Introduction
Titanium (Ti) and its alloys have been widely used
as implant materials in oromaxillofacial and other regions of bone
tissue, for example, as prostheses in implantodontics and anchors
in orthodontics, owing to their good biocompatibility,
osseointegration, resistance to corrosion and low allergenicity
(1,2). The criterion of a successful implant
in vivo is osseointegration between biomaterial and bone
tissue, a process in which osteoblasts are important. Various
surface modifications of Ti implants have been explored to promote
osseointegration and the RGD (Arg-Gly-Asp) sequence has been widely
employed as a bioactive coating to improve osteoblast adhesion and
proliferation, accelerate osteogenic differentiation and
extracellular matrix (ECM) mineralization (3,4).
The immobilization of the RGD peptide is of
considerable fundamental and practical importance. Commonly, RGD
covalently binds to Ti via functional moieties, including hydroxyl,
amino and carboxyl radicals (5),
however, this method of immobilization is associated with a number
of issues, including complex treatment procedures, cytotoxity of
the coupling reagent and deactivation of the active group rapidly
by hydrolysis (6). Previously,
peptide aptamers (i.e., binders) which interact with inorganic
materials have been artificially created and used as a ‘glue’ to
link various biomolecules to the surface of inorganic metals
(7). TBP-1 (RKLPDAPGMHTW), a novel
peptide aptamer isolated from a linear 12-mer peptide phage
library, has been confirmed to be capable of specifically
recognizing and interacting with Ti, by its N-terminal sequence,
RKLPDA (minTBP-1) (8). In our
previous study, a novel chimeric peptide RKLPDAPRGDN
(minTBP-1-PRGDN) was synthesized by connecting minTBP-1 to the
N-terminal of PRGDN. This chimeric peptide was found to exhibit
favorable affinity for the Ti surface and the modified Ti was
associated with improved attachment and spreading of MC3T3-E1 cells
(9).
The MC3T3-E1 cell, derived from neonatal mouse
calvaria, has been widely used as a model osteoprogenitor cell in
bone tissue engineering (10).
MC3T3-E1 cells on implant surfaces are known to undergo three
phases: initial adhesion (from minutes to hours), proliferation
(days) and differatiation (weeks to months) (11). In the present study, the biological
behavior of MC3T3-E1 cells following adhesion to minTBP-1-PRGDN
modified Ti discs was further investigated. Cell proliferation and
differentiation were estimated by cell numbers, relative gene
expression and alkaline phosphatase activity (ALP). In addition,
ECM mineralization was assessed.
Materials and methods
Preparation of Ti discs and peptides
Commercially pure Ti discs (diameter, 15 mm;
thickness, 1 mm; Cp Ti; Baoji Nonferrous Metal Industry Co., Baoji,
China) were polished, cleaned, oxidized and sterilized as described
previously (12). The chimeric
peptide RKLPDAPRGDN (minTBP-1-PRGDN; 1,238.38 g/mol) was
synthesized by connecting RKLPDA (minTBP-1) to the N-terminal of
PRGDN (derived from the consecutive sequence of human BSP, GenBank
accession no. AAA60549.1, residues 285–289). RKLPDA (698.83 g/mol),
PRGDN (557.57 g/mol) and RKLPDAPRGDN were all synthesized by
ChinaPeptides Co., Ltd. (Shanghai, China). All peptides were
synthesized using the solid phase peptide synthesis method,
purified by HPLC and identified by amino acid analysis. The
lyophilized peptides were reconstituted in sterile deionized water
to a final concentration of 100 μg/ml.
Ti disc coating
Sterilized Ti discs were placed at the bottom of
24-well culture plates which had the same diameter as the Ti disc
to prevent the non-specific binding of cells to the bottom of the
culture plate. The discs were then divided into 4 groups and were
coated with minTBP-1-PRGDN/Ti, minTBP-1/Ti or PRGDN/Ti overnight at
4°C, or were uncoated (control/Ti). Prior to cell seeding, all
discs were washed several times with phosphate-buffered saline
(PBS; Gibco-BRL, Carlsbad, CA, USA) to remove unbound peptides and
pre-warmed in an incubator at 37°C.
MC3T3-E1 cell culture
Mouse osteoblastic MC3T3-E1 cells were cultured as
described previously (9).
MC3T3-E1 cell proliferation
To investigate cell proliferation, MC3T3-E1 cells
were cultured onto Ti discs at a density of 2×104
cells/cm2 in 24-well tissue culture plates and incubated
in medium containing 10% fetal bovine serum for 24, 48 and 72 h.
Following each incubation period, quantification of proliferated
cells was performed by colorimetry using alamarBlue (Biosource
International, Inc., Camarillo, CA, USA). Briefly, medium was
aspirated and proliferative cells were incubated in serum-free
culture medium containing 10% (v/v) fresh alamarBlue for 5 h. Cell
number was measured as the difference in absorbance at 570 and 600
nm by a microplate spectrophotometer (μQuant; BioTek Instruments,
Inc., Winooski, VT, USA).
MC3T3-E1 cell differentiation
For ALP activity, osteogenic marker gene expression
and matrix mineralization assays, MC3T3-E1 cells were seeded onto
the various modified Ti surfaces at a density of 1×104
cells/cm2 in 24-well tissue culture plates. After 24 h,
the cells were cultured in osteogenic induction medium, which
contained 50 mg/l ascorbic acid, 10 mmol/l β-glycerophosphate and
10−8 mol/l hexadecadrol. The medium was replaced every
48 h.
ALP activity assay
ALP activity was determined by a colorimetric
endpoint assay, in which the enzymatic conversion of p-nitrophenyl
phosphate (pNPP; N9389; Sigma-Aldrich, St. Louis, MO, USA) to the
yellowish product, p-nitrophenol (pNP), was measured. Following
osteogenic induction for 28 days, cells on Ti discs were rinsed
with PBS, detached with 0.25% (w/v) trypsin and then collected with
1% Triton X-100 buffer solution (pH 7.6) containing 1 mM
MgCl2·6H2O and 50 mM Tris. Following
treatment with hypersound on ice for 30 min, an aliquot (20 μl) of
the cell lysate was incubated with 1 ml reaction solution
(containing 1 mM MgCl2, 1 mM ZnCl2 and 1
mg/ml pNPP) in the dark for 30 min at room temperature. To
terminate the enzymatic conversion of pNPP to pNP in the presence
of ALP, 3 N NaOH was added, followed by incubation for an
additional 5 min. ALP activity was determined by
spectrophotometrical quantification of pNP at 405 nm using an ELISA
reader (model 550; Bio-Rad, Hercules, CA, USA). A bicinchoninic
acid protein assay reagent (#23227; Pierce Biotechnology, Inc.,
Rockford, IL, USA) was used to determine the total intracellular
protein content. ALP activity was normalized to U pNP/mg
protein.
Quantitative real-time polymerase chain
reaction (q-RT-PCR)
At the end of each osteogenic induction (7, 14 and
21 days), total RNA was isolated with an RNeasy kit (Qiagen, Tokyo,
Japan) according to the manufacturer's instructions. Reverse
transcription was performed following standard procedures with
SuperScript Reverse Transcriptase (Invitrogen Life Technologies,
Carlsbad, CA, USA). SYBR-Green-based q-RT-PCR amplifications were
performed using the following primer sets: osteopontin (OPN),
5′-CTT TCA CTC CAA TCG TCC CTA C-3′ and 5′-CTG CCC TTT CCG TTG TTG
TC-3′ (58°C, 238 bp); osteocalcin (OC), 5′-TCT CTG CTC ACT CTG CTG
GC-3′ and 5′-GGG ACT GAG GCT CCA AGG TA-3′ (60°C, 155 bp); and
β-actin, 5′-GTG GGC CGC TCT AGG CAC CAA-3′ and 5′-CTC TTT GAT GTC
ACG CAC GAT TTC-3′ (58°C, 249 bp). Triplicate reactions were
performed for each sample. Expression values of each sample were
normalized against β-actin expression levels.
Alizarin red-S (AR-S) staining and
analysis of mineralized deposits by metallurgical microscopy
Following the 28-day osteogenic induction period,
cells were fixed with 4% paraformaldehyde in PBS for 15 min at 4°C
and rinsed with PBS. Fixed cells were stained for 10 min with 0.1%
AR-S (A5533; Sigma-Aldrich) at room temperature with gentle
rotation and washed with PBS. For each group, three visual fields
of the same magnification (x100) were randomly selected and images
were captured using a metallurgical microscope (Axiovert 200MAT;
Carl Zeiss Light Microscopy, Göttingen, Germany).
Image-Pro® Plus software package (6.0.0 edition, Media
Cybernetics, Inc., Rockville, MD, USA) was employed for image
analysis. Briefly, the mineralized deposits in each group were
counted and their relative sizes were quantified by measuring the
average number of pixels captured using the Image-Pro®
Plus software package.
Statistical analysis
All experiments were performed at least in
triplicate. Results were analyzed by Student's t-test between 2
groups of experiments and one-way ANOVA in >2 groups of
experiments. If the ANOVA test identified a significant difference,
further post hoc Fisher's LSD multiple comparison test was applied.
Data are presented as mean ± SD (bars). P<0.05 was considered to
indicate a statistically significant difference.
Results
MC3T3-E1 cell proliferation
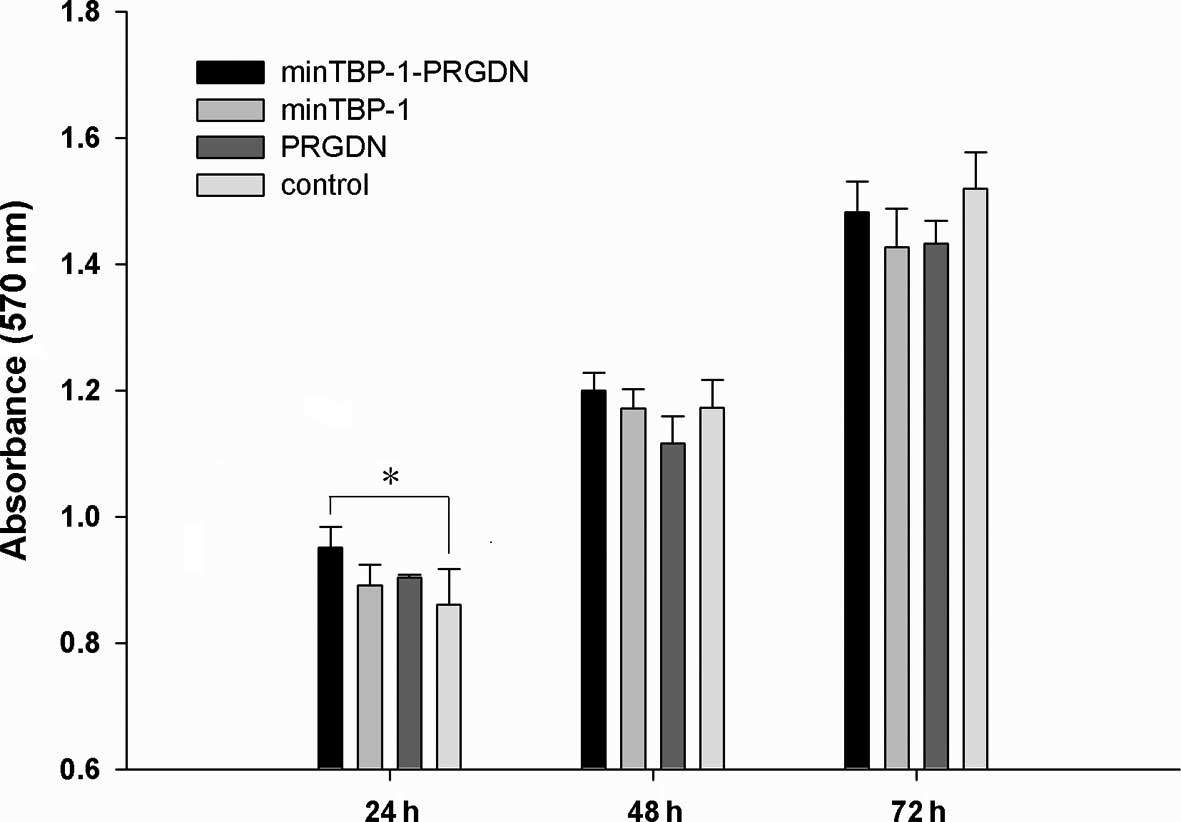
The alamarBlue assay was used to determine relative
cell proliferation levels at 24, 48 and 72 h. As demonstrated in
Fig. 1, increased absorbance was
observed in all groups at the end of the culture period indicating
that MC3T3-E1 cell proliferation occurred in all 4 groups. In
particular, the proliferation on minTBP-1-PRGDN/Ti was observed to
be significantly greater than that on control/Ti after 24 h
(P<0.05).
ALP activity assay
For each group, ALP activity was at a low basal
level on day 3 and increased steadily until day 14, this was
followed by a rapid increase which peaked on day 28 (Fig. 2). These observations indicate that
MC3T3-E1 cells in all groups differentiated towards mature
osteoblasts. Of note, the ALP activity of cells on control/Ti was
much higher than those on minTBP-1-PRGDN/Ti on day 14
(P<0.05).
Expression of osteogenic marker
genes
Gene expression profiling data derived from q-RT-PCR
for osteogenic markers were assessed and are shown in Fig. 3. Cells on minTBP-1-PRGDN/Ti were
found to express the highest levels of OPN (Fig. 3A) and OC (Fig. 3B) mRNA after 14 days of osteogenic
induction (P<0.05).
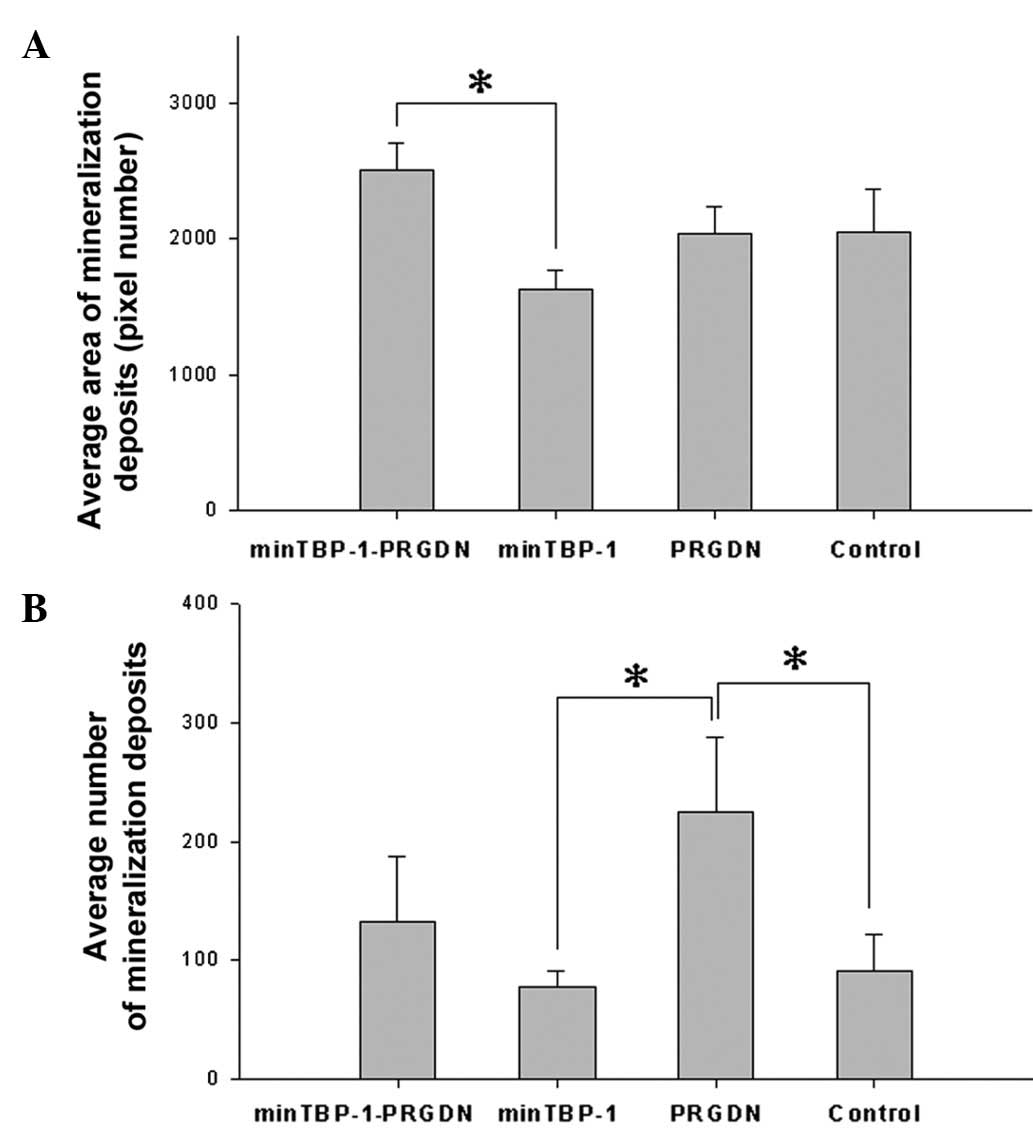
Mineralized deposits
On day 28, thin spherical deposits, which were
stained red by AR-S, were distributed uniformly on the Ti discs in
all groups (Fig. 4). The average
area of mineralized deposits was highest on minTBP-1-PRGDN/Ti and
lowest on minTBP-1/Ti and the difference between these two groups
was significant (P<0.05; Fig.
5A). The average number of mineralization deposits on PRGDN/Ti
was the highest and was significantly higher than the numbers on
minTBP-1/Ti and control/Ti (P<0.05; Fig. 5B).
Discussion
To assistant RGD peptide immobilization on the Ti
surface, the chimeric peptide minTBP-1-PRGDN was previously
designed and synthesized with the aim of achieving a double binding
function by minTBP-1 specifically recognizing and binding Ti and
PRGDN associating with integrin receptors on the osteoblast
membrane (9). In our previous
study, the chimeric peptide was found to exhibit an affinity for Ti
and the ability to facilitate the adhesion of MC3T3-E1 cells. In
the present study, the effect of minTBP-1-PRGDN on the
bioactivities of MC3T3-E1 cells following adhesion was
explored.
Favorable adhesion and fast proliferation has been
demonstrated to aggregate osteoblasts into multilayers which
provide collagen support as the basis of mineralized deposit
formation (13). In the present
study, cell numbers increased with time in all groups, which
indicated that MC3T3-E1 cells proliferated well. In particular,
minTBP-1-PRGDN rapidly accelerated the proliferation of the cells
(at 24 h), indicating that this chimeric peptide may favor the
differentiation of MC3T3-E1 cells.
ALP represents a standard marker of ECM development
and maturation for in vitro experiments and is expressed
from the early stages of osteoblast differentiation, decreases
slightly when mineral deposition occurs and is then maintained at
relatively high levels (14). In
the present study, ALP activity increased persistently with time in
all groups. and did not decrease. This result is consistent with
the findings of Oya et al that the ALP activity of MC3T3 E1
cells kept increasing with time from the beginning of osteogenic
induction to day 28 (15).
Elevated ALP activity is an indicator of improved MC3T3-E1 cell
function and matrix production. In the present study, cells on
PRGDNT/Ti exhibited a marked increase in ALP activity by day 10 and
14. This observation is not considered to be unusual since RGD, a
common element of the majority of ECM proteins, has been reported
to advance the ALP activity induced by RGD-ligand binding to
osteoblast integrin receptors in a number of studies (16,17).
OPN and OC, secreted by differentiated osteoblasts,
are the main noncollagen proteins and participate in ECM
mineralization. In the current study, OPN and OC rapidly peaked on
minTBP-1-PRGDN/Ti in the middle stage of differentiation (day 14).
It should be noted that the middle stage of osteoblast
differentiation is extremely important as, during this stage, the
ECM matures gradually and mineralized deposits begin to form
(18). The results of the current
study indicate that the chimeric peptide, minTBP-1-PRGDN,
accelerated MC3T3-E1 cell osteogenic differentiation, which is
likely to promote the osseointegration of modified Ti implants
in vivo.
Mineralization of the ECM is a late marker of
osteoblast differentiation and an important indicator of the
osteoinductive ability of implant biomaterial (19). During mineralization, amorphous
Ca/P materials are deposited extracellularly and propagate into the
collagen fibril matrix, leading to formation of mineralized
deposits (18). In the present
study, the average area of mineralized deposits on
minTBP-1-PRGDN/Ti was the highest at the end of osteogenic
induction (day 28). We hypothesize that the chimeric peptide
facilitated the accumulation of hydroxyapatite on the modified Ti
surface. Of note, MC3T3-E1 cell attachment to minTBP-1-PRGDN/Ti was
the most evident (9) and the cell
proliferation in the minTBP-1-PRGDN/Ti group was higher than that
in the other groups in the initial 24 h. In addition, this chimeric
peptide contributed to the expression of mineralization-related
genes (OPN/OC) in the middle stage of MC3T3-E1 cell
differentiation. Collectively, these observations are consistent.
We hypothesize that increased attachment and active movement
facilitated the rapid confluency of MC3T3-E1 cells on
minTBP-1-PRGDN/Ti, which contributed to the rapid proliferation,
differentiation and mineralization of the ECM. On day 14, ALP
activity of MC3T3-E1 cells on PRGDN/Ti was the most conspicuous in
this study, which may contribute to the highest mineralized deposit
number on the PRGDN/Ti surface. This result was consistent with
reports that RGD-containing coatings may promote osteoblast
activities and mineralization on biomaterials (3,4).
In conclusion, the present study indicated that the
chimeric peptide minTBP-1-PRGDN facilitated the biological
behaviour of MC3T3-E1 cells, to some extent, following adhesion to
the Ti surface and mineralization of the ECM. This study may
provide an important insight into the value of the biomimetic
coating of Ti implants and the application of functional adhesion
peptides which may facilitate bone remodeling. Further in
vivo studies are required to fully understand the role of
minTBP-1-PRGDN in osseointegration between Ti implants and bone
tissue and this investigation is currently in progress.
Acknowledgements
This study was supported by a grant from the
National Science Foundation of China (No. 81171450).
References
|
1
|
Long M and Rack HJ: Titanium alloys in
total joint replacement - a materials science perspective.
Biomaterials. 19:1621–1639. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu XY, Chu PK and Ding CX: Surface
modification of titanium, titanium alloys and related materials for
biomedical applications. Mater Sci Eng R. 47:49–121. 2004.
View Article : Google Scholar
|
|
3
|
Ferris DM, Moodie GD, Dimond PM, Gioranni
CW, Ehrlich MG and Valentini RF: RGD-coated titanium implants
stimulate increased bone formation in vivo. Biomaterials.
20:2323–2331. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cavalcanti-Adam EA, Shapiro IM, Composto
RJ, Macarak EJ and Adams CS: RGD peptides immobilized on a
mechanically deformable surface promote osteoblast differentiation.
J Bone Miner Res. 17:2130–2140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sawyer AA, Weeks DM, Kelpke SS, McCracken
MS and Bellis SL: The effect of the addition of a polyglutamate
motif to RGD on peptide tethering to hydroxyapatite and the
promotion of mesenchymal stem cell adhesion. Biomaterials.
26:7046–7056. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hersel U, Dahmen C and Kessler H: RGD
modified polymers: biomaterials for stimulated cell adhesion and
beyond. Biomaterials. 24:4385–4415. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baneyx F and Schwartz DT: Selection and
analysis of solid-binding peptides. Curr Opin Biotechnol.
18:312–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sano K and Shiba K: A hexapeptide motif
that electrostatically binds to the surface of titanium. J Am Chem
Soc. 125:14234–14235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang D, Mao J, Zhou B, Liao XF, Gong SQ,
Liu Y and Zhang JT: A chimeric peptide that binds to titanium and
mediates MC3T3-E1 cell adhesion. Biotechnol Lett. 33:191–197. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from new born mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Quarles LD, Yohay DA, Lever LW, Caton R
and Wenstrup RJ: Distinct proliferative and differentiated stages
of murine MC3T3-E1 cells in culture: An in vitro model of
osteoblast development. J Bone Miner Res. 7:683–692. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Mao J, Zhou B, Wei W and Gong S:
Peptide aptamers against titanium-based implants identified through
phage display. J Mater Sci Mater Med. 21:1103–1107. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pockwinse SM, Stein JL, Lian JB and Stein
GS: Developmental stage-specific cellular responses to vitamin D
and glucocorticoids during differentiation of the osteoblast
phenotype: interrelationship of morphology and gene expression by
in situ hybridization. Exp Cell Res. 216:244–260. 1995. View Article : Google Scholar
|
|
14
|
Choi JY, Lee BH, Song KB, Park RW, Kim IS,
Sohn KY, Jo JS and Ryoo HM: Expression patterns of bone-related
proteins during osteoblastic differentiation in MC3T3-E1 cells. J
Cell Biochem. 61:609–618. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oya K, Tanaka Y, Saito H, Kurashima K,
Nogi K, Tsutsumi H, Tsutsumi Y, Doi H, Nomura N and Hanawa T:
Calcification by MC3T3-E1 cells on RGD peptide immobilized on
titanium through electrodeposited PEG. Biomaterials. 30:1281–1286.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruoslahti E: RGD and other recognition
sequences for integrins. Annu Rev Cell Dev Biol. 12:697–715. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sawyer AA, Hennessy KM and Bellis SL: The
effect of adsorbed serum proteins, RGD and proteoglycan-binding
peptides on the adhesion of mesenchymal stem cells to
hydroxyapatite. Biomaterials. 28:383–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rohde M and Mayer H: Exocytotic process as
a novel model for mineralization by osteoblasts in vitro and in
vivo determined by electron microscopic analysis. Calcif Tissue
Int. 80:323–336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Declercq HA, Verbeeck RM, De Ridder LI,
Schacht EH and Cornelissen MJ: Calcification as an indicator of
osteoinductive capacity of biomaterials in osteoblastic cell
cultures. Biomaterials. 26:4964–4974. 2005. View Article : Google Scholar : PubMed/NCBI
|