysis using a reagent (ELITech™, Provence, France).
Statistical analysis
SPSS for Windows (version 12.0, SPSS, Inc., Chicago,
IL, USA) was used as the statistical analysis program. Continuous
variables were analyzed using ANOVA when the variables followed a
normal distribution, and the Kruskal-Wallis test (a non-parametric
method) when they did not follow a normal distribution. P<0.05
was considered to indicate a statistically significant
difference.
Results
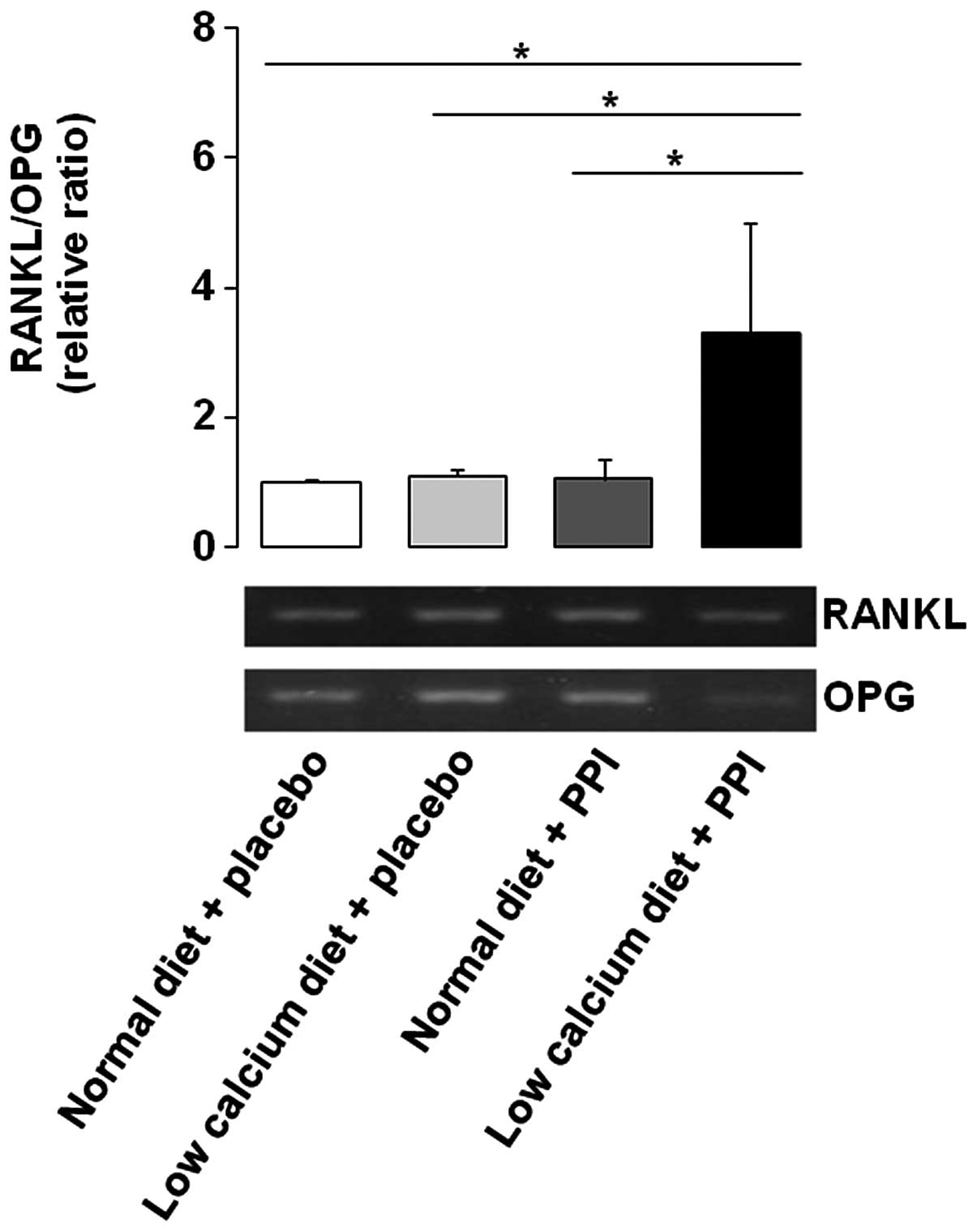
RANKL/OPG ratio is increased by the
combination of a low calcium diet and PPI
To evaluate the relative ratio of RANKL/OPG, the
RT-PCR method was performed. Initially, the gene expression level
of RANKL was upregulated by low calcium diet alone and PPI alone,
compared with normal diet alone. Expression levels of OPG were also
upregulated by PPI alone, compared to that of normal diet alone,
which showed a similar pattern to RANKL. However, OPG was markedly
downregulated by a low calcium diet with PPI. Consequently, the
RANKL/OPG ratio was significantly increased by a low calcium diet
with PPI, compared to the other three groups (Fig. 1). Given that OPG binds
competitively to RANKL to inhibit RANKL-RANK binding, if the
administration of PPI is combined with a low calcium diet, it may
enhance the signal transduction through RANK.
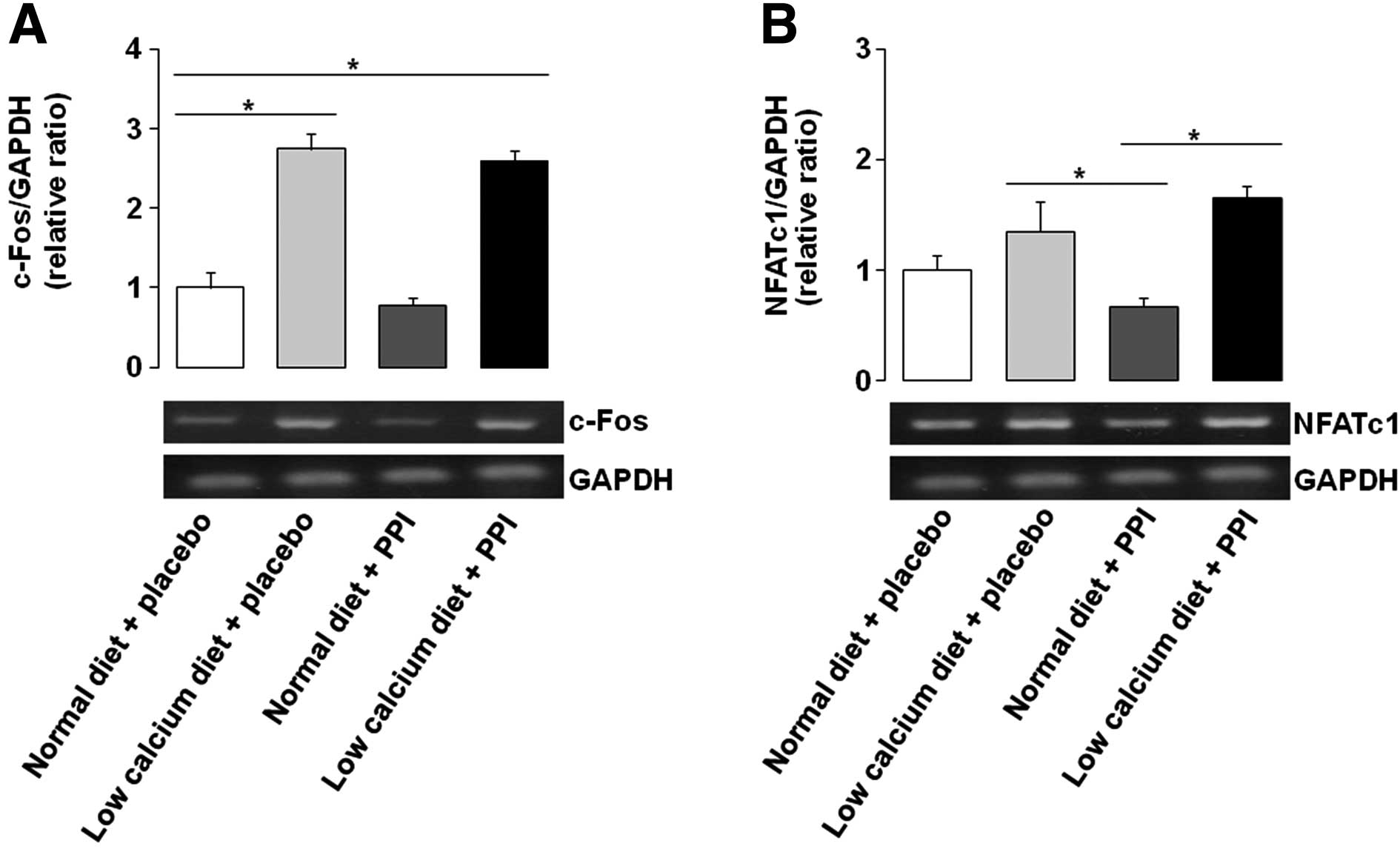
Gene expression of c-Fos and NFATc1 is
upregulated by low calcium diet with or without PPI
Relative mRNA expression levels of c-Fos, a
transcription factor of the RANKL/RANK/NF-κB signaling pathway, was
also measured by the RT-PCR method. C-Fos levels were increased by
a low calcium diet with or without PPI, compared with that of a
normal diet alone, by 131 and 115%, respectively (Fig. 2A). NFATc1 is a target gene for
c-Fos activation, and showed a similar pattern for c-Fos
expression, which was increased by a low calcium diet with or
without PPI, compared with that of PPI alone, by 87 and 107%,
respectively (Fig. 2B). When
considered together with the RANKL/OPG ratio, it is suggested that
PPI alone may not cause differentiation and activation of
osteoclasts, as the autoamplification of NFATc1 mediates completion
of the differentiation process as the final step to several
signaling pathways (20).
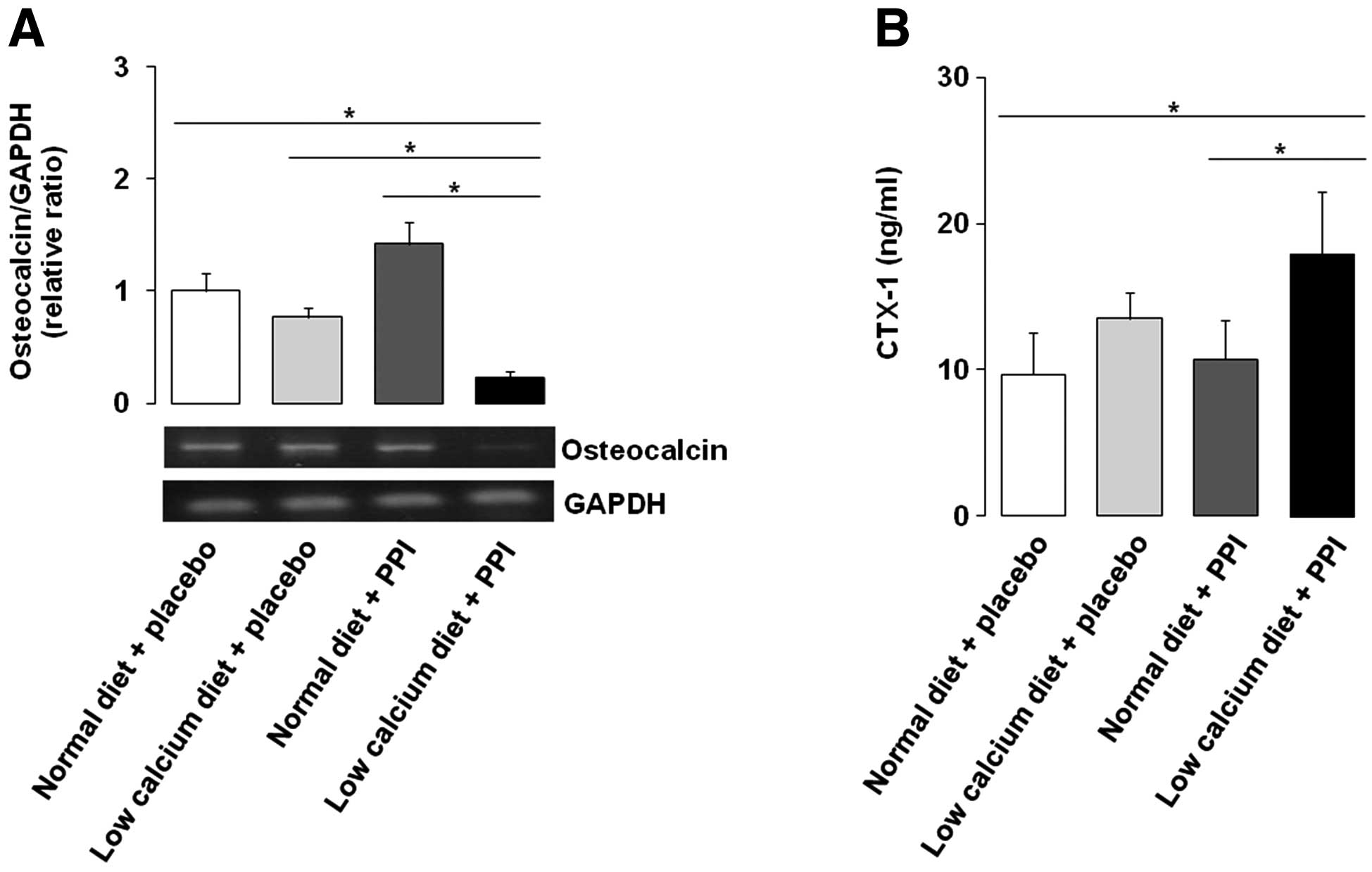
Osteocalcin is decreased and CTX-1 is
increased by the combination of a low calcium diet and PPI
Relative mRNA expression levels of osteocalcin, a
well-known bone formation biochemical marker, were significantly
downregulated by a low calcium diet with PPI, compared with the
other three groups, by −83, −80 and −89%, respectively (Fig. 3A). By contrast, the bone resorption
marker serum CTX-1 was significantly higher in the group with a low
calcium diet and PPI compared with the normal diet alone or PPI
alone groups (Fig. 3B). It is
suggested that PPI, together with a low calcium diet, may induce
alteration of macro- or micro-structure and contribute to the
pathogenesis of bone metabolism.
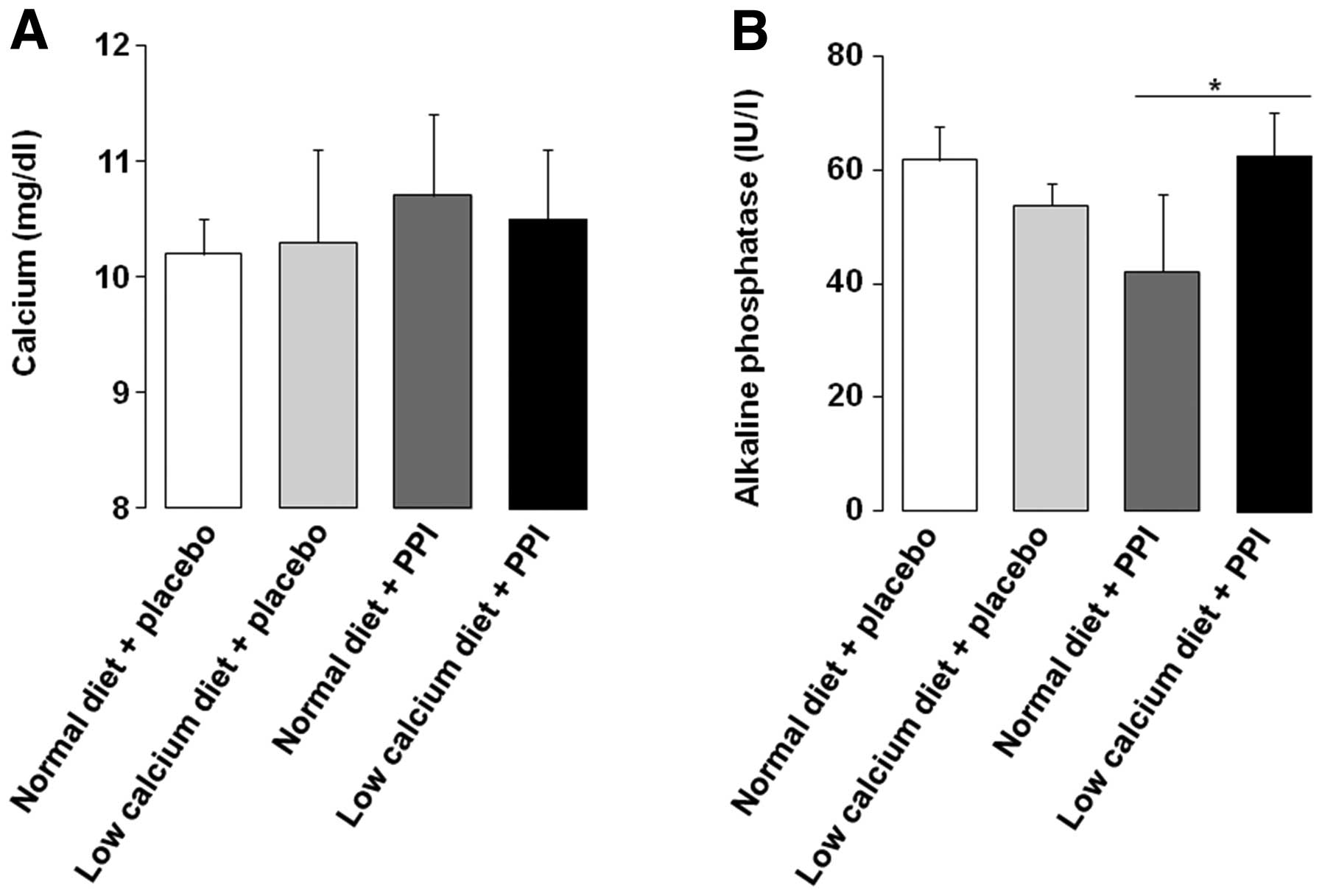
Serum concentration of calcium and
ALP
Serum calcium levels were not significantly
different among the four groups (Fig.
4A). However, the serum ALP level was found to be significantly
higher in the group with a low calcium diet and PPI, compared with
PPI alone (Fig. 4B). Generally,
serum calcium levels do not change greatly beyond the normal limit
despite ovariectomization in the rat model. Our result is thought
to be consistent with previous data, which suggested that serum
calcium remains constant under internal or external experimental
conditions (12,26). Considering that serum ALP usually
acts as an early biomarker of osteoblast differentiation and bone
formation (27), the difference in
serum ALP in our study conflicts with that of osteocalcin, which
was markedly decreased by the low calcium diet with PPI. However,
we believe that the osteocalcin result in our study is more
specific in reflecting the alteration to bone formation than that
of serum ALP, due to the fact that we used extracts taken directly
from femoral bone marrow cells for the analysis of the osteocalcin
activity.
Discussion
In this in vivo study we aimed to show how
PPI leads to bone turnover and to provide an explanation of the
exact mechanism. Based on clinical data suggesting that PPI
increased fracture risk in a duration- or dose-dependent manner, we
induced the long-term effects of PPI by administering 30 mg/kg for
8 weeks, which was longer than in previous rat model studies where
rats ingested omeprazole 20–30 mg/kg for 4 days to 3 weeks
(28–30).
In this study, we evaluated the effect of PPI on
differentiation and activation of osteoclasts and bone resorption.
RANKL/RANK binding and its sequential cascade of c-Fos and NFATc1
was not increased in the group with a regular diet and PPI. By
contrast, the relative ratio of RANKL/OPG and mRNA levels of c-Fos
and NAFATc1 were increased by the combination of a low calcium diet
and PPI administration, and we suggest that osteoclast activity may
be enhanced by the combination of a low calcium diet and PPI. Such
consideration is supported by the finding that serum CTX-1 levels,
a reliable bone resorption marker, were also increased with a low
calcium diet and PPI, compared with a regular diet with or without
PPI administration. Another notable finding is that the expression
level of osteocalcin was reduced significantly by the combination
of a low calcium diet with PPI compared with the other three
groups. However, it is controversial as to whether low calcium diet
and PPI have a synergic effect on the activation of osteoclasts and
the inhibition of osteoblasts. Experimental data supporting the
hypothesis for bone resorption or formation is still lacking, and
further studies are required.
We should be cautious about interpreting the
outcomes of this study after the consideration of several aspects.
Firstly, from the point of osteoclasts, there is a limit to the
direct comparison of the effect of omeprazole from that of other
synthetic V-ATPase inhibitors (i.e., FR167356, FR202126, SB242784),
which showed inhibitory effects on bone resorption and activation
of osteoclasts in previous in vivo studies (14,26),
as their potency on the V-ATPase of an osteoclast may be different.
Secondly, PPI can be a double-edged sword in reducing intestinal
absorption of calcium or inhibiting activation of osteoclasts, and
profound acid suppression with high-dose and long-term PPI therapy
may be necessary to have a significant impact on bone metabolism
(10). Thirdly, it would have been
helpful in supporting our data if we had scanned images from more
than two sites by micro-CT, in order to reveal alterations to
trabecular microstructure. Previous studies using ovariectomized
rats reported that significant changes of trabecular
microstructures were detected from both the femur neck and
vertebral body by micro-CT (31,32).
In conclusion, our results suggest that the
combination of a low calcium diet with PPI produces a possible
synergic effect on bone resorption, and calcium supplements are
necessary when a patient takes PPIs for a long time. Further
studies are required in the near future to investigate whether
their synergism clinically affects bone resorption and increases
the risk of osteoporosis and pathologic fracture.
Abbreviations:
|
GERD
|
gastroesophageal reflux disorder
|
|
PPI
|
proton pump inhibitor
|
|
NSAID
|
non-steroidal anti-inflammatory
drug
|
|
V-ATPase
|
vacuolar-ATPase
|
|
RANKL
|
receptor activator of NF-κB ligand
|
|
OPG
|
osteoprotegerin
|
|
OCP
|
osteoclast precursor
|
|
BMD
|
bone marrow density
|
|
NFATc1
|
nuclear factor of activated T cell
c1
|
|
CTX-1
|
crosslinking telopeptide of type 1
|
|
RT-PCR
|
reverse transcriptase-polymerase chain
reaction
|
|
ALP
|
alkaline phosphatase
|
References
|
1
|
El-Serag HB: Time trends of
gastroesophageal reflux disease: a systematic review. Clin
Gastroenterol Hepatol. 5:17–26. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeVault KR and Castell DO: Updated
guidelines for the diagnosis and treatment of gastroesophageal
reflux disease. Am J Gastroenterol. 100:190–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soll AH: Consensus conference. Medical
treatment of peptic ulcer disease Practice guidelines Practice
Parameters Committee of the American College of Gastroenterology.
JAMA. 275:622–629. 1996. View Article : Google Scholar
|
|
4
|
Talley NJ and Vakil N: Guidelines for the
management of dyspepsia. Am J Gastroenterol. 100:2324–2337. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Zanten SV, Armstrong D, Chiba N, et
al: Esomeprazole 40 mg once a day in patients with functional
dyspepsia: the randomized, placebo-controlled ‘ENTER’ trial. Am J
Gastroenterol. 101:2096–2106. 2006.PubMed/NCBI
|
|
6
|
Becker V, Bajbouj M, Waller K, Schmid RM
and Meining A: Clinical trial: persistent gastro-oesophageal reflux
symptoms despite standard therapy with proton pump inhibitors - a
follow-up study of intraluminal-impedance guided therapy. Aliment
Pharmacol Ther. 26:1355–1360. 2007. View Article : Google Scholar
|
|
7
|
Dent J, Brun J, Fendrick A, et al: An
evidence-based appraisal of reflux disease management - the Genval
Workshop Report. Gut. 44(Suppl 2): S1–S16. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jacobson BC, Ferris TG, Shea TL, Mahlis
EM, Lee TH and Wang TC: Who is using chronic acid suppression
therapy and why? Am J Gastroenterol. 98:51–58. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Targownik LE, Lix LM, Metge CJ, Prior HJ,
Leung S and Leslie WD: Use of proton pump inhibitors and risk of
osteoporosis-related fractures. CMAJ. 179:319–326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang YX, Lewis JD, Epstein S and Metz DC:
Long-term proton pump inhibitor therapy and risk of hip fracture.
JAMA. 296:2947–2953. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chonan O, Takahashi R, Yasui H and
Watanuki M: Effect of L-lactic acid on calcium absorption in rats
fed omeprazole. J Nutr Sci Vitaminol. 44:473–481. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O’Connell MB, Madden DM, Murray AM, Heaney
RP and Kerzner LJ: Effects of proton pump inhibitors on calcium
carbonate absorption in women: a randomized crossover trial. Am J
Med. 118:778–781. 2005.PubMed/NCBI
|
|
13
|
Karsdal MA, Henriksen K, Sorensen MG, et
al: Acidification of the osteoclastic resorption compartment
provides insight into the coupling of bone formation to bone
resorption. Am J Pathol. 166:467–476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niikura K, Takeshita N and Takano M: A
vacuolar ATPase inhibitor, FR167356, prevents bone resorption in
ovariectomized rats with high potency and specificity: potential
for clinical application. J Bone Miner Res. 20:1579–1588. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kocsis I, Arato A, Bodánszky H, et al:
Short-term omeprazole treatment does not influence biochemical
parameters of bone turnover in children. Calcif Tissue Int.
71:129–132. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizunashi K, Furukawa Y, Katano K and Abe
K: Effect of omeprazole, an inhibitor of H+,K(+)-ATPase,
on bone resorption in humans. Calcif Tissue Int. 53:21–25. 1993.
View Article : Google Scholar
|
|
17
|
Suda T, Takahashi N, Udagawa N, Jimi E,
Gillespie MT and Martin TJ: Modulation of osteoclast
differentiation and function by the new members of the tumor
necrosis factor receptor and ligand families. Endocr Rev.
20:345–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang H, Ryu J, Ha J, et al: Osteoclast
differentiation requires TAK1 and MKK6 for NFATc1 induction and
NF-kappaB transactivation by RANKL. Cell Death Differ.
13:1879–1891. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boyce BF and Xing L: The RANKL/RANK/OPG
pathway. Curr Osteoporos Rep. 5:98–104. 2007. View Article : Google Scholar
|
|
21
|
Kwan Tat S, Pelletier JP, Lajeunesse D,
Fahmi H, Lavigne M and Martel-Pelletier J: The differential
expression of osteoprotegerin (OPG) and receptor activator of
nuclear factor kappaB ligand (RANKL) in human osteoarthritic
subchondral bone osteoblasts is an indicator of the metabolic state
of these disease cells. Clin Exp Rheumatol. 26:295–304. 2008.
|
|
22
|
Matsuo K, Galson DL, Zhao C, et al:
Nuclear factor of activated T-cells (NFAT) rescues
osteoclastogenesis in precursors lacking c-Fos. J Biol Chem.
279:26475–26480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takayanagi H, Kim S, Koga T, et al:
Induction and activation of the transcription factor NFATc1 (NFAT2)
integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamashita T, Yao Z, Li F, et al: NF-kappaB
p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL)
and tumor necrosis factor-induced osteoclast precursor
differentiation by activating c-Fos and NFATc1. J Biol Chem.
282:18245–18253. 2007. View Article : Google Scholar
|
|
25
|
Lee BJ, Park JJ, Joo MK, et al: Effect of
proton pump inhibitor on the bone turnover in the ovariectomized
ICR mice. J Gastroenterol Hepatol. 23(Suppl 5): A102008.
|
|
26
|
Niikura K, Takeshita N and Chida N: A
novel inhibitor of vacuolar ATPase, FR202126, prevents alveolar
bone destruction in experimental periodontitis in rats. J Toxicol
Sci. 30:297–304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sorensen MG, Henriksen K, Schaller S and
Karsdal MA: Biochemical markers in preclinical models of
osteoporosis. Biomarkers. 12:266–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cadir FO, Bicakci U, Tander B, et al:
Protective effects of vitamin E and omeprazole on the
hypoxia/reoxygenation induced intestinal injury in newborn rats.
Pediatr Surg Int. 24:809–813. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Diogo Filho A, Santos PS, Duque AS,
Cezário RC and Gontijo Filho PP: Experimental model in the
qualitative and quantitative assessment of non-Helicobacter
gastric microflora under proton pump inhibitors action. Acta Cir
Bras. 21:279–284. 2006.PubMed/NCBI
|
|
30
|
Elseweidy MM, Younis NN, Amin RS, Abdallah
FR, Fathy AM and Yousif ZA: Effect of some natural products either
alone or in combination on gastritis induced in experimental rats.
Dig Dis Sci. 53:1774–1784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Otomo H, Sakai A, Ikeda S, et al:
Regulation of mineral-to-matrix ratio of lumbar trabecular bone in
ovariectomized rats treated with risedronate in combination with or
without vitamin K2. J Bone Miner Metab. 22:404–414. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song YH, Lee W, Lee CJ, Ji JH and Lee BD:
Study of bony trabecular characteristics using bone morphometry and
micro-CT. Korean J Oral Maxillofac Radiol. 37:27–33. 2007.
|