Introduction
The latest advances in computed tomography (CT) make
it possible to non-invasively and intuitively obtain the anatomical
morphology of the coronary artery tree, particularly contributing
to the identification of the magnitude, distribution and
composition of coronary atherosclerosis (1,2). It
has been documented that CT coronary angiography (CTCA) has a high
accuracy (AC) for the detection of obstructive coronary artery
disease (CAD) compared with invasive coronary angiography (CAG)
(2–5). CTCA reflects the anatomical
morphology of the coronary arteries, without directly providing
functional or prognostic information on CAD, that is to say it does
not directly provide the pathophysiological significance correlated
with the coronary lesions. As the extent and severity of ischemia
are usually the key factors for deciding whether to select
revascularization or medical therapy for CAD, a non-invasive
evaluation of the anatomical and functional information on the
coronary lesions prior to performing CAG becomes a necessary and
reasonable requirement. Myocardial perfusion imaging (MPI) using
single photon emission CT (SPECT) is a well-established,
non-invasive method that has been widely used for decades to
provide functional information on coronary lesions. Numerous
studies have proved that MPI is a cost-effective, non-invasive
method for CAD management (6–8).
Combined CTCA and SPECT MPI should have positive
incremental values and play a complementary role in CAD management
by revealing the coronary anatomy and its relative functional
significance, factors which are extremely important for the
decision-making process and for improving the cost effectiveness
with regard to CAD therapy. The present study aimed to discuss and
evaluate the diagnostic performance and incremental clinical values
of combined CTCA and MPI for the detection of functionally relevant
coronary stenoses (FRCS).
Materials and methods
Study population
A total of 54 consecutive patients with suspected or
known CAD were enrolled in the present study. All the cases
underwent CTCA, MPI and CAG within 30 days. CAG was performed
following CTCA and MPI, however, the sequence of CTCA and MPI
depended on the individual clinical situation. The mean age of the
study population was 57.5±9.4 years and 36 patients were male
(66.7%). The exclusion criteria were as follows: unstable angina,
acute coronary syndrome (ACS), frequently premature heartbeat,
atrial fibrillation, contraindications for iodinated contrast
agent, X syndrome, severe coronary calcification and motion
artifacts affecting the measurement of the stenoses and patients
with bypass grafts. All the patients provided written informed
consent and the study was approved by the ethics committee of the
Tianjin Medical University Cardiovascular Clinical Institute and
TEDA International Cardiovascular Hospital, Tianjin, China.
CTCA
Image acquisition
All the scans were performed on a 64-slice CT
scanner (GE Light Speed VCT, GE Healthcare, Amersham, UK). The
patients were required to fast for 4 h prior to the CT imaging. The
patients with pre-scan heart rates of >65 b.p.m were
administered β-blockers (Betaloc tablets, 25–100 mg). Prior to
starting the scans, all the patients were required to take 0.5 mg
nitroglycerin sublingually. The acquisition parameters included a
rotation time of 350 msec, a tube voltage of 120 kV, a tube current
of 650–800 mA and a collimation of 0.6 mm. The scan scope ranged
from the carina of the trachea to 2 cm below the diaphragm. The
total scan time was ~12 sec. Briefly, for the pre-scan, a bolus of
20 ml iohexol contrast agent (350 mg/ml) was delivered
intravenously through the antecubital vein at a flow rate of 4
ml/sec using a high pressure injector. Bolus tracking techniques
were performed with a region of interest placed in the ascending
aorta in order to record the peak time of enhancement and confirm
the delay time for starting the acquisition. A bolus of 70–80 ml of
iohexol was then intravenously injected into the bloodstream at a
speed of 4–5 ml/sec through the antecubital vein. The patients were
required to hold their breath for the duration of the scan.
Image reconstruction and
interpretation
The acquisition data were transferred to a GE
Advanced Workstation (GE Healthcare) and a late diastole phase (75%
R-R time) was automatically reconstructed. If the quality of the
reconstructed images was not satisfactory, then images of a 45–85%
R-R time were reconstructed and reviewed in order to select the
best reconstructed images, i.e., those which clearly depicted the
coronary arteries. The images were visualized on the screen in the
form of multiple planar reformations (MPRs), with virtual rendering
volume (VRT) and maximal intensity projection (MIP). The images
were visually and independently evaluated by two experienced
readers (with >3 years of experience in cardiovascular imaging),
who were blinded to the results of the MPI. The final diagnosis was
reached when a consensus was agreed by the two readers. The
coronary arteries were subdivided according to the 15-segment model
proposed by the American Heart Association (9). Each segment was visually assessed and
reported as a ≥50 or <50% narrowing and allocated to the left
main (LM), left anterior descending (LAD), left circumflex (LCX)
and right coronary arteries (RCA). The narrowest lesion was
regarded as the final diagnosis for diffuse or multiple stenoses in
a single vessel.
MPI
Imaging acquisition
Exercise stress/rest-gated MPIs were performed on
all the patients prior and subsequent to CTCA. β-blockers, calcium
channel blockers and nitrates were discontinued for ≥24 h prior to
MPI. The exercise stress tests were performed according to the
modified Bruce protocol on a bicycle ergometer with a 12-lead ECG.
Blood pressure measurements were taken at the baseline and then
every 2 min during the procedure. The endpoints for the stress
tests included any of the following indices: reaching the target
heart beats [(220 - age in years) × 85%], ischemic ST-segment
horizontal or downslope depression of ≥2 mm, the emergence of
typical angina, severe cardiac arrhythmia, hypertension (≥240/120
mmHg) or a fall in systolic pressure by ≥40 mmHg. At the peak of
exercise, a 925-MBq dose of technetium-99m-labelled methoxyisobutyl
isonitrile (99mTc-MIBI) was injected into the
bloodstream through a vein and the patient continued to pedal for
an additional 1 min. The ECG and blood pressure were monitored
prior to, throughout and subsequent to the injection. The
acquisition for the stress-gated SPECT study was performed 1 h
subsequent to the injection. The rest studies began acquisition 1.5
h subsequent to an injection using the same doses. The GE Millenium
VG and Hawkeys dual-detector SPECT imaging system (GE Healthcare)
was used. The acquisition parameters were as follows: a low-energy,
high-resolution collimator; a 20% symmetric window at 140 keV; a
64×64 matrix; an elliptical orbit with step-and-shoot acquisition
at 6° intervals over 180° from the right anterior oblique (RAO) 45°
angle to the left posterior oblique (LPO) 45° angle; and a 25 sec
dwell time per stop. The acquisitions were gated at 8 frames per
R-R cycle with a 50% window of the accepted heart rate.
Image reconstruction and
interpretation
All the data were transferred to an eNTEGRA
workstation (GE Healthcare) and reconstructed using an iterative
reconstruction algorithm (2 iterations and 10 subsets; Butterworth
pre-filtering function, a gradient order of 5.0 and a frequent
cut-off of 0.25) without x-ray attenuation correction. The images
were reconstructed into short, horizontal and vertical long axial
sections. At the same time, polar maps of wall motion and wall
thickening were obtained by a special software package (ECToolbox
and Multi-dim).
SPECT image interpretations were visually performed,
with a consensus agreed by two experienced nuclear physicians; the
readers were unaware of the CTCA results. The tomographic slices
were divided into 17 segments according to the American Heart
Association model. The MPI results were divided into two
categories: normal perfusion, which was defined as homogenous
radioactive distributions in the myocardium and no defective
segments; and perfusion defects, including reversible and fixed
perfusion defects. The reversible pattern showed that the localized
segments of decreased perfusion observed during stress were no
longer apparent or that they demonstrated a partial improvement at
rest. The fixed pattern showed that the localized segments of
decreased perfusion during stress were unchanged between the stress
and rest images. The reversible perfusion defects were considered
to represent myocardial ischemia and the fixed perfusion defects
with concurrent regional wall motion abnormalities were considered
to be myocardial scars. The perfusion defects were allocated to
coronary artery territories as previously repoted (10). The defects in the anterior and
septal walls were allocated to the LAD and defects in the lateral
wall were allocated to the RCA. The defects affecting the LAD and
LCX regions together were considered as LM lesions. In the
watershed region, allocation was determined according to the main
extension of the defect onto the lateral, anterior or inferior
wall.
CAG
Conventional CAG was performed according to the
standard Judkins catheterization technique. Multiple views of each
coronary artery were obtained and digitally stored on a designated
workstation following intracoronary application of an iodinated
contrast agent. The angiograms were observed and evaluated by two
experienced interventional cardiologists who were blinded to the
results of the CTCA and MPI. The final diagnosis was reached by
consensus between the two readers. A visual assessment of a vessel
narrowing of ≥50% was defined as significant stenosis.
Data analysis and statistics
FRCS was defined as a vessel narrowing of ≥50% on
CAG with a corresponding perfusion defect on MPI (10). The performance indices of CTCA for
the detection of significant stenosis on the patient- or
vessel-based level were calculated using CAG as a reference. The
indices of CTCA alone or combined with MPI in the detection of FRCS
on the patient- or vessel-based level were also calculated using
CAG plus MPI as a reference. The gold standard defines FRCS as a
≥50% stenosis on CAG with a corresponding perfusion defect on MPI
in the same vessel territory. A true positive (TP) CTCA or combined
CTCA and MPI segment was defined according to the gold standard.
Comparisons of the statistical differences between groups were
performed using a Pearson Chi-square test or a Fisher's exact
probability test. P<0.05 was considered to indicate a
statistically significant difference. The data statistics were
automatically completed using the SPSS 13.0 statistical
software.
Results
Patient characteristics
CTCA, MPI and CAG were performed without
complications in all patients. The patient characteristics are
described in detail in Table
I.
 | Table IClinical characteristics of patients
referred for invasive angiography. |
Table I
Clinical characteristics of patients
referred for invasive angiography.
| Parameters | Overall (n=54) |
|---|
| Gender, n (M/F) | 36/18 |
| Age, mean ± SD
(years) | 57.5±9.4 |
| Body mass index, mean
± SD (kg/m2) | 26.6±3.0 |
| Risk factors for
CAD |
| Diabetes mellitus, n
(%) | 3 (12) |
| Hypertension, n
(%) | 33 (61) |
|
Hypercholesterolemia, n (%) | 12 (22) |
| Positive family
history, n (%) | 8 (15) |
| History of smoking,
n (%) | 22 (41) |
| Symptoms |
| Aymptomatic, n
(%) | 3 (6) |
| Dyspnea, n (%) | 22 (41) |
| Non-anginal chest
pain, n (%) | 5 (9) |
| Atypical angina
pectoris, n (%) | 12 (22) |
| Typical angina
pectoris, n (%) | 13 (24) |
CTCA versus CAG
With CAG regarded as the reference, the performance
indices of CTCA for detecting significant stenoses on the patient-
or vessel-based level are listed in Table II. The data showed that CTCA had a
high sensitivity (SN), specificity (SP) and AC for the detection of
obstructive CAD compared with CAG.
 | Table IIDiagnostic indices of CTCA for the
detection of ≥50% coronary narrowing in 216 segments of 54
patients. |
Table II
Diagnostic indices of CTCA for the
detection of ≥50% coronary narrowing in 216 segments of 54
patients.
| Indices | Patient-based level
(n=54) | Vessel-based level
(n=216) |
|---|
| True positive, n | 28 | 51 |
| True negative, n | 21 | 161 |
| False positive,
n | 1 | 2 |
| False negative,
n | 4 | 2 |
| SN, % | 87.5 | 96.2 |
| SP, % | 95.5 | 98.8 |
| PPV, % | 96.6 | 96.2 |
| NPV, % | 84.0 | 98.8 |
| AC, % | 90.7 | 98.2 |
CTCA alone or combined with MPI compared
with CAG plus MPI
According to the predefined reference standard, the
performance indices of CTCA alone or combined with MPI are listed
in Table III and the comparisons
between them analyzed. Whether on the patient- or vessel-based
level, the positive predictive value (PPV), SP and AC values of the
combined CTCA and MPI for the detection of FRCS were significantly
improved compared with CTCA alone, but the SN and negative
predictive value (NPV) did not change greatly.
 | Table IIIDiagnostic indices and comparisons of
CTCA alone or combined with MPI in 216 segments of 54 patients. |
Table III
Diagnostic indices and comparisons of
CTCA alone or combined with MPI in 216 segments of 54 patients.
| Patient-based level
(n=54) | | Vessel-based level
(n=216) | |
|---|
|
| |
| |
|---|
| Indices | CTCA (%) | CTCA+MPI (%) | P-values, n
(χ2) | CTCA (%) | CTCA+MPI (%) | P-values, n
(χ2) |
|---|
| True positive | 20 | 20 | | 28 | 28 | |
| True negative | 23 | 31 | | 162 | 183 | |
| False positive | 9 | 1 | | 23 | 2 | |
| False negative | 2 | 2 | | 3 | 3 | |
| SN | 90.9 | 90.9 | 1.00a | 90.3 | 90.3 | 1.00a |
| SP | 71.9 | 96.9 | 0.01a | 87.6 | 98.9 | 0.00b
(18.92) |
| PPV | 69.0 | 95.2 | 0.03a | 54.9 | 93.3 | 0.00b
(13.07) |
| NPV | 92.0 | 93.9 | 1.00a | 98.2 | 98.4 | 1.00a |
| AC | 79.6 | 94.4 | 0.02b
(5.3) | 88.0 | 97.7 | 0.00b
(15.33) |
Fig. 1 shows a
typical case of a 51-year-old male with angina pectoris. The
anatomical and physiological information provided by CTCA and MPI
showed that interventional therapy should be directed at the
lesions in the LAD and RCA. The patient consequently received
successful stent therapy for these lesions.
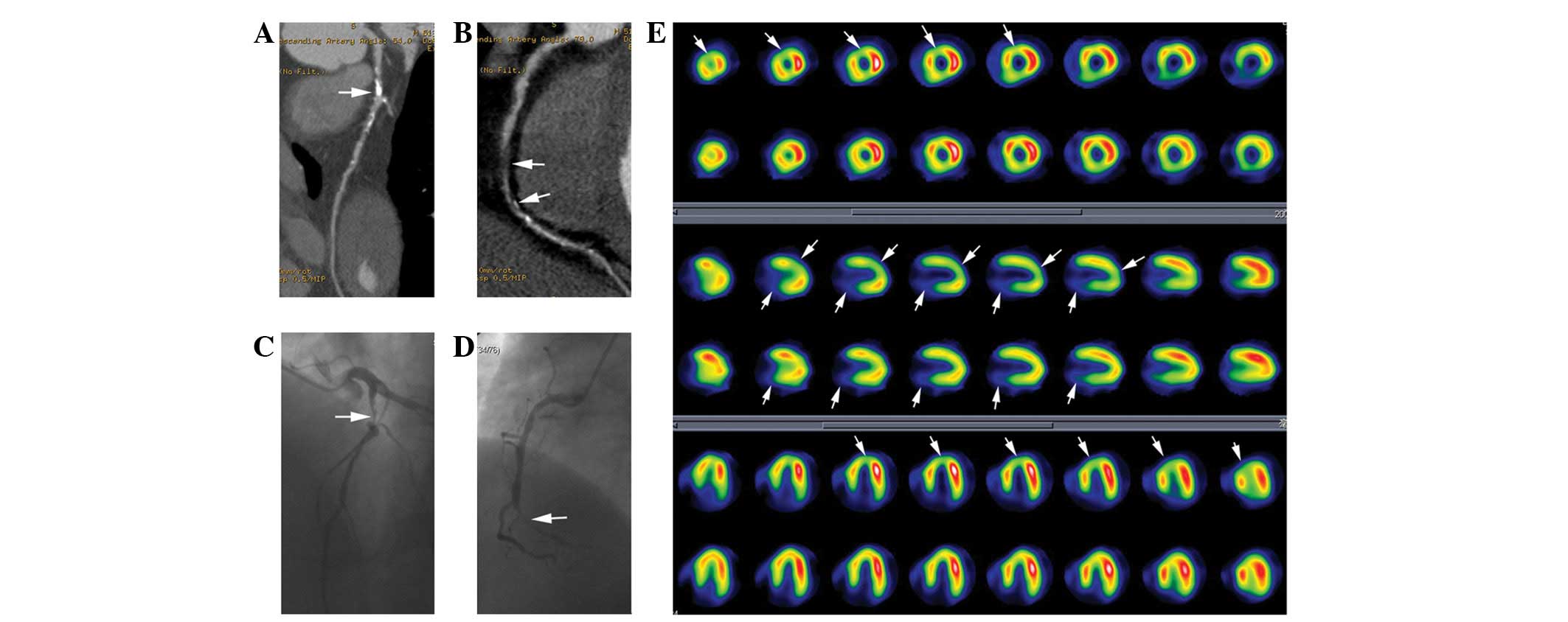 | Figure 1Patient with stenosis of the LAD
artery classified as having ≥50% stenosis and RCA classified as
nearly occluded on CTCA, associated with reversible perfusion
defects in the anterior wall and partial apex and fixed perfusion
defects in the posterior wall. (A) Multiple planar reformation
(MPR) on CTCA shows the proximal and middle segment of the LAD
artery with partially calcified, narrowed lumen (arrow). (B) MPR on
CTCA shows the whole course of RCA with multiple, non-calcified,
narrowed lumen and near occlusion in the middle segment of the RCA
(arrows). (C) CAG of LAD shows severe stenosis (arrow), which
corresponded to the CTCA results. (D) CAG of RCA shows near
occlusion (arrow), which is also consistent with the CTCA results.
(E) SPECT MPI study during stress (odd rows) and rest (even rows)
shows reversible perfusion defects (arrows) in the anterior wall
and partial apex corresponding to the territory of the LAD artery
and fixed perfusion defects (arrows) in the posterior wall
corresponding to the territory of the RCA. LAD, left anterior
descending; RCA, right coronary artery; CTCA, computed tomography
coronary angiograhy; CAG, coronary angiography; SPECT MPI, single
photon emission CT myocardial perfusion imaging. |
According to the predefined standard, 31 vessels
with FRCS were detected among 54 patients. A total of 7 patients
with three-vessel artery stenoses were included in the present
study, 6 of which were unbalanced three-vessel stenoses, with 18
vessels narrowed by ≥50% among the total 24 vessels. Only 5 vessels
with FRCS were identified by CAG plus MPI in 6 of the patients with
unbalanced three-vessel stenoses, which inferred that even more
vessels with FRCS were missed due to negative MPIs. One patient
with a balanced three-vessel stenosis was included in the present
study whose MPI result was negative.
Discussion
The most significant results from the present study
are as follows: First, CTCA had a high accuracy for the detection
of significant coronary artery stenoses, as shown in previous
studies (11–13). Second, in the detection of FRCS,
CTCA alone had a relatively low SP, PPV and AC. Third, in the
detection of FRCS, combining CTCA and SPECT MPI significantly
improved the SP and PPV and maintained the SN and NPV when compared
with CTCA alone.
In recent years, with the marked development of CT
techniques, particularly the emergence of multi-slice CT (MSCT),
which has a faster gantry rotation, multi-detector array and
dual-source system, it is possible to overcome the motion artifacts
of the heart and non-invasively create morphological images of the
coronary arteries, usually identified by CTCA. CTCA is able to
directly reveal the site and severity of a coronary lesion. In
addition, the length of the stenosis and the distribution,
magnitude and even the composition of the plaque may be precisely
revealed and classified (calcified versus non-calcified) (2,3,12,14).
CTCA does not directly provide the hemodynamic significance
correlated with the abnormalities of the coronary arteries, which
would be extremely important to the therapeutic strategies for CAD.
The main limitations for considering coronary narrowing only are as
follows: First, the severity of the stenosis is only a modest
surrogate of coronary resistance that does not include other lesion
characteristics, including the length of the narrowing, the shape
and eccentricity of the plaque and serial stenosis. These factors
may also greatly impede the blood perfusion to the myocardium.
Second, the vasomotor tone and coronary collateral flow, which are
known to affect myocardial perfusion, may not be assessed by a
simple measurement of stenosis severity (15). Therefore, functional imaging is
required in order to reveal the pathophysiological changes
correlated with the coronary lesions.
MPI is a well-established and documented
non-invasive cardiac imaging method used for the diagnosis,
prognosis and risk stratification of CAD, which has been practiced
in the clinic for decades. Numerous results from evidence-based
medicinal studies have confirmed that MPI is extremely effective,
possessing guided significance and a better cost-benefit ratio for
patient management (6,8,11,16).
A normal myocardial perfusion scan study indicated that patients
were at a low risk for subsequent cardiac events. Therefore, no
interventional therapy is usually required, but risk increases
exponentially with worsening perfusion abnormality (17,18).
Numerous study results have led to a large number of class I
indications for MPS in the risk assessment of patients with an
intermediate or high likelihood of CAD (8,11).
CTCA and MPI provide differing information for CAD
from respective angles, thus it is difficult to directly compare
CTCA with MPI as they show different things. The correlation
between CTCA and MPI is supplemental rather than substitutional.
For example, a positive MPI plus significant coronary stenoses are
confident indicators for revascularization. According to the
American Heart Association guidelines for cardiac intervention
therapy and radionuclide imaging, a non-invasive morphological and
functional method should be used prior to revascularization in
patients with chronic stable angina. The guidelines also state that
coronary stenosis with corresponding ischemia is one of the major
indications for performing revascularization and that conservative
therapy should be adopted where there is no significant stenoses or
corresponding ischemia (8,11,19).
These guidelines aid in making the correct decision to ensure that
patients benefit from treatments when non-invasively obtaining the
functional and anatomical information on the coronary lesions. Of
note is the fact that for patients with a high pre-test likelihood
of CAD (>85%), a negative MPI study does not exclude a diagnosis
of coronary atherosclerosis. For example, in the present study,
only 5 vessels with FRCS were identified by CAG plus MPI in 6
patients with unbalanced three-vessel stenoses, while MPI was
negative for one patient with a balanced three-vessel stenosis. The
reasons for this include the fact that the diagnostic criteria of
MPI are classified as normal or abnormal depending on the relative
distribution of the radiopharmaceuticals in the myocardium. MPI may
just detect the abnormal territories corresponding to one or two
relatively severe coronary artery stenoses in the patients with
three-vessel disease. MPI may even be negative for the balanced
reduction of perfusion to the myocardium in patients with balanced
three-vessel stenoses. Therefore, MPI may miss certain patients
with balanced three-vessel disease, whereas CTCA is unlikely to
miss severe or extensive coronary atherosclerosis. It may be
inferred that for patients with three-vessel disease, the SN for
the detection of FRCS is lower due to negative MPI results,
however, the extensive and diffuse lesions of the multiple coronary
arteries presented on the CTCA remain highly indicative of the
patients with a high risk. To avoid these potential clinical
limitations, CTCA combined with MPI is required and is valuable for
obtaining a deeper knowledge of CAD.
The incremental values that guide therapeutic
strategies using combined CTCA and MPI for the detection of FRCS
are summarized as follows: A patient without significant coronary
stenoses or perfusion defects should undergo primary prevention and
control of risk factors; a patient with significant coronary
stenoses and without corresponding perfusion defects should turn to
aggressive medical therapy and control of risk factors; and a
patient with significant coronary stenoses and corresponding
perfusion defects should actively undergo revascularization in
order to obtain an improved prognosis rather than undergoing
conservative therapy (18,20). To evaluate the diagnostic
performance of CTCA combined with MPI for the detection of FRCS,
the use of MPI is the accepted reference standard, which has been
previously used for the evaluation of the clinical values of hybrid
heart imaging devices in patients with known or suspected CAD
(21,22).
Although CTCA alone has a high accuracy for the
detection of obstructive CAD and an excellent NPV for ruling out
FRCS, its PPV and SP remain relatively low. Using combined CTCA and
MPI may markedly increase the diagnostic performance for the
detection of FRCS when compared with CTCA alone, which may provide
comprehensive information and play a significant role in the
decision-making process for CAD management.
Acknowledgements
This study was supported by grants from the Binhai
Health Bureau Medicine Health Science and Technology Project
(2011BHKY004) and the Tianjin Health Bureau Technology Fund
(2011KZ12).
References
|
1
|
Ropers D, Baum U, Pohle K, et al:
Detection of coronary artery stenoses with thin-slice
multi-detector row spiral computed tomography and multiplanar
reconstruction. Circulation. 107:664–666. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoffmann MH, Shi H, Schmitz BL, et al:
Noninvasive coronary angiography with multislice computed
tomography. JAMA. 293:2471–2478. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mollet NR, Cademartiri F, van Mieghem CA,
et al: High-resolution spiral computed tomography coronary
angiography in patients referred for diagnostic conventional
coronary angiography. Circulation. 112:2318–2323. 2005. View Article : Google Scholar
|
|
4
|
Leber AW, Knez A, von Ziegler F, et al:
Quantification of obstructive and nonobstructive coronary lesions
by 64-slice computed tomography: a comparative study with
quantitative coronary angiography and intravascular ultrasound. J
Am Coll Cardiol. 46:147–154. 2005. View Article : Google Scholar
|
|
5
|
Maffei E, Palumbo A, Martini C, et al:
Diagnostic accuracy of 64-slice computed tomography coronary
angiography in a large population of patients without
revascularization: registry data and review of multicentre trials.
Radiol Med. 115:368–384. 2010. View Article : Google Scholar
|
|
6
|
Underwood SR, Anagnostopoulos C, Cerqueira
M, et al; British Cardiac Society; British Nuclear Cardiology
Society; British Nuclear Medicine Society; Royal College of
Physicians of London; Royal College of Radiologists. Myocardial
perfusion scintigraphy: the evidence. Eur J Nucl Med Mol Imaging.
31:261–291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Go V, Bhatt MR and Hendel RC: The
diagnostic and prognostic value of ECG-gated SPECT myocardial
perfusion imaging. J Nucl Med. 45:912–921. 2004.PubMed/NCBI
|
|
8
|
Klocke FJ, Baird MG, Lorell BH, et al;
American College of Cardiology; American Heart Association Task
Force on Practice Guidelines; American Society for Nuclear
Cardiology. ACC/AHA/ASNC guidelines for the clinical use of cardiac
radionuclide imaging - executive summary. A report of the American
College of Cardiology/American Heart Association Task Force on
Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995
Guidelines for the Clinical Use of Cardiac Radionuclide (Imaging).
Circulation. 108:1404–1418. 2003.
|
|
9
|
Austen WG, Edwards JE, Frye RL, et al: A
reporting system on patients evaluated for coronary artery disease.
Report of the Ad Hoc Committee for Grading of Coronary Artery
Disease, Council on Cardiovascular Surgery, American Heart
Association. Circulation. 51(Suppl 4): 5–40. 1975. View Article : Google Scholar
|
|
10
|
Gaemperli O, Schepis T, Valenta I, et al:
Functionally relevant coronary artery disease: comparison of
64-section CT angiography with myocardial perfusion SPECT.
Radiology. 248:414–423. 2008. View Article : Google Scholar
|
|
11
|
Hendel RC, Patel MR, Kramer CM, et al;
American College of Cardiology Foundation Quality Strategic
Directions Committee Appropriateness Criteria Working Group;
American College of Radiology; Society of Cardiovascular Computed
Tomography; Society for Cardiovascular Magnetic Resonance; American
Society of Nuclear Cardiology; North American Society for Cardiac
Imaging; Society for Cardiovascular Angiography and Interventions;
Society of Interventional Radiology.
ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness
criteria for cardiac computed tomography and cardiac magnetic
resonance imaging: a report of the American College of Cardiology
Foundation Quality Strategic Directions Committee Appropriateness
Criteria Working Group, American College of Radiology, Society of
Cardiovascular Computed Tomography, Society for Cardiovascular
Magnetic Resonance, American Society of Nuclear Cardiology, North
American Society for Cardiac Imaging, Society for Cardiovascular
Angiography and Interventions, and Society of Interventional
Radiology. J Am Coll Cardiol. 48:1475–1497. 2006.
|
|
12
|
Sato A, Hiroe M, Tamura M, et al:
Quantitative measures of coronary stenosis severity by 64-Slice CT
angiography and relation to physiologic significance of perfusion
in nonobese patients: comparison with stress myocardial perfusion
imaging. J Nucl Med. 49:564–572. 2008. View Article : Google Scholar
|
|
13
|
Wijns W: Anatomic-functional imaging by
single-photon emission computed tomography/computed tomography as
the corner stone of diagnosis and treatment for coronary patients:
a glimpse into the (near) future? J Am Coll Cardiol. 49:1068–1070.
2007. View Article : Google Scholar
|
|
14
|
Cyrus T, Gropler RJ and Woodard PK:
Coronary CT angiography (CCTA) and advances in CT plaque imaging. J
Nucl Cardiol. 16:466–473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dorbala S, Hachamovitch R and Di Carli MF:
Myocardial perfusion imaging and multidetector computed tomographic
coronary angiography: appropriate for all patients with suspected
coronary artery disease? J Am Coll Cardiol. 48:2515–2517. 2006.
View Article : Google Scholar
|
|
16
|
Hachamovitch R, Hayes SW, Friedman JD,
Cohen I and Berman DS: Stress myocardial perfusion single-photon
emission computed tomography is clinically effective and cost
effective in risk stratification of patients with a high likelihood
of coronary artery disease (CAD) but no known CAD. J Am Coll
Cardiol. 43:200–208. 2004. View Article : Google Scholar
|
|
17
|
Berman DS, Abidov A, Kang X, et al:
Prognostic validation of a 17-segment score derived from a
20-segment score for myocardial perfusion SPECT interpretation. J
Nucl Cardiol. 11:414–423. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hachamovitch R, Hayes SW, Friedman JD,
Cohen I and Berman DS: Comparison of the short-term survival
benefit associated with revascularization compared with medical
therapy in patients with no prior coronary artery disease
undergoing stress myocardial perfusion single photon emission
computed tomography. Circulation. 107:2900–2907. 2003. View Article : Google Scholar
|
|
19
|
Smith SC Jr, Dove JT, Jacobs AK, et al;
American College of Cardiology; American Heart Association Task
Force on Practice Guidelines. Committee to Revise the 1993
Guidelines for Percutaneous Transluminal Coronary Angioplasty.
ACC/AHA guidelines of percutaneous coronary interventions(revision
of the 1993 PTCA guidelines) - executive summary. A report of the
American College of Cardiology/American Heart Association Task
Force on Practice Guidelines (committee to revise the 1993
guidelines for percutanous transluminal coronary angioplasty). J Am
Coll Cardiol. 37:2215–2239. 2001.
|
|
20
|
Berman DS, Hachamovitch R, Shaw LJ, et al:
Roles of nuclear cardiology, cardiac computed tomography, and
cardiac magnetic resonance: Noninvasive risk stratification and a
conceptual framework for the selection of noninvasive imaging tests
in patients with known or suspected coronary artery disease. J Nucl
Med. 47:1107–1118. 2006.
|
|
21
|
Rispler S, Keidar Z, Ghersin E, et al:
Integrated single-photon emission computed tomography and computed
tomography coronary angiography for the assessment of
hemodynamically significant coronary artery lesions. J Am Coll
Cardiol. 49:1059–1067. 2007. View Article : Google Scholar
|
|
22
|
Namdar M, Hany TF, Koepfli P, et al:
Integrated PET/CT for the assessment of coronary artery disease: a
feasibility study. J Nucl Med. 46:930–935. 2005.PubMed/NCBI
|















