Introduction
Apoptosis or programmed cell death, is a
physiological process responsible for the removal of cells that
have completed their specific functions or are harmful to the
organism. Apoptosis plays a key role in combatting cancer cells
(1). Therefore, the induction of
apoptosis in cancer cells is a strategy used in anticancer therapy.
Over the past two decades a number of studies have reported the
apoptotic activities of members of the triterpene saponin group
(2–4).
Nortriterpene saponin is an important triterpene
saponin and numerous studies have reported the anti-inflammatory
(5), anticancer (6), anticomplement (7), anti-HIV (8,9),
insecticidal (10), melanogenesis
inhibitory (11),
gastroprotective, platelet aggregative (12), antioxidant (13), antiviral (14) and protein tyrosine phosphatase
inhibitory (15) activities of
nortriterpenoids. Although various bioactivity studies of
nortriterpenoids have been performed, the molecular mechanism by
which nortriterpene saponin acts on cancer cells remains
unclear.
Previously, we reported the isolation and structural
elucidation of a new nor-Oleanane type triterpene saponin
(Bigelovii A; Fig. 1) from the
dried herbs of Salicornia bigelovii Torr (16). In addition, Bigelovii A was shown
to inhibit the proliferation of HL-60 (leukemia), MCF-7 (breast)
and HepG2 (liver) cells, with the HL-60 cells being the most
sensitive (16). The present study
used the HL-60 cells to further investigate the cytotoxic mechanism
of Bigelovii A and found that the mechanism may be dependent on,
not only membrane permeabilisation, but also on the
apoptosis-inducing effect of Bigelovii A, which was observed to be
involved in Bcl-2 suppression and caspase-3 activation.
Materials and methods
Materials
Bigelovii A was isolated from the whole herbs of
Salicornia bigelovii Torr and the structure is presented in
Fig. 1. Bigelovii A was dissolved
in dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO, USA) to generate
a stock solution which was stored at −20°C and diluted with medium
prior to each experiment. The final DMSO concentration was ≥0.1%
DMSO throughout the study (all control groups were composed of 0.1%
DMSO). Iscove’s modified Dulbecco’s medium (IMDM) was purchased
from Gibco-BRL (Carlsbad, CA, USA) and fetal bovine serum was
purchased from Hyclone Laboratories, Inc. (Logan, UT, USA).
Penicillin, streptomycin and DMSO were purchased from Sunshine
Biotechnology (Nanjing, China). RNase A and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
were obtained from Amersco LLC (Solon, OH, USA). Propidium iodide
(PI) was purchased from Sigma. Hoechst 33258 and the CaspACETM
Assay System were purchased from KeyGen Biotechnology (Nanjing,
China) and Promega Corporation (Madison, WI, USA), respectively. A
lactate dehydrogenase (LDH) detection kit was obtained from Roche
Diagnostics GmbH (Mannheim, Germany). Primary antibodies against
Bcl-2, Bax, caspase-3 and β-tubulin were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Horse-radish peroxidase
(HRP)-conjugated secondary antibody was purchased from Wuhan Boster
Biological Technology, Ltd. (Wuhan, China) and the enhanced
chemiluminescence (ECL) kit was obtained from Cell Signaling
Technology (Beverly, MA, USA).
Cell culture
The human leukocyte cancer cell line, HL-60, was
obtained from the Cell Bank of the Shanghai Institute of
Biochemistry and Cell Biology, Chinese Academy of Sciences
(Shanghai, China). The cells were maintained in IMDM supplemented
with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml
streptomycin. The media were changed every other day. All the cells
were incubated at 37°C in a humidified atmosphere of 95% air and 5%
CO2. The study was approved by the ethics committee of
the Institute of Botany, Jiangsu Province and Chinese Academy of
Sciences
MTT assay
The effect of Bigelovii A on the viability of the
tumor cells was assessed by a MTT assay, which was based on the
reduction of MTT by the mitochondrial dehydrogenase of intact cells
to form a purple formazan product. Briefly, the HL-60 cells were
seeded at 5×104 cells/well in a 96-well plate and
treated with various concentrations of Bigelovii A or vehicle
(control). Each of the treated or control groups contained 6
parallel wells. Following incubation for 24 h at 37°C in a
humidified incubator, cell viability was determined. MTT [5 mg/ml
in phosphate-buffered saline (PBS)] was added to each well and
incubated for 4 h. Then, 100 μl solubilization solution (10% SDS in
0.012 M HCl) was added to each well and the plate was left to stand
overnight in the incubator. Absorbance was recorded on a microplate
reader (Tecan US, Inc., Morrisville, NC, USA) at a wavelength of
570 nm (reference wavelength, 690 nm). The percentage cell
proliferation was calculated as a ratio of the sample to control OD
values. Experiments were performed under the same conditions at
least three times. The cell inhibitory ratio was calculated using
the following formula: inhibitory ratio (%) = (1 − average
absorbance of treated group/average absorbance of control group) ×
100. IC50 was defined as the concentration that caused a
50% inhibition of cell viability.
LDH release assay
The HL-60 cells were seeded in 96-well round bottom
plates at a density of 5×104 cells/well in 100 μl IMDM
with 1% FBS (serum contains natural LDH activity) and containing
1–10 μl/ml Bigelovii A. Following a 24-h incubation at 37°C, the
plates were centrifuged at 250 × g (Eppendorf AG, Hamburg, Germany)
for 10 min. Aliquots (100 μl) of the supernatants were collected. A
mixture of diaphorase/NAD+ and iodotetrazolium chloride
(100 μl) was added to each well. Following 30 min at room
temperature in the dark, absorbance was recorded on a microplate
reader at a wavelength of 490 nm. Spontaneous LDH release from
untreated normal cells (Lc) and high level maximum release by
disrupting the cells with Triton X-100 (Hc) were analyzed. The
percentage of LDH release compared with the control was calculated
as: [(treated mean − Lc)/(Hc − Lc)] × 100.
Morphological analysis of apoptosis by
Hoechst 33258 staining
Hoechst 33258 staining of the HL-60 cells was
performed to evaluate the cell death pattern induced by increasing
the Bigelovii A concentration. The morphology of the HL-60 cells
treated with Bigelovii A for 24 h was observed under an inverted
microscope. Hoechst 33258 staining was then used to determine
apoptotic morphology. Briefly, the cells were seeded at a
concentration of 1×106 cells/ml in 6-well tissue culture
plates and treated with the indicated concentration of Bigelovii A.
The cells were harvested, washed twice with PBS, fixed with 4%
formaldehyde for 10 min and stained with Hoechst 33258 staining
solution according to the manufacturer’s instructions. The stained
nuclei were examined and immediately photographed under a
fluorescence microscope (Olympus IX51, Tokyo, Japan) with a peak
excitation wavelength of 340 nm.
Cell cycle analysis and
sub-G0/G1 measurement
The HL-60 cells were seeded in a 6-well culture
plate at a density of 1×106 cells/ml and treated with
Bigelovii A for 12 and 24 h. The cells were then collected, washed
with PBS and fixed in 1 ml 70% ice-cold ethanol at 4°C. After being
left to stand overnight, cell pellets were collected by
centrifugation, resuspended in 100 μl RNase (100 μg/ml) and
incubated at 37°C for 30 min. Then, 400 μl PI solution (50 μg/ml)
was added and the mixture was allowed to stand on ice for 30 min.
The fluorescence emitted from the PI-DNA complex was quantified
following excitation of the fluorescent dye by FACScan flow
cytometry (Becton-Dickinson, Franklin Lakes, NJ, USA). The
percentage of cells in the G0/G1, S,
G2/M and sub-G1 phases was analysed using the
CellQuest software program.
Semi-quantitative RT-PCR
The effect of Bigelovii A treatment on the
expression of Bcl-2 and Bax was determined by RT-PCR analysis.
Following 24 h incubation, total RNA was isolated using an RNA
extraction kit according to the manufacturer’s instructions (Takara
Biotechnology, Dalian, China). The RNA concentration was determined
by the absorption at 260 nm. cDNA was synthesized by extension of
oligo (dT) primers with 10 units AMV reverse transcriptase in a
mixture containing 1 μg total RNA. Amplification of the cDNA was
performed using the PCR kit. The oligonucleotide primer sequences
were as follows: GADPH, 5′-GGTCGGAGTCAACGGATTTGGTCG-3′ (sense) and
5′-CCTCCGACGCCTGCTTCACCAC-3′ (antisense); Bcl-2,
5′-TGCCACGGTGGTGGAGGAGC-3′ (sense) and 5′-GCATGTTGACTTCACTTGTGGC-3′
(antisense); and Bax, 5′-TCCACCAAGAAGCTGAGCGAG-3′ (sense) and
5′-GTCCAGCCCATGATGGTTCT-3′ (antisense). The samples for Bcl-2 were
inactivated for 3 min at 94°C prior to hotstart amplification. The
amplification cycle was performed at 94°C for 30 sec, 65°C for 1
min and 72°C for 2 min for 35 cycles. The samples for Bax were
inactivated for 7 min at 97°C prior to hotstart amplification. This
amplification cycle was performed at 94°C for 1 min, 60°C for 45
sec and 72°C for 45 sec for 35 cycles. The PCR products were
separated by electrophoresis in 1% agarose gels and visualized by
ethidium bromide staining. For quantitation, images of the agarose
gels were scanned and the bands were densitometrically analyzed
with Scan Analysis software (Tanon Science and Technology,
Shanghai, China).
Western blot analysis
The HL-60 cells were treated for 24 h with various
concentrations of Bigelovii A (1, 2 and 4 μg/ml) and then
collected. The cells were lysed in lysis buffer [50 mM Tris-Cl (pH
7.6), 150 mM NaCl, 1 mM EDTA, 1% (m/v) NP-40, 0.2 mM PMSF, 0.1 mM
NaF and 1.0 mM DTT] and the lysates were clarified by
centrifugation at 4°C for 15 min at 13,000 × g. The protein
concentration was measured by the Bradford assay. Following this,
equal concentrations of protein were separated by SDS-PAGE and
transferred onto the PVDF membranes (Millipore, Billerica, MA,
USA). The blots were incubated with specific antibodies against the
indicated primary antibodies overnight at 4°C, followed by
HRP-conjugated secondary antibody for 1 h at 37°C. Immunoreactive
proteins were detected with the ECL kit. All blots were stripped
and reprobed with polyclonal anti-tubulin antibody to ascertain
equal loading of the proteins.
Measurement of caspase-3 activity
The activation of caspase-3 was determined using a
caspase-3 assay kit. This assay is based on the spectrophotometric
detection of chromophore p-nitroanilide (pNA) released following
cleavage of the labeled substrate, DEVD-pNA. The absorbance of pNA
from an apoptotic sample was compared with that of an uninduced
control. For the inhibited apoptosis samples, the inhibitor,
Z-VAD-FMK, was added to the cells at the same time as Bigelovii A.
Briefly, the HL-60 cells (1×106) were harvested by
centrifugation. The cell pellets were resuspended in cold lysis
buffer and placed on ice for 15 min and then the resuspension was
centrifuged at 15,000 × g for 20 min. The supernatant was collected
as cell lysate and incubated with Z-VAD-FMK according to the
manufacturer’s instructions for 4 h at 37°C. The release of pNA was
measured at 405 nm using a 96-well microplate reader. The
quantification of viable cells was determined by a cytometer.
Statistical analysis
Experiments were repeated at least three times.
Results are presented as mean ± SD. Statistically significant
differences compared with untreated controls were calculated using
the Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cytotoxic effect of Bigelovii A on the
HL-60 cells
To evaluate the effect of Bigelovii A on cell
growth, proliferation assays were performed on the HL-60 cells
using increasing drug concentrations (1–10 μg/ml) and cell
viability was determined using the MTT assay. As demonstrated in
Fig. 2, incubation of the cells
for 24 h with Bigelovii A was found to significantly decrease cell
survival in a dose-dependent manner. A reduction in cell viability
by 50% in comparison with the control was achieved at a dose of
2.15 μg/ml. In contrast with the Bigelovii A treated cells, the
cells treated with DMSO showed limited or no cytotoxicity. Since
the ability to interact with cell membranes is a characteristic of
saponins, using the LDH release test we investigated whether
Bigelovii A altered the cellular membrane. The test must be
performed with a low level of serum in the medium. The results
indicated that the release of LDH was concentration-dependent.
Collectively, the results of the MTT assay and LDH release test
indicate that cell death caused by Bigelovii A may not only be due
to membrane permeabilization.
Bigelovii A-induced apoptotic cell death
in the HL-60 cells
To determine whether the HL-60 cells treated with
Bigelovii A underwent apoptosis, two apoptotic assays, including
Hoechst 33258 staining and a hypodiploid DNA assay, were performed.
Marked apoptosis was observed by both assays. Hoechst 33258 dye
selectively bound the DNA and enabled monitoring of nuclear
morphological changes under a fluorescence microscope. The nuclei
of the untreated cells stained blue with Hoechst were observed to
exhibit loose chromatin and be distributed throughout the cell.
Bigelovii A at 2 and 4 μg/ml induced significant nuclear
fragmentation and condensation in the HL-60 cells following 24 h of
treatment (Fig. 3). The majority
of the cells decreased in size and had a relatively small size
compared with the untreated cells. To investigate the effects of
Bigelovii A on cell cycle status, the HL-60 cells were treated with
various concentrations of Bigelovii A for 12 and 24 h and then
analyzed for alterations in the cell cycle by flow cytometry.
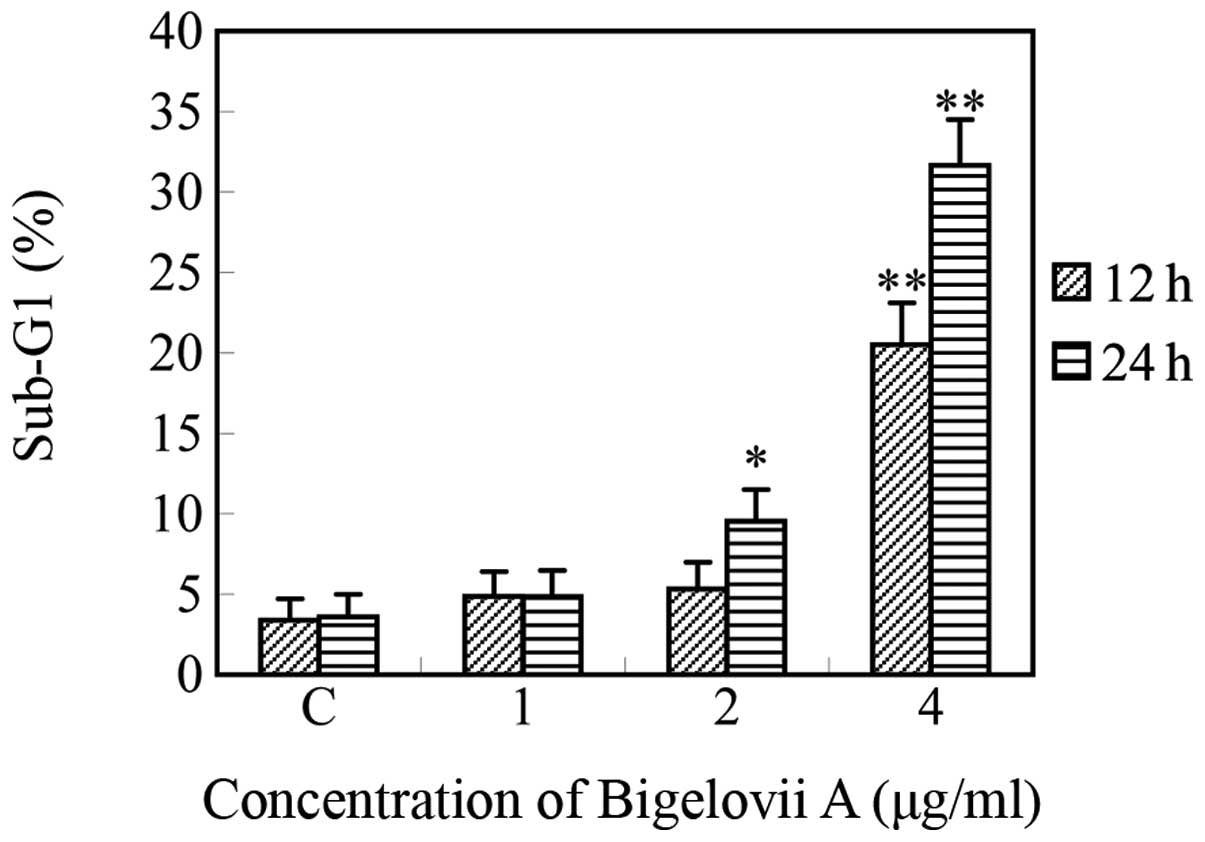
Bigelovii A caused dose- and time-dependent increases in the
hypodiploid sub-G0/G1 DNA fraction (<2n
DNA), indicating apoptosis due to DNA fragmentation (Fig. 4). The
sub-G0/G1 fraction was 3.6% in untreated
cells, which gradually increased to 31.7% following 4 μg/ml
Bigelovii A treatment for 24 h. Bigelovii A treatment had almost no
effect on the G2/M fraction, indicating that the
compound does not produce a mitotic block or delay in the cell
cycle.
Intrinsic pathway involvement in
Bigelovii A-induced apoptosis
Bcl-2 family proteins, including anti-apoptotic
members (e.g., Bcl-2) and pro-apoptotic members (e.g., Bax), play a
pivotal role in apoptosis (17).
Bcl-2 has been identified to form a heterodimeric complex with the
proapoptotic member Bax, neutralizing its proapoptotic effects.
Therefore, the Bcl-2/Bax ratio is a decisive factor and plays a
significant role in determining whether cells are likely to undergo
death or survival. To elucidate the mechanisms underlying Bigelovii
A-induced apoptosis, the effect of Bigelovii A treatment on the
gene and protein expression levels of Bcl-2 and Bax was examined.
The HL-60 cells were treated for 24 h and the total RNA was
isolated, reverse-transcribed and amplified using Bcl-2 and Bax
specific primers. As demonstrated in Fig. 5, treatment with Bigelovii A
decreased the Bcl-2 expression and increased the Bax mRNA
expression in a dose-dependent manner, leading to a decrease in the
Bc1-2/Bax ratio. In addition, further western blot analysis was
used to evaluate the protein expression levels of the Bcl-2 family
members and caspase-3. The Bcl-2 protein levels were decreased and
caspase-3 was activated, however, the Bax protein levels were not
markedly changed following treatment with Bigelovii A for 24 h
(Fig. 6). Overall, the reduction
of Bcl-2 expression, together with the activation of caspase-3,
indicated that Bigelovii A-induced apoptotic cell death was
mediated through the intrinsic pathway.
Caspase activation involved in Bigelovii
A-induced apoptosis
Activation of the caspase family is a crucial
mechanism for the induction of death signals in apoptosis. The
caspases are a family of cysteine proteases in mammalian cells that
cleave proteins following an aspartic acid residue. Caspases may be
divided into initiators and effectors. Caspase-3, an effector
caspase, is central to the mediation of various apoptotic
responses. In addition to being observed by western blot analysis,
activated caspase-3 was also observed by colorimetric assay in the
present study (Table I). To
determine whether the activation of caspase-3 was required for the
induction of cell death by Bigelovii A, the HL-60 cells were
pretreated with the pan-caspase inhibitor, Z-DEVD-FMK, which
completely reversed Bigelovii A-induced growth inhibition. These
results indicate that caspase-3 is activated in response to
apoptosis induced by Bigelovii A.
 | Table IEffect of 24 h Bigelovii A treatment
on caspase-3 activity in HL-60 cells. |
Table I
Effect of 24 h Bigelovii A treatment
on caspase-3 activity in HL-60 cells.
| Parameters | N | A | I |
|---|
| OD405 | 0.0452±0.0035 | 0.1084±0.0041a | 0.0267±0.0016b |
| Cell viability
(%) | 100±2.7 | 51.4±3.2a | 100±4.1b |
Discussion
Acute leukaemia, as with other types of cancer, is a
progressive clonal disorder driven by mutation. Leukaemic cells
proliferate primarily in the bone marrow and lymphoid tissues,
where they interrupt normal hematopoiesis and immunity. Regimens
for the induction of remission, consolidation therapy and
bone-marrow transplantation have improved the outlook for patients
with acute leukaemia (18).
However, there remains a considerable number of incidences of
relapse and unsatisfactory results in patients with extramedullary
disease (19). Therefore, the
research and development of new and safe drugs for leukaemia is
required by the pharmaceutical industry. The present study
evaluated the chemopreventive potential of Bigelovii A in HL-60
human acute promyelocytic leukaemia cells, as well as their
cytotoxic mechanism.
There are several assays currently available for the
assessment of cell cytotoxicity, including the MTT reduction assay
and the LDH release assay. A colorimetric MTT assay is available
for measuring the mitochondrial-related reduction capacity and LDH
release assays are used to evaluate cell membrane damage (20). In the present study, the novel
30-nortriterpenoid glycoside, Bigelovii A, was shown to exhibit
marked cytotoxic effects on HL-60 cells by MTT and LDH assays. MTT
assay suggested that the growth inhibitory effects were
dose-dependent: for example, 34.9, 42.1 and 64.7% cytotoxicity at
1, 2 and 4 μg/ml, respectively, with an IC50 value of
2.15 μg/ml. Also, LDH release was slowly increased following
treatment with Bigelovii A, indicating cell membrane
impairment.
Apoptosis is an evolutionarily conserved process
that eliminates irreversibly damaged or potentially harmful cells
in order to protect the organism. In the present study, we found
that treatment with Bigelovii A rapidly induced HL-60 cell death.
Bigelovii A-induced cell death in these cells exhibited the typical
characteristics of apoptotic cells, including membrane blebbing and
cell and chromatin condensation. Flow cytometry confirmed that the
treatment with Bigelovii A increased the fraction of cells with
hypodiploid DNA and that this effect was concentration- and
time-dependent. Bigelovii A induced apoptosis in a similar manner
to macranthoside B in HepG2 (21)
and in HL-60 cells (22).
Extrinsic and intrinsic-mediated pathways lead to
apoptosis. The intrinsic or mitochondria-mediated pathways may be
activated directly without being triggered by a death receptor. The
Bcl-2 protein family are the most important regulators of the
intrinsic apoptotic processes and this family includes proapoptotic
members, such as Bax, and anti-apoptotic members, such as Bcl-2.
Bcl-2 functions as a repressor of apoptosis by blocking the release
of cytochrome c. Bcl-2 is also important for the resistance to
chemotherapy and radiotherapy (23,24).
However, Bax has been demonstrated to play a key role in initiating
mitochondrial dysfunction (25).
Therefore, the apoptotic process is regulated by the ratio of Bax
and Bcl-2, which is recognized to initiate caspase signaling
(26,27). In the present study, treatment with
Bigelovii A decreased Bcl-2 expression while increasing Bax mRNA
expression in a dose-dependent manner. The Bcl-2/Bax ratio was
evidently lower than that of the control at 4 μg/ml Bigelovii A.
The Bcl-2 protein levels were also decreased, however, the Bax
levels were not markedly changed.
The caspases may be structurally divided on the
basis of the presence or absence of an N-terminal prodomain. The
caspases containing long prodomains are the first to be activated
in response to apoptotic stimuli. The activated caspases destroy
the cellular architecture and ultimately result in cell death
(28). Caspase-3 is the most
important member of the caspase family. Western blot analysis and
the colorimetric assay indicated that 4 μg/ml Bigelovii A activated
caspase-3. Taken together, these observations reveal that the
downregulation of Bcl-2 may also lead to the activation of
caspase-3 and the induction of apoptosis in Bigelovii A-mediated
HL-60 cells.
In the present study, Bigelovii A was demonstrated
for the first time to exhibit potent anticancer activity in
vitro and induce apoptosis in HL-60 cells, indicating that
Bigelovii A may be a promising novel anticancer drug.
Acknowledgements
This study was supported by grants from the Fund of
the Institute of Botany, Jiangsu Province and the Chinese Academy
of Sciences (no. 201001), as well as the Jiangsu Provincial Natural
Science Foundation of China (no. BK2011424), the National Natural
Science Foundation of China (no. 31100251) and the Open Funds of
Jiangsu Center for Research and Development of Medicinal Plants
(201201).
References
|
1
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang SR and Fang WS: Pentacyclic
triterpenoids and their saponins with apoptosis-inducing activity.
Curr Top Med Chem. 9:1581–1596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Z, Gao J, Cai XT, et al: Escin
sodium induces apoptosis of human acute leukemia Jurkat T cells.
Phytother Res. 25:1747–1755. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen DY, Kang JH, Song W, Zhang WQ, Li WG,
Zhao Y and Chen QX: Apoptosis of human cholangiocarcinoma cell
lines induced by β-escin through mitochondrial caspase-dependent
pathway. Phytother Res. 25:1519–1526. 2011.
|
|
5
|
Thao NT, Hung TM, Cuong TD, et al:
28-nor-oleanane-type triterpene saponins from Camellia
japonica and their inhibitory activity on LPS-induced NO
production in macrophage RAW264.7 cells. Bioorg Med Chem Lett.
20:7435–7439. 2010.PubMed/NCBI
|
|
6
|
Thao NT, Hung TM, Lee MK, Kim JC, Min BS
and Bae K: Triterpenoids from Camellia japonica and their
cytotoxic activity. Chem Pharm Bull (Tokyo). 58:121–124.
2010.PubMed/NCBI
|
|
7
|
Min BS, Oh SR, Ahn KS, et al:
Anti-complement activity of norlignans and terpenes from the stem
bark of Styrax japonica. Planta Med. 70:1210–1215. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao WL, Yang SY, Yang LM, et al: Chemical
constituents from the leaves and stems of Schisandra
rubriflora. J Nat Prod. 73:221–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei Y, Ma CM, Chen DY and Hattori M:
Anti-HIV-1 protease triterpenoids from Stauntonia
obovatifoliola Hayata subsp intermedia. Phytochemistry.
69:1875–1879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Avilla J, Teixidò A, Velázquez C,
Alvarenga N, Ferro E and Canela R: Insecticidal activity of
Maytenus species (Celastraceae) nortriterpene quinone
methides against codling moth, Cydia pomonella (L.)
(Lepidoptera, tortricidae). J Agric Food Chem. 48:88–92. 2000.
|
|
11
|
Akihisa T, Takahashi A, Kikuchi T, et al:
The melanogenesis-inhibitory, anti-inflammatory and chemopreventive
effects of limonoids in n-hexane extract of Azadirachta
indica A. Juss. (neem) seeds. J Oleo Sci. 60:53–59. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshikawa M, Morikawa T, Fujiwara E,
Ohgushi T, Asao Y and Matsuda H: New noroleanane-type triterpene
saponins with gastroprotective effect and platelet aggregation
activity from the flowers of Camellia japonica: Revised
structures of camellenodiol and camelledionol. Heterocycles.
55:1653–1657. 2001. View Article : Google Scholar
|
|
13
|
Wang H, Tian XA and Ying GQ: A new
nortriterpene from the root of Celastrus hypoleucus. Helv
Chim Acta. 93:1628–1633. 2010. View Article : Google Scholar
|
|
14
|
Cheng YB, Liao TC, Lo YW, et al:
Nortriterpene lactones from the fruits of Schisandra
arisanensis. J Nat Prod. 73:1228–1233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kwon JH, Chang MJ, Seo HW, et al:
Triterpenoids and a sterol from the stem-bark of Styrax
japonica and their protein tyrosine phosphatase 1B inhibitory
activities. Phytother Res. 22:1303–1306. 2008.PubMed/NCBI
|
|
16
|
Wang QZ, Liu XF, Shan Y, et al: Two new
nortriterpenoid saponins from Salicornia bigelovii Torr. and
their cytotoxic activity. Fitoterapia. 83:742–749. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that med iate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burnett AK and Eden OB: The treatment of
acute leukaemia. Lancet. 349:270–275. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmid C, Schleuning M, Schwerdtfeger R,
et al: Long-term survival in refractory acute myeloid leukemia
after sequential treatment with chemotherapy and reduced-intensity
conditioning for allogeneic stem cell transplantation. Blood.
108:1092–1099. 2006. View Article : Google Scholar
|
|
20
|
Kim H, Yoon SC, Lee TY and Jeong D:
Discriminative cytotoxicity assessment based on various cellular
damages. Toxicol Lett. 184:13–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Zhao XZ, Qi Q, et al:
Macranthoside B, a hederagenin saponin extracted from Lonicera
macranthoides and its anti-tumor activities in vitro and in
vivo. Food Chem Toxicol. 47:1716–1721. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guan F, Shan Y, Zhao X, et al: Apoptosis
and membrane permeabilisation induced by macranthoside B on HL-60
cells. Nat Prod Res. 25:332–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tortora G, Caputo R, Damiano V, et al:
Combined targeted inhibition of bcl-2, bcl-XL, epidermal growth
factor receptor, and protein kinase A type I causes potent
antitumor, apoptotic, and antiangiogenic activity. Clin Cancer Res.
9:866–871. 2003.
|
|
24
|
Tsujimoto Y: Cell death regulation by the
Bcl-2 protein family in the mitochondria. J Cell Physiol.
195:158–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei MC, Zong WX, Cheng EH, et al:
Proapoptotic BAX and BAK, a requisite gateway to mitochondrial
dysfunction and death. Science. 292:727–730. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kirkin V, Joos S and Zörning M: The role
of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta.
1644:229–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manion MK and Hockenbery DM: Targeting
BCL-2-related proteins in cancer therapy. Cancer Biol Ther. 2(4
Suppl 1): S105–S114. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|