Introduction
Human cytomegalovirus (HCMV) is a large
double-stranded DNA virus of the human herpesvirus family; it
belongs to the β-herpesviridae subfamily of the herpes viruses.
HCMV is a significant human pathogen which infects the majority of
the world's population (1).
Although HCMV infection in adults and healthy children is usually
asymptomatic, it causes severe disease, including retinitis,
pneumonitis, hepatitis, gastroenteritis and end-organ disease, in
immunodeficient and/or immunocompromised individuals, such as organ
transplant patients, AIDs patients, congenitally infected neonates
and cancer patients undergoing chemotherapy (2,3).
Kenneson and Cannon (4) have
reported that the rates of congenital CMV infection are between 0.3
and 6.2% of all live births in developed countries.
Since there is no vaccine for HCMV, antiviral drugs
constitute the current treatment for CMV infection; the available
antiviral drugs include ganciclovir (GCV), valganciclovir,
foscarnet, cidofovir and fomivirsen (5,6).
However, these antiviral therapies have limited efficacy and a high
incidence of side-effects. Therefore, it is necessary to develop a
novel drug of low toxicity and high efficiency for the treatment of
HCMV infection. Garlic bulb has long been used as a natural
fungicide, and it is also known as a ‘natural antibiotic’.
Allitridin is an active constituent of garlic bulbs, and a number
of studies have demonstrated its pharmaceutical effects (7–9).
These studies have shown that allitridin has a variety of actions
potentially useful for human health, such as lowering hepatic
cholesterol storage, detoxification, anti-oxidant, antifungal and
antibacterial activities, as well as tumor suppression and
prevention of heart disease (10,11).
The antiviral effects of allitridin against HCMV have also been
shown in previous studies (12).
A previous study performed in our laboratory
demonstrated that allitridin reduces viral loads in the liver and
improves the histopathological lesions and functions of the liver
(13). Another, more recent study
by our group has shown that allitridin treatment reduces viral
levels in the salivary glands and facilitates the healing of
pathologic lesions in organs, particularly during the chronic
infection phase, and that it has mild anti-MCMV properties in
vivo(14). To further assess
the target and mechanisms of action of allitridin against HCMV,
immediate-early (IE; ul122 and ul123), early (E; ul54) and late (L;
ul83) gene transcription was investigated following the treatment
of HCMV-infected cells with allitridin or GCV. SYBR real-time PCR
was used to analyze the expression levels of the mRNAs of these
genes in various treatment groups; the HCMV IE72 deletion mutant
was evaluated as a control in order to investigate the inhibitory
effect of allitridin against IE72 as well as the synergistic effect
of IE72 and IE86. Finally, we aimed to ascertain whether allitridin
inhibits the expression of IE genes and then the expression of E
and L genes, to confirm the main link of the antiviral mechanism
and to provide an experimental basis for its anti-HCMV therapeutic
effects.
Materials and methods
Cell culture and virus
Human embryo lung fibroblast (HEL) cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco,
Carlsbad, CA, USA) supplemented with 10% (v/v) heat-inactivated
fetal calf serum (FCS), 100 U/ml penicillin and 100 μg/ml
streptomycin in a humidified incubator at 37°C in the presence of
5% CO2. The HCMV AD169 strain was provided by the
Institute of Virology, Chinese Academy of Preventive Medical
Sciences (Beijing, China) and the HCMV IE72 deletion mutant strain
(ΔIE72), which is obtained by deletion of exon 4 from the major IE
gene and does not express IE72 protein, was provided by M Zhang
(Medical College of Georgia, Augusta, GA, USA). The mean infective
viral quantities of AD169 and ΔIE72 were ~4.56×106 and
3.52×106 PFU/ml, respectively. The multiplicity of
infection (MOI) is the viral infectious titer divided by the cell
count; a high MOI (MOI=2.5) was used in this experiment. HEL cells
were digested with 0.20% pancreatic enzyme and seeded in 6-well
plates at a concentration of 2×105/ml; the monolayer
cells were then infected with the appropriate amount of HCMV AD169
or ΔIE72 strain. The starting time (0 h) was after 1 h of virus
adsorption, and normal cells were evaluated as the control.
Following 0.5, 2, 4, 6, 12 and 24 h of infection, the cells were
harvested, and maintained in refrigerators at −80°C until use.
Drugs
Allicin injection was purchased from Shanghai
Harvest Pharmaceutical Co., Ltd. (cat. no. 100801, 15 mg/ml;
Shanghai, China). GCV injection was obtained from Hubei Keyi
Pharmaceutical Co., Ltd. (cat. no. 100714; Hubei, China).
Cytotoxicity assay
The MTT assay was used to determine the cytotoxicity
of allitridin to HEL cells. HEL cells were cultured in a 96-well
plate in the absence or presence of various concentrations of
allitridin; each concentration of allitridin was used in 4 wells.
Following a 72-h culture, the plates were read at 560 nm in a
microplate reader. The percentage of surviving cells in each test
group was then calculated and the maximum tolerated concentrations
(MTCs) of allitridin and GCV in the HEL cells were determined to be
9.6 and 2.3 μg/ml, respectively.
Establishment of the infected cell
model
HEL cells were seeded in 6-well plates at a
concentration of 2×105/ml, and the monolayer cells were
then infected with the appropriate amount of HCMV AD169 or ΔIE72
strain. The viruses were adsorbed for 1 h and the end of the viral
adsorption process was considered to be the zero point of the time
course. The nutrient medium was then changed to a drug-free (with
4% FCS) or drug-containing (9.6 μg/ml allitridin or 2.3 μg/ml GCV)
maintenance medium. In this experiment, the cell groups were: i)
the experimental group, HCMV AD169 strain-infected cells treated
with allitridin; ii) the control groups: a) HCMV AD169
strain-infected cells; b) HCMV AD169 strain-infected cells treated
with GCV; c) HCMV ΔIE72 strain-infected cells; d) HCMV ΔIE72
strain-infected cells treated with allitridin; and e) HCMV ΔIE72
strain-infected cells treated with GCV. Each culture was harvested
0.5, 2, 4, 6, 12 and 24 h post-infection (p.i.) separately and
maintained at −80°C until RNA extraction was conducted.
Transcriptional level analyis of the
HCMV-related genes
Total RNA extraction was performed on these samples
using the TRIzol reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) following the manufacturer's instructions. To exclude
cellular DNA contamination, the samples were treated with DNase I
(Qiagen, Hilden, Germany). Dry RNA pellets were dissolved in
nuclease-free water and the concentration was estimated using a
NanoDrop ND-1000 Spectrophotometer (Nano Drop Technologies, Inc.,
Wilmington, DE, USA). cDNA was synthesized from 300 ng RNA using
Prime-Script™ RT Enzyme Mix I (Takara Bio, Inc, Shiga, Japan). In
each cDNA sample, the target and reference genes were detected
simultaneously by SYBR real-time PCR using the Mx3000P®
real-time PCR system (Stratagene, La Jolla, CA, USA). The primers
of the IE, E and L genes of HCMV were as follows: ul122 (78 bp)
forward, 5′-ATCATGCTGCCCCTCATC AAA-3′ and reverse,
5′-GATAATCTTGTTGCGGTACTGGAT-3; ul123 (86 bp) forward,
5′-GCTCCTCTGATTCTCTGGTGTC-3′ and reverse,
5′-ACTGTTCTCAGCCACAATTACTG-3′; ul54 (148 bp) forward,
5′-GACCTATTCGTTTTCACACCTACG-3′ and reverse,
5′-ATACTGTAGCCGTGTTCTGTGG-3′; ul83 (159 bp) forward,
5′-GCAGCCACGGGATCGTACT-3′ and reverse,
5′-GGCTTTTACCTCACACGAGCATT-3′. The human glyceraldehyde phosphate
dehydrogenase (GAPDH, 92 bp) gene was used as an amplification
control. The GAPDH primers were: forward, 5′-GGTTTATGGAGGTCCTCT
TGTGT-3′ and reverse, 5′-AACTACCCATGACTCAGC TTCTC-3′. SYBR
real-time PCR and melting curve analysis were performed using the
SYBR® Premix Ex Taq™ II (Perfect Real-Time; Takara Bio,
Inc.) according to the manufacturer's instructions. The standard
curves for the real-time PCR were generated using HCMV DNA. The
amounts of HCMV mRNA were quantified relative to GAPDH
expression.
Statistical analysis
The 2−ΔΔCT method was used to investigate
the different transcriptional levels of the IE, E and L genes of
HCMV, as well as the inhibitory effects of drugs against the
HCMV-related genes (15). All the
tests were repeated three times. The data were analyzed using SPSS
15.0 software and expressed as the mean ± standard error of the
mean (SEM). Comparisons between two groups were by analysis of
variance and statistical differences between groups were determined
using the Student's t-test. All tests were two-sided and P<0.05
was considered to indicate a statistically significant
difference.
Results
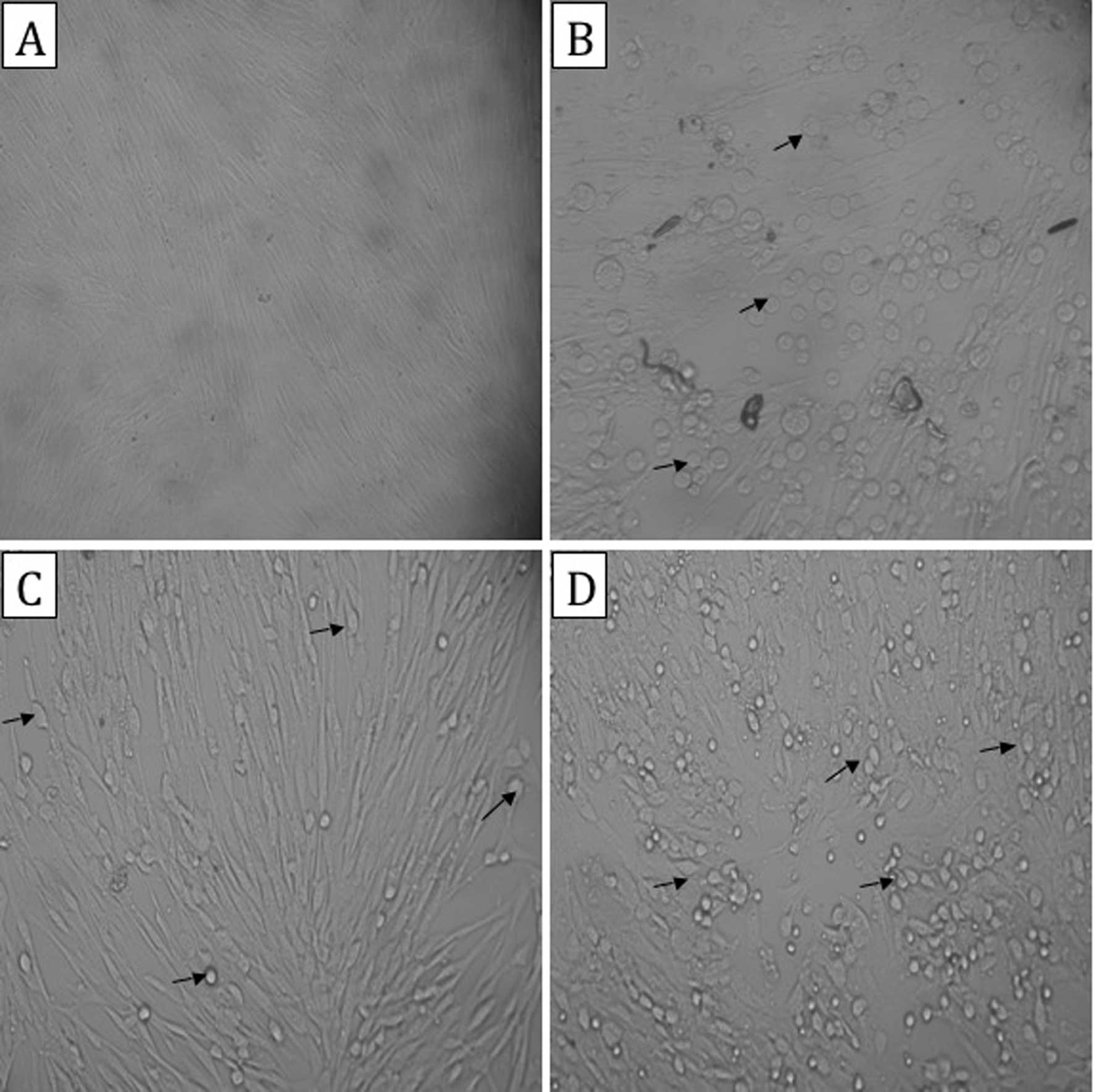
Observation of cells in each group
Allitridin is a potent irritant to HEL cells. When
the cell morphology was observed under an inverted microscope, cell
contraction and gaps between the cells were noted following the
addition of allitridin, but the cell morphology soon recovered in
the infected HEL cells. Lesions were observed in the cells of the
HCMV AD169 strain-infected control and allitridin- or GCV-treated
groups 8 h p.i. More specifically, the cells were swollen, had
increased in size and become rounder in shape, and were arranged in
a disorderly manner. Twenty-four hours p.i., the cells of the
AD169-infected control and GCV-treated groups remained markedly
swollen. In the allitridin-treated group, some of the cells
remained markedly swollen, however, most of the cells had decreased
in size. Moreover, the arrangement of cells was disordered and
there were some floating cells. Lesions were also observed in the
cells of the HCMV ΔIE72 strain-infected control and allitridin- or
GCV-treated groups 16 h p.i., including swelling and a disorderly
arrangement (Fig. 1).
HCMV gene expression
The expression pattern of HCMV genes described in
the present study is consistent with that reported in previous
studies (16). Fig. 2 shows the gene expression in HEL
cells following HCMV infection. The expression pattern of ul122
mRNA in the cells of the HCMV AD169 strain-infected groups was
similar to that in the cells of the HCMV ΔIE72 strain-infected
groups (Fig. 2A). The expression
began quickly and the levels rapidly increased following infection
in the two virus-infected groups. The transcription of the ul123
gene in the HCMV AD169 strain-infected cells rose from 0.5 to 6 h
p.i. and then increased modestly from 6 to 24 h p.i. (Fig. 2B). However, there was no ul123 gene
expression in the HCMV ΔIE72 strain-infected cells. The ul54 mRNA
levels in the two types of virus-infected cells were low as
infection began; however, the expression level in the AD169
strain-infected cells was clearly increased and continued to
increase 4 h p.i., while in the HCMV ΔIE72 strain-infected cells it
increased slowly and the growth rate was significantly lower than
that in the HCMV AD169 strain-infected cells (P<0.05; Fig. 2C). The expression pattern of ul83
in the HCMV AD169 strain-infected cells was similar to that in the
HCMV ΔIE72 strain-infected cells (Fig.
2D). The expression levels in the two types of virus-infected
cells were low during the first 4 h p.i. but significantly
increased 6 h p.i. The expression level of ul83 mRNA in the
ΔIE72-infected cells was lower than that in the AD169-infected
cells 6 h p.i. The levels of ul83 mRNA expression in the two types
of virus-infected cells were significantly different at the last
two time points (P<0.05).
Allitridin significantly suppresses the
expression of HCMV IE genes
The present study demonstrated that the expression
levels of the ul122 gene of the two virus strains in the
allitridin- and the GCV-treated cells were lower than those in the
virus-infected control groups (without drug treatment) following
infection (Fig. 3A). The
expression levels of the ul122 gene in the allitridin-treated cells
were significantly lower than those in the GCV-treated and
virus-infected control groups (P<0.01). The ul122 mRNA
expression levels were significantly decreased in the AD169-and
ΔIE72-infected cells following treatment with allitridin, and were
lower in the ΔIE72-infected cells. In the AD169-infected cells, the
expression of the ul123 gene following allitridin treatment was
significantly lower than that in the AD169-infected control cells
(P<0.01; Fig. 3B), while there
was no difference between the GCV-treated and the AD169-infected
control groups (P>0.05). In the ΔIE72-infected cells, the
expression of ul123 mRNA was not detected since exon 4 of the ul123
coding region was knocked down (Fig.
3B).
Allitridin suppresses the expression of
the HCMV E gene
The expression levels of the AD169 ul54 mRNA in the
allitridin- and GCV-treated cells were lower than those in the
virus-infected control cells (Fig.
3C), and the differences between the expression levels were
statistically significant (P<0.05). The expression levels of the
ΔIE72 ul54 mRNA in the allitridin- and the GCV-treated cells were
also lower than those in the virus-infected control cells. The
inhibitory effects of allitridin and GCV against HCMV ul54 mRNA
were more potent in the ΔIE72-infected cells than in the
AD169-infected cells (Fig.
3C).
Allitridin suppresses the expression of
the HCMV L gene
During the first 6 h following HCMV infection, the
expression levels of the ul83 gene were not different among the six
groups (P>0.05). However, 6 h following infection, the
expression levels of the ul83 gene in the drug-treated groups (GCV
or allitridin) were lower than those in the virus-infected control
groups, whether infected by the AD169 or the ΔIE72 strain. A more
potent inhibitory effect was observed in the allitridin-treated
cells 12 and 24 h p.i. (P<0.05), particularly in the
allitridin-treated ΔIE72 strain-infected cells, when compared with
the GCV-treated cells (Fig.
3D).
Discussion
The results of the present study regarding the
expression of HCMV genes were consistent with the results of
previous studies, and indicate that a gene expression cascading
effect occurred (16). The HCMV
gene family includes IE, E and L genes. The IE genes encode
immediate-early antigens (IEAs), mainly containing IE72 (ul122) and
IE86 (ul123) proteins, which have the highest expression levels and
the broadest range of functional activities. The E gene encodes
early antigens (EAs) which are enzymes and regulatory factors
essential for the synthesis of progeny DNA and proteins, such as
the viral DNA polymerase (UL54). UL54 is the catalytic subunit of
HCMV DNA polymerase, and the expression of ul54 gene is mainly
regulated by IE proteins (IE 72 and IE 86). Late antigens (LAs) are
viral structural proteins, such as the capsule matrix proteins
(UL83, pp65) (17). In the present
study, the HCMV ΔIE72 strain was used as a control virus strain in
order to investigate the inhibitory effect of allitridin against
IE72, the synergistic action of IE72 and IE86, and the regulation
of the transcription of ul54 and ul83 by IE72. The ΔIE72 strain was
not able to express IE72 since the exon 4 of the ul123 coding
region was knocked down. At a low MOI, only a small amount of IE86
was expressed, while the early and late proteins were not
expressed. Thus, the ΔIE72 strain was not able to replicate,
proliferate and form plaques (18). Therefore, the ΔIE72 strain and
adenovirus Ad5-infected HEK cells (adenovirus packaging cell) were
simultaneously used, since the cofactor required for the
proliferation of the ΔIE72 strain was provided by Ad5 (19).
In the present study, it was found that the
expression level of ul122 mRNA in the two virus strains was
similar; it was not influenced by the deficiency of IE72, in
accordance with the findings of a previous study (20). When treated with allitridin and
GCV, the expression levels of ul122 mRNA in the two types of virus
strain-infected cells were decreased, while the reduction in the
GCV-treated cells was lower than that in the allitridin-treated
cells. Notably, the inhibitory effect of allitridin against the
types of two virus-infected cells increased in a time-dependent
manner, and was significantly higher in the HCMV ΔIE72
strain-infected cells. The difference between the effects of
allitridin on the expression of the ul122 genes of the two viruses
suggests that allitridin may affect additional links, such as other
virus IE proteins, to inhibit the expression of ul122 mRNA.
Moreover, this may also be associated with the regulatory function
of IE86. IE86 acts alone or in synergy with IE72 to trans-activate
the IE promoter; it may also be combined with a DNA regulatory
sequence, such as the TATA box upstream of main immediate early
genes (MIE), to realize its own negative feedback control (21,22).
We hypothesized that the significant inhibitory effect of
allitridin against the transcription of ΔIE72 strain ul122 is
associated with several reasons: the strong and long-lasting
inhibitory effect of allitridin against the transcription of ul122,
the ΔIE72 strain lacks the synergy of IE72, and the regulatory
mechanism of IE86 when challenged by drug exposure is not able to
compensate for or resist the inhibitory effect of allitridin. We
subsequently analyzed the expression of the HCMV ul123 gene. It was
observed that the expression of ul123 mRNA in the
allitridin-treated cells was significantly lower than that in the
control cells in the HCMV AD169 strain-infected groups. The
expression of ul123 mRNA was not detected in the ΔIE72-infected
cells since the exon 4 of the ul123 coding region was knocked down.
Our results demonstrated that allitridin clearly inhibits the
expression of HCMV ul123 mRNA, while there was no significant
difference between the ul123 mRNA levels in the GCV-treated and
control cells.
These results indicate that the inhibitory effect of
allitridin on HCMV IE gene expression began early and was
prolonged. It was also observed that allitridin inhibited the
expression of ul122 mRNA to a greater extent than ul123 mRNA (24 h
p.i.), which was consistent with the results of our previous study
(23). Consequently, whether at
the transcriptional or the protein level, the inhibitory effect of
allitridin on IE86 was greater than that on IE72, while the
differences between the inhibitory effects at the DNA and protein
levels indicated that the drug is effective at the transcriptional,
post-transcriptional, translational and post-translational levels.
The IE86 protein is essential since it creates a favorable cellular
environment for viral replication; the MIE gene locus consists of
an enhancer-containing promoter upstream of the IE1 (ul123) and IE2
(ul122) genes (24). Our results
indicated that allitridin had a significant inhibitory effect
against the transcription of ul122 and ul123, which may occur
during the transcription process of the MIE gene to the precursor
RNA. There were no significant differences between these two genes
in the GCV-treated and the control cells, suggesting that the IE
genes may not be the key targets of GCV against HCMV.
The product of ul54 mRNA is the catalytic subunit of
HCMV DNA polymerase (symbolic early gene), which interacts with
UL44 (the auxiliary subunit of HCMV DNA polymerase) through the C
terminal of its polypeptide chain, is phosphorylated and then has
enzymatic activity (25). Our
results demonstrated that the level of expression of the ul54 gene
in the ΔIE72-infected cells was lower than that in the
AD169-infected cells, indicating that the deletion of IE72 resulted
in a reduction in ul54 mRNA expression, and that the transcription
of the virus downstream genes may be affected by IE72 deletion. It
was noted that the expression levels of AD169 and ΔIE72 ul54 mRNA
in the drug-treated cells were lower than those in the
virus-infected control cells; the expression level in the
ΔIE72-infected cells was lower, so it was concluded that the
inhibitory effects of allitridin and GCV against ΔIE72 ul54 mRNA
were stronger. The inhibitory effects of allitridin against the
ul54 mRNA of the two virus strains were significantly lower than
those against ul122 and ul123 mRNA. This may be due to the fact
that the expression of the ul54 gene is regulated by the IE
protein, suggesting that the IE genes may be the key functional
link between the actions of allitridin. Compared with GCV,
allitridin significantly inhibited the expression of IE genes, and
subsequently affected the expression of the E gene. When compared
with IE genes, GCV can inhibit the E gene more markedly, which is
consistent with the mechanism of GCV which is the competitive
inhibition of viral DNA polymerase.
pp65 (ul83) often serves as one of the target
proteins in the clinical diagnosis of HCMV active infection due to
its high levels of expression. Previous studies have shown that the
pp65 protein is an important protein which takes part in viral gene
expression and regulation, altering the metabolism of host cells
and viral replication (17). Our
results showed that the expression level of ul83 mRNA in the
ΔIE72-infected cells was lower than that in the AD169-infected
cells 6 h p.i., suggesting that the deletion of IE72 also affects
the expression of the virus L gene. The reduction was not as marked
as that of the E gene of the ΔIE72 strain; this may be due to the
fact that the synthesis of late virus protein occurs after the
replication of viral genes, and the expression may be influenced by
additional factors. Following treatment with allitridin or GCV, the
expression of ul83 mRNA was clearly decreased compared with that in
the respective control group 12 and 24 h p.i. Furthermore, the
inhibitory effect of allitridin against the L gene was similar to
that against the E gene, while the affects against both were lower
than those against the IE genes. Since the expression of HCMV genes
occurred in a sequential cascade, IE genes may be the key sites at
which allitridin acts. While GCV had no inhibitory effect on IE
genes but inhibited E (ul54) and L genes (ul83), this was
consistent with its main mechanism, the inhibition of DNA
polymerase activity.
It was hypothesized that allitridin significantly
inhibits the expression of HCMV IE genes (ul122 and ul123) at the
transcriptional level. The inhibitory effects against the two IE
genes were similar, which indicates that the action of allitridin
against the transcription of IE genes affected the progress of the
transcription of the MIE gene to the precursor RNA. By using the
ΔIE72 strain as a control, it was observed that the expression of
ul122 and ul123 mRNA have a clear synergistic association. The
inhibitory effects of allitridin against the ΔIE72 strain ul122,
ul54 and ul83 mRNA were higher than those against the respective
mRNA of the AD169 strain. The degree of inhibition decreased in the
order ul122 mRNA > ul54 mRNA > ul83 mRNA, indicating that
there were many factors involved in the regulation of the upstream
to downstream gene transcription and the regulation of the
expression of the HCMV IE, E and L genes. Following analysis of the
degree of inhibition of allitridin against ul54 and ul83 mRNA, as
well as the influence of IE72 deficiency on the expression of
ul122, ul54 and ul83 mRNA, it was hypothesized that the inhibitory
action of allitridin against the expression of ul54 and ul83 mRNA
may occur indirectly through the inhibition of the expression of IE
gene mRNA. It is well known that GCV acts by inhibiting the viral
DNA polymerase and incorporates viral DNA directly to terminate the
extension of the viral DNA chain (26). In the replication cycle of HCMV,
the expression of IE protein occurs prior to the replication of
viral DNA and is followed by the expression of the E gene (ul54),
which encodes DNA polymerase. Following completion of the DNA
replication, the L gene begins to express encoded virus structural
proteins and the replication cycle is then finished. Therefore, the
effect of GCV was consistent with its mechanism. The observation
that the inhibitory effect of GCV against HCMV was different from
that of allitridin, and less potent, demonstrates that the
antiviral mechanisms of GCV and allitridin are different.
In conclusion, the present study showed that the
expression pattern of related gene mRNAs in the AD169
strain-infected cells was similar to the pattern in the ΔIE72
strain-infected cells, and that the deletion of IE72 affects the
expression of virus downstream genes. Following drug treatment, it
was concluded that allitridin significantly inhibits the
transcription of HCMV-related genes, and that IE genes constitute
the main target; the key link may be the progress of the MIE gene
transcript to the precursor RNA. This may provide a theoretical
basis for the effect of allitridin against HCMV infection, and
support the advantageous antiviral properties of traditional
Chinese medicine. Since circumstances did not allow the
investigation of the cytotoxicity of allitridin in vivo, the
concentration of allitridin in the blood is not known. Future
studies will aim to focus on the treatment of infections caused by
HCMV and the investigation of additional potential inhibitory
pathways.
Acknowledgements
This study was supported by a grant (no. 30070928)
from the National Natural Science Foundation of China, awarded to
Feng Fang.
References
|
1
|
Cannon MJ, Schmid DS and Hyde TB: Review
of cytomegalovirus seroprevalence and demographic characteristics
associated with infection. Rev Med Virol. 20:202–213. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan G, Nogalski MT, Stevenson EV and
Yurochko AD: Human cytomegalovirus induction of a unique signalsome
during viral entry into monocytes mediates distinct functional
changes: a strategy for viral dissemination. J Leukoc Biol.
92:743–752. 2012. View Article : Google Scholar
|
|
3
|
Crough T and Khanna R: Immunobiology of
human cytomegalovirus: from bench to bedside. Clin Microbiol Rev.
22:76–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kenneson A and Cannon MJ: Review and
meta-analysis of the epidemiology of congenital cytomegalovirus
(CMV) infection. Rev Med Virol. 17:253–276. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Santos LF and Carratalà J: Treatment of
cytomegalovirus disease. Enferm Infecc Microbiol Clin. 29:65–69.
2011.
|
|
6
|
Pescovitz MD: Valganciclovir: recent
progress. Am J Transplant. 10:1359–1364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Powolny AA and Singh SV: Multitargeted
prevention and therapy of cancer by diallyl trisulfide and related
Allium vegetable-derived organosulfur compounds. Cancer
Lett. 269:305–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Durak I, Kavutcu M, Aytac B, Avci A,
Devrim E, Ozbek H and Ozturk S: Effects of garlic extract
consumption on blood lipid and oxidant/antioxidant parameters in
humans with high blood cholesterol. J Nutr Biochem. 15:373–377.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leuschner RGK and Ielsch V: Antimicrobial
effects of garlic, clove and red hot chilli on Listeria
monocytogenes in broth model systems and soft cheese. Int J
Food Sci Nutr. 54:127–133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu Y, He Z, Shen X, Xu X, Fan J, Wu S and
Zhang D: Cholesterol-lowering effect of allicin on
hypercholesterolemic ICR mice. Oxid Med Cell Longev.
2012:4896902012.PubMed/NCBI
|
|
11
|
Agarwal KC: Therapeutic actions of garlic
constituents. Med Res Rev. 16:111–124. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo NL, Lu DP, Woods GL, Reed E, Zhou GZ,
Zhang LB and Waldman RH: Demonstration of the anti-viral activity
of garlic extract against human cytomegalovirus in vitro. Chin Med
J. 106:93–96. 1993.PubMed/NCBI
|
|
13
|
Liu ZF, Fang F, Dong YS, Li G and Zhen H:
Experimental study on the prevention and treatment of murine
cytomegalovirus hepatitis by using allitridin. Antiviral Res.
61:125–128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu XL, Wang H, Li YN, Ge HX, Shu SN and
Fang F: Effects of allitridin on acute and chronic mouse
cytomegalovirus infection. Arch Virol. 156:1841–1846. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murphy E and Shenk T: Human
cytomegalovirus genome. Human Cytomegalovirus: Current Topics in
Microbiology and Immunology. Shenk TE and Stinski MF: Springer; New
York, NY: pp. 1–19. 2008, View Article : Google Scholar
|
|
17
|
White EA, Del Rosario CJ, Sanders RL and
Spector DH: The IE2 60-kilodalton and 40-kilodalton proteins are
dispensable for human cytomegalovirus replication but are required
for efficient delayed early and late gene expression and production
of infectious virus. J Virol. 8125:2573–2583. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gawn JM and Greaves RF: Absence of IE1 p72
protein function during low-multiplicity infection by human
cytomegalovirus results in a broad block to viral delayed-early
gene expression. J Virol. 76:4441–4455. 2002. View Article : Google Scholar
|
|
19
|
Wilkinson GW and Akrigg A: Constitutive
and enhanced expression from the CMV major IE promoter in a
defective adenovirus vector. Nucleic Acids Res. 20:2233–2239. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Greaves RF and Mocarski ES: Defective
growth correlates with reduced accumulation of a viral DNA
replication protein after low-multiplicity infection by a human
cytomegalovirus ie1 mutant. J Virol. 72:366–379. 1998.PubMed/NCBI
|
|
21
|
Arlt H, Lang D, Gebert S and Stamminger T:
Identification of binding sites for the 86-kilodalton IE2 protein
of human cytomegalovirus within an IE2-responsive viral early
promoter. J Virol. 68:4117–4125. 1994.PubMed/NCBI
|
|
22
|
Ahn JH and Hayward GS: The major
immediate-early proteins IE1 and IE2 of human cytomegalovirus
colocalize with and disrupt PML-associated nuclear bodies at very
early times in infected permissive cells. J Virol. 71:4599–4613.
1997.
|
|
23
|
Zhen H, Fang F, Ye DY, et al: Experimental
study on the action of allitridin against human cytomegalovirus in
vitro: inhibitory effects on immediate-early genes. Antiviral Res.
72:68–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stinski MF and Petrik DT: Functional roles
of the human cytomegalovirus essential IE86 protein. Curr Top
Microbiol Immunol. 325:133–152. 2008.PubMed/NCBI
|
|
25
|
Aitken C, Barrett-Muir W, Millar C, et al:
Use of molecular assays in diagnosis and monitoring of
cytomegalovirus disease following renal transplantation. J Clin
Microbiol. 37:2804–2807. 1999.
|
|
26
|
De Clercq E and Neyts J: Antiviral agents
acting as DNA or RNA chain terminators. Antiviral Strategies.
Kräusslich HG and Bartenschlager R: Springer; New York: pp. 53–84.
2008
|