Introduction
Carcinoma of the cervix is the second most common
cancer among women worldwide. During 2009, ~11,270 new cases of
invasive cervical cancer and ~4,070 cervical cancer-related
mortalities occurred in the US (1).
Radical hysterectomy (RH) has been the treatment of
choice for early-stage cervical carcinomas for more than a century,
with an overall 5-year survival rate of >80% (2–4).
However, it is well known that the classical RH may be accompanied
by early and late postoperative morbidity, such as bladder
dysfunction, anorectal mobility disorders and sexual
dissatisfaction, which seriously influence the quality of life
(QOL) of surviving patients. These complications are believed to be
the results of surgical injury involving the autonomic innervation
of the pelvic organs (5–8). The nerve-sparing technique,
originally developed in Japan by Okabayashi in 1921 and
subsequently modified by other authors (3,7,8), had
been considered as the solution to these complications. Since then,
the anatomy of the pelvic autonomic nerves has been verified by
open surgery, and the rapid recovery of bladder function following
nerve-sparing radical hysterectomy (NSRH) has also been
demonstrated. Although the autonomic nerves of the pelvic organs
and their origins are well-described in anatomy textbooks, these
structures are rarely visualized in operating rooms during surgery,
and no consensus has been reached on which parts of the uterine
supporting ligaments [cardinal ligament (CL), uterosacral ligament
(USL) or vesicouterine ligament (VUL)] should be targeted to
minimize the potentially damaging effects of NSRH.
Promising results have been reported regarding the
disease-free and overall survival (OS) following NSRH for cervical
cancer (9,10). However, concerns remain regarding
the radicality and safety of this method. It has been suggested
that parametrial lymph nodes may remain within the uterine
supporting ligaments following NSRH, which may threaten the
radicality of the procedure. Some researchers have suggested that
the parametrial lymph nodes distributed in these tissues may cause
early tumor spread, since fatty tissue in the lateral parts of the
paracervix are not completely removed following NSRH (3,4,11).
However, it has been reported that parametrial involvement in RH
specimens was observed in <1% of patients with early-stage
cervical cancer, and so extensive parametrectomy was not
recommended (12,13). Whether or not lymph nodes are
located in the supporting ligaments around the cervix is
controversial, and a deep knowledge of the anatomical relationship
between lymphatic tissues and pelvic nerve components, with
particular reference to NSRH techniques, is not available. Tailored
surgery has become a major issue in modern cervical cancer surgery.
It is our opinion that filling this gap may significantly help
surgeons to choose the most appropriate surgical intervention for
each patient.
In the present study, the distribution of lymphatic
tissues, autonomic nervous fibers and sympathetic fibers in the
supporting ligaments around the cervix were investigated in a
series of adult female cadavers and the topographical association
between them was described.
Materials and methods
Materials
Nine formalin-fixed donated adult female cadavers
with no evidence of previous pelvic operations were used and
macroscopic dissection of normal uterine cervix was performed at
the Institute of Anatomy of Suzhou University (Suzhou, China)
between December 2008 and December 2010. All the cadavers were
adults; the mean age at death was 69.1±7.4 years (range, 52–81
years). The study was approved by the ethics committee of Soochow
University.
Dissection procedures
Firstly, the parietal peritoneum was opened. The
peritoneum of the vesicouterine pouch was then incised. The bladder
was separated from the central wall of the cervix down to the
cranial level of the trigone of the urinary bladder. The peritoneum
on the pouch of Douglas and posterior peritoneal leaves of the
broad ligament were transversely incised. The connective tissue on
the vaginal wall was separated from the central wall of the rectum.
Then, the pararectal and paravesical spaces were developed.
Following separation of the paravesical and pararectal spaces on
each side, the CL (between the pararectal and paravesical space),
USL (between the pararectal space and rectovaginal ligament) and
VUL (between the paravesical space and vesicocervical space) were
isolated. These ligaments were then cut starting from their origin
at the uterine cervix.
Immunohistochemistry
The mean lengths of the CLs, USLs and VULs were
4.66±0.34, 6.04±0.28 and 2.32±0.30 cm, respectively. After labeling
the direction, serial macroscopic slices (each 10 mm) were cut from
the specimens. Following dehydration in graded ethanol and transfer
to xylene, the tissues were embedded in paraffin. Three serial
slices of 5 μm, cut at 1-cm intervals, were used for
immunohistochemical analysis. One 5-μm slice cut at a 2-mm interval
was used for standard hematoxylin and eosin (H&E) staining.
Sections analyzed using immunohistochemistry were heated in a
pressure cooker (LD2X-30KBS, Shanghai Shengan Medical Equipment
Factory, Shanghai, China) for 2 min in 0.01-mol/l citrate buffer
(pH 6.0) for antigen retrieval and then cooled at room temperature
(RT) for 20 min. Following several washes in phosphate-buffered
saline (PBS; Sigma, St. Louis, MO, USA), the sections were quenched
with 3% hydrogen peroxide solution for 10 min to block endogenous
peroxidase activity. Following several washes in PBS, the sections
were incubated with the first antibody: S100 protein (a general
nerve marker, 1:400; Dako Goldbridge, Beijing, China), TH (an
antibody against tyrosine hydroxylase, a specific marker of
sympathetic fibers, 1:400; Dako Goldbridge) or D2–40 (a specific
marker of lymphatic endothelium cells, 1:400; Dako Goldbridge). The
incubation with the first antibody had a duration of 2 h. Then, all
the sections were incubated for 0.5 h in a Novolink Polymer
detection system (PV-9000; Novocastra, Goldbridge, Beijing, China).
With diaminobenzidine (DBA) as the chromogen, the sections were
briefly counterstained with Mayer's hematoxylin, dehydrated via
ethanol, transferred to xylene and coverslipped. All the stained
sections were assessed by a pathologist.
Results
H&E staining
Four lymph nodes were identified in three cadavers.
One and three lymph nodes were observed in the USLs and CLs,
respectively. The former was located in the cranial side of the USL
at a distance of 4.0 cm from the uterine cervix, and the latter
were present in the cranial side of the CLs at a distance of 2.0 cm
from the uterine cervix (Fig. 1A and
B). No lymph nodes were identified in the VULs. Lymph nodes in
the CLs were present bilaterally only in one cadaver.
Immunohistochemistry
Lymphatic vessels
The features of the lymphatic vessels in all the
ligaments were similar. The lumen was large and irregular and the
wall was thin and intermittent. There were large spaces between the
endothelial cells and the basement membrane was not complete. The
lymphatic vessels were dispersed in the CLs (Fig. 1C), scattered in the cervical side
of the USLs, and occasionally distributed in the VULs.
Autonomic nervous and sympathetic
fibers
Autonomic nerves consist of sympathetic and
parasympathetic fibers. S100 is a general nerve marker and TH is a
specific marker of sympathetic fibers, which are both expressed in
the cytomembrane, cytoplasm and nucleus of Schwann cells.
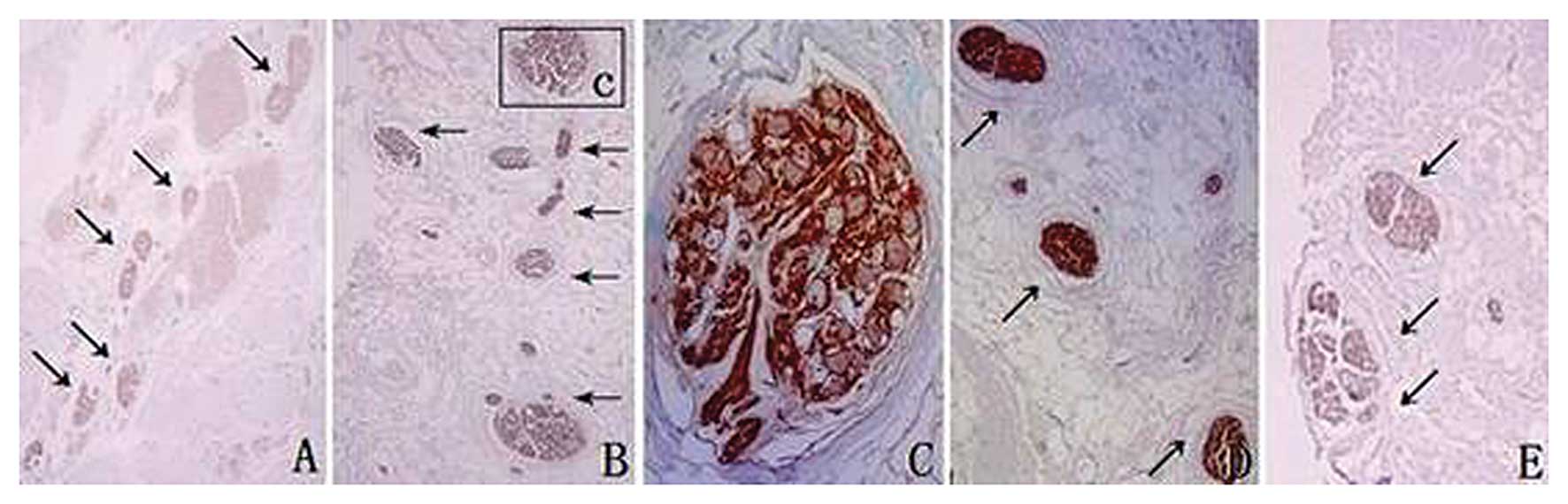
In CLs, S100-positive fibers were located in the
middle part at a distance of 4.5 cm from the cervix (Fig. 2A), in the middle and inferior parts
at 3.0 cm, close to the ventral side at 2.0 cm (Fig. 2B-D) and in the caudal and ventral
sides at 0.5 cm (Fig. 2E). There
were no TH-positive fibers located in the ligaments at distances of
4.5, 4.0, 3.5, 3.0, 2.5 or 2.0 cm from the cervix (Fig. 3A-C). The TH-positive fibers were in
the middle of the ligaments at a distance of 1.5 cm from the cervix
(Fig. 3D) and in the caudal side
of the ligaments at 1.0 and 0.5 cm (Fig. 3E). In CLs, parasympathetic nerves
were located at the pelvic wall and went downwards and medially
into the cervix, while sympathetic fibers were located in the
middle and lower parts of the ligaments.
In USLs, S100-positive fibers were located in the
medial and top part at a distance of 6.0 cm from the cervix
(Fig. 4A), in the middle and
inferior parts and close to the lateral side at 4.5, 3.0 and 1.5 cm
(Fig. 4B-D) and in the caudal and
lateral sides at 0.5 cm (Fig. 4E).
TH-positive fibers were located in the medial and cephalic parts of
the ligaments at distances of 6.0 and 5.5 cm from the cervix
(Fig. 5A), in the middle and
inferior parts of the ligaments at distances of 5.5, 4.5, 4.0, 3.5,
3.0, 2.5 and 2.0 cm (Fig. 5B-D)
and in the caudal and lateral sides of the ligaments at distances
of 1.5, 1.0 and 0.5 cm (Fig. 5E).
In USLs, the autonomic nerves, which consisted primarily of
sympathetic fibers, went downwards and laterally from the pelvic
wall to the cervix.
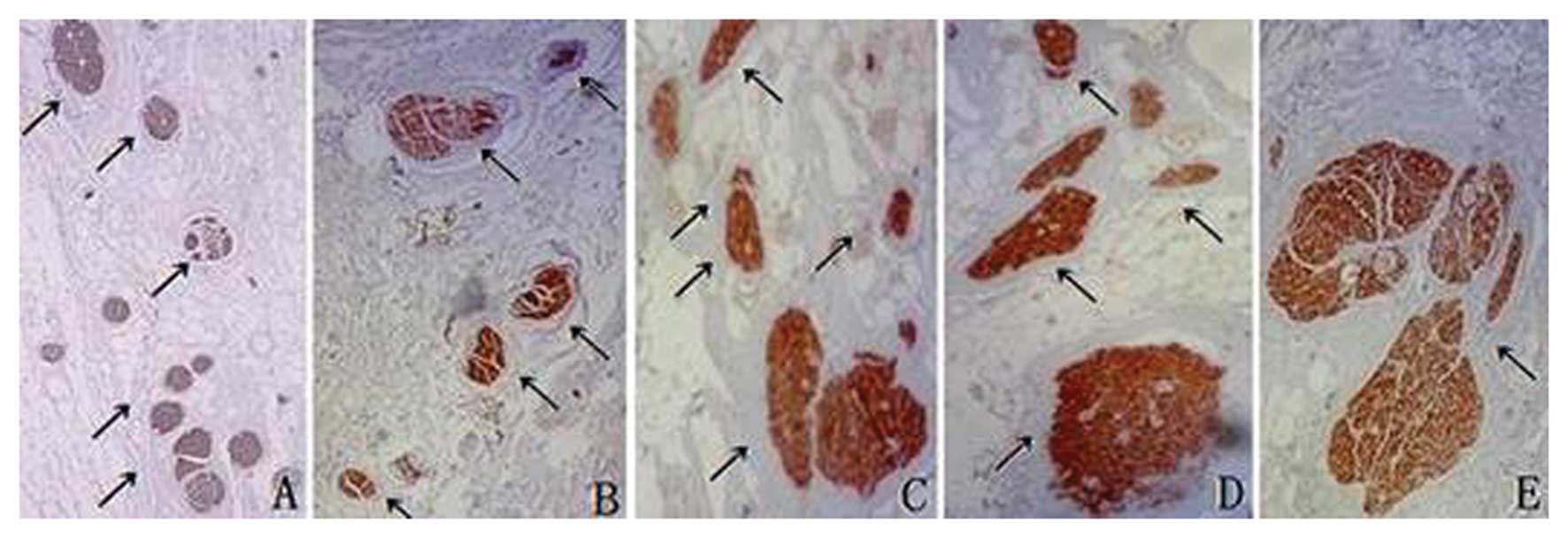 | Figure 4In uterosacral ligaments,
S100-positive fibers located in the medial and top parts at
distances of (A, left) 6.0, (B, right) 4.5, (C, left) 3.0, (D,
right) 1.5 and (E, right) 0.5 cm from the uterine cervix (arrows).
Magnification, ×4. Immunohistochemical staining with S100 antibody.
Right and left represent right side and left side of human
body. |
 | Figure 5In uterosacral ligaments, TH-positive
fibers located in the ligaments at distances of (A, right) 5.5, (B,
left) 5.0, (C, left) 3.0, (D, left) 2.0 and (E, right) 0.5 cm from
the uterine cervix (arrows). Magnification, ×4. Immunohistochemical
staining with TH antibody. Right and left represent right side and
left side of human body. |
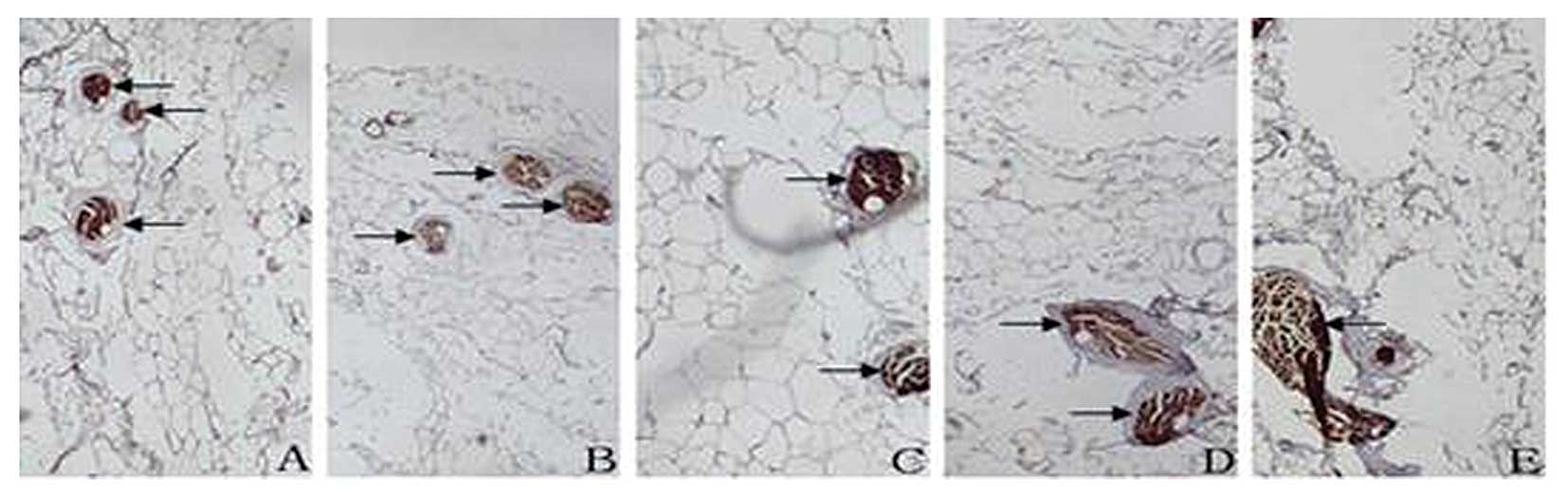
In the VULs, S100-positive fibers were located in
the medial side of the vesical veins in deep layers of the VULs at
distances of 2.0, 1.5, 1.0 and 0.5 cm from the cervix (Fig. 6). TH-positive fibers were located
in the medial side of the vesical veins in deep layers of the VULs
at distances of 2.0, 1.5, 1.0 and 0.5 cm from the cervix (Fig. 7). In VULs, the greatest number of
parasympathetic and sympathetic nerves were located in the medial
side of the vesical veins in deep layers of the ligaments.
Discussion
Individualization of treatment to reduce
therapy-associated early and late morbidity is the current trend in
cervical cancer surgery. The main aim of surgery is to minimize
injury to the pelvic autonomic innervation. The ideal surgical
management of cervical cancer should reduce morbidity without
compromising oncological disease control. The novel classification
of RH by Querleu and Morrow (Q-M classification) (11) has been proposed to balance curative
effects with the risk of adverse consequences. It distinguishes
between a type C1 procedure, which corresponds to the nerve-sparing
modification, and a type C2 procedure, which aims for a complete
parametrial resection (11). It is
well known that the normal function of the pelvic organs depends on
autonomic nerves. These autonomic nerves have important functions
for orgasm, sexual arousal, urinary functions and anorectal
motility. Several studies describing the anatomical background of
NSRH based on gross, macroscopic dissections have been published
(14–16).
The superior hypogastric plexus contains mostly
sympathetic nerves from the abdominal aortic plexus and lumbar
ganglia of the sympathetic trunk. The superior hypogastric plexus
caudally and laterally leads to the right and left hypogastric
nerves. The hypogastric plexus runs through the lateral layer of
the USL to the dorsal part of the paracervix in a craniocaudal
fashion. Hypogastric nerves bilaterally incorporate the
dorsocranial part of the inferior hypogastric plexus. The pelvic
plexus is composed of sympathetic fibers from the hypogastric and
parasympathetic fibers, arising from the second, third and fourth
sacral roots of the spinal cord (pelvic splanchnic nerves). These
fibers then run into the inferior pelvic plexus, which is the most
important deep neural network of the lateral part of the cervix.
The efferent fibers from the pelvic plexus continue to the rectum
(rectal fibers), uterus (uterine fibers), rectovaginal ligament
ventrally (rectal and vaginal fibers), and finally to the deep VUL.
Preservation of the distal part of the inferior hypogastric plexus
keeps postoperative morbidity to a minimum, particularly the fine
bladder branch located in the deep layer of the VUL lateral and
caudal to the ureter.
Butler-Manuel et al(17) quantitatively and qualitatively
assessed the nerve content of the uterine-supporting ligaments
following radical or simple hysterectomy using immunocytochemistry.
They reported that more nerve tissues were present in the USL than
in the lateral third of the CL, and more nerve fibers were present
in large trunks in the USL than in the CL. According to a recent
study by Mantzaris et al(18), fewer nerve fibers were present in
the paracervix in specimens from NSRH than in specimens from
standard RH. The results of the present study are consistent with
those reported previously. Technical details of NSRH using
illustrations and step-by-step instructions were provided by Fujii
(8). Injuries to the autonomic
pelvic nerves may be encountered particularly during the following
phases: hypogastric nerves during the resection of the USL;
inferior hypogastric plexus during division of the USL and
rectovaginal ligaments; pelvic splanchnic nerve during the division
of the deep uterine vein in the CL; and the bladder branch from the
inferior hypogastric plexus during the resection of the VUL and the
paracolpium.
However, whether the modification of NSRH with
respect to the uterine supporting ligaments threatens radicality
remains to be elucidated. Lymphatic spread is the most important
metastatic pathway of cervical cancer. The removal of parametrial
tissue has been considered to be of high importance in the
treatment of cervical cancer, since tumors may spread to this area
through direct microscopic extension or tumor embolization from the
primary lesion to lymph nodes embedded in the parametrial tissue
(12). The frequency of lymph node
metastases in early-stage cervical cancer has been reported to be
0% in certain studies and as high as 9.7% in others (19). The high-risk factors of lymph node
metastases include large tumor size, cervical stromal invasion to
the middle or deep one-third, as well as parametrial involvement
and lymphovascular space invasion (LVSI) (20–23).
Women with tumors <2 cm in the largest diameter, or stromal
infiltration less than half of the stroma, or invasion <10 mm,
had a significantly lower risk regarding involvement of the
paracervix and pelvic lymph nodes. Isolated parametrial metastases
without positive pelvic nodes have been rare (11,24–27).
Several studies have shown that <1% of patients with early
cervical cancer with favorable pathologic characteristics have
parametrial involvement, and that the final pathologic specimens of
~60% of patients who underwent radical trachelectomy contained no
residual disease. Wright et al(26) studied 594 patients who underwent
RH. Parametrial involvement was observed in 64 patients (10.8%) and
was associated with advanced grade, deep cervical stromal invasion,
LVSI, large tumor size and lymph node metastases. In a subgroup of
270 patients with negative lymph nodes, no LVSI, and tumors <2
cm, the incidence of parametrial involvement was only 0.4%.
Frumovitz et al(12)
reported similar findings in 350 women who underwent RH.
Parametrial involvement was noted in 7.7% of patients. Patients
with favorable pathological characteristics (such as those
previously mentioned) had no parametrial involvement.
Parametrial lymph nodes constitute the first site of
extracervical involvement. However, they have been ignored by
clinical pathologists due to their small size and special location.
During 1996, Benedetti-Panici et al(27) used a giant sections technique, in
which the three parametria were spread, the entire specimen
including the cervix was processed, and the sections were cut on
the coronal plane. Parametrial nodes were found in 65 (60%) of the
109 giant sections. The superficial and deep layers of the VUL, USL
and the lateral parametrium revealed the presence of nodes in 13
(33%), 10 (26%), 2 (5%) and 28 patients (70%), respectively. This
study showed that parametrial nodes and their metastases were
unevenly scattered between the cervix and the pelvic wall,
confirming that paracervical tissue constitutes a major route for
the lymphatic spread of cervical cancer. These findings confirmed
that the lymph from the cervix was drained by three main trunks:
the lateral, anterior and posterior. The lateral trunk runs through
the lateral parametrium and is the main lymphatic drainage from the
uterine cervix. In 2000, another study by the same group confirmed
that there were many lymph nodes in the inner and outer parts of
the lateral parametrium as well as lymphatic vessels, both of which
may be potential metastatic sites (28).
Sentinel lymph node (SLN) mapping allows
perioperative assessment of the condition of the lymph nodes of
patients by using patent blue dye and/or radiocolloid injected into
the cervix on the day prior to and on the day of surgery. Plentl
and Friedman (29) described a
typical pattern of lymphatic drainage from the cervix to regional
lymph nodes. According to this pattern, the parametrial node is the
first draining node in cervical cancer, although it is too close to
the cervix to be detected. In previous studies, the most common SLN
site was in the topography of the superficial obturator (30,31),
while parametrial lymph nodes were rarely found. Ercoli et
al(32) used open macroscopic
or laparoscopic pelvic dissections in fresh adult female cadavers
following lymphatic channel and node staining with Lipiodol dye
solution injected into the uterine cervix. Stained lymphatic
structures were observed into the lateral parametrium, but not in
VULs and USLs. The present study confirmed that there are lymphatic
channels lateral, anterior and posterior to the normal cervix. The
lymphatic vessels were dispersed in the CLs, scattered in the
cervical side of USLs, and occasionally distributed in the VULs.
There were few lymphatic tissues in the supporting ligaments of the
normal cervix.
In the present study, four lymph nodes were
identified in three of nine cadaver specimens; the frequency is
lower than that reported by Benedetti-Panici et al(27). This discrepancy has several
potential explanations. Firstly, our specimens were restricted to
the supporting ligaments of the cervix and did not include the
extensive parametrium, where lymphatic tissue may be abundantly
found. Moreover, a classical morphological study (33) was specifically conducted on the
cadavers of newborn infants and children in their first years of
life due to the particular abundance of lymphoreticular tissue,
which is known to become atrophic with age, while the present study
was conducted on the cadavers of old women. It may be hypothesized
that the existence of lymph nodes should be considered an exception
rather than a rule. Finally, our findings are hardly comparable to
those by Benedetti-Panici et al(27), since this earlier study was
performed on surgical specimens from cervical cancer patients,
while the present study was performed on subjects without evidence
of cervical cancer. Further studies are required to gain a better
understanding of the anatomy and pathology of the paracervix and
pelvic lymph nodes of cervical cancer patients.
In our opinion, there are few lymphatic tissues in
the supporting ligaments of the cervix. Comparing the locations of
the lymph nodes with those of the nerve components, indicates that
the extent of radicality, including CLs, VULs and USLs, is not
affected by the preservation of the autonomic nerves. Observational
and comparative clinical data have also shown that there is no
difference in the local recurrence rate, disease-free and overall
survival following NSRH and conventional techniques (10,11).
Moreover, with the development of robotic radical techniques, a
laparoscopic NSRH may minimize the impairment of radicality by
precise neurovascular dissection using the magnified laparoscopic
view, direct access with the tip of the laparoscope and the
laparoscopic instruments to the deep pelvic structures, and by
securing a clear resection margin during the dissection of the deep
part of the CLs, USLs, and the posterior leaf of the VULs (34).
In conclusion, NSRH is a safe and feasible method,
which does not compromise radicality, and may be considered as the
standard treatment for early-stage cervical cancer.
Acknowledgements
This study was supported by a grant from Jiangsu
Province.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2
|
Piver MS, Rutledge F and Smith JP: Five
classes of extended hysterectomy for women with cervical cancer.
Obstet Gynecol. 44:265–272. 1974.PubMed/NCBI
|
|
3
|
Raspagliesi F, Ditto A, Hanozet F,
Martinelli F, Solima E, Zanaboni F, et al: Nerve-sparing radical
hysterectomy in cervical cancer: evolution of concepts. Gynecol
Oncol. 107:119–121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pluta M, Rob L, Charvat M, Chmel R,
Halaska M Jr, Skapa P, et al: Less radical surgery than radical
hysterectomy in early stage cervical cancer: a pilot study. Gynecol
Oncol. 113:181–184. 2009. View Article : Google Scholar
|
|
5
|
Likic IS, Kadija S, Ladjevic NG,
Stefanovic A, Jeremic K, Petkovic S, et al: Analysis of urologic
complications after radical hysterectomy. Am J Obstet Gynecol.
99:6442008.
|
|
6
|
Jackson KS and Naik R: Pelvic floor
dysfunction and radical hysterectomy. Int J Gynecol Cancer.
16:354–363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yabuki Y, Sasaki H, Hatakeyama N and
Murakami G: Discrepancies between classic anatomy and modern
gynecologic surgery on pelvic connective tissue structure:
harmonization of those concepts by collaborative cadaver
dissection. Am J Obstet Gynecol. 193:7–15. 2005. View Article : Google Scholar
|
|
8
|
Fujii S: Anatomic identification of
nerve-sparing radical hysterectomy: a step-by-step procedure.
Gynecol Oncol. 111:33–41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van den Tillaart SAHM, Kenter GG, Peters
AA, Dekker FW, Gaarenstroom KN, Fleuren GJ, et al: Nerve-sparing
radical hysterectomy: local recurrence rate, feasibility, and
safety in cervical cancer patients stage IA to IIA. Int J Gynecol
Cancer. 19:39–45. 2009.PubMed/NCBI
|
|
10
|
Espino-Strebel EE, Luna JT and Domingo EJ:
Comparison of the feasibility and safety of nerve-sparing radical
hysterectomy with the conventional radical hysterectomy. Int J
Gynecol Cancer. 20:1274–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Querleu D and Morrow CP: Classification of
radical hysterectomy. Lancet Oncol. 9:297–303. 2008. View Article : Google Scholar
|
|
12
|
Frumovitz M, Sun CC, Schmeler KM, Deavers
MT, Dos Reis R, Levenback CF and Ramirez PT: Parametrial
involvement in radical hysterectomy specimens for women with
early-stage cervical cancer. Obstet Gynecol. 114:93–99. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmeler K, Frumovitz M and Ramirez P:
Conservative management of early stage cervical cancer: is there a
role for less radical surgery? Gynecol Oncol. 120:321–325. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mauroy B, Demondion X, Bizet B, Claret A,
Mestdagh P and Hurt C: The female inferior hypogastric (=pelvis)
plexus: anatomical and radiological description of the plexus and
its afferences - applications to pelvic surgery. Surg Radiol Anat.
29:55–66. 2007.
|
|
15
|
Kato T, Suzaka K and Osaki T: Unilateral
or bilateral nerve-sparing radical hysterectomy: a surgical
technique to preserve the pelvic autonomic nerves while increasing
radicality. Int J Gynecol Cancer. 17:1172–1178. 2007. View Article : Google Scholar
|
|
16
|
Hazewinkel MH, Sprangers MA, van der
Velden J, van der Vaart CH, Stalpers LJ, Burger MP and Roovers JP:
Long-term cervical cancer survivors suffer from pelvic floor
symptoms: a cross-sectional matched cohort study. Gynecol Oncol.
117:281–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Butler-Manuel SA, Buttery LD, A'Hern RP,
Polak JM and Barton DP: Pelvic nerve plexus trauma at radical and
simple hysterectomy: a quantitative study of nerve types in the
uterine supporting ligaments. J Soc Gynecol Investig. 9:47–51.
2002. View Article : Google Scholar
|
|
18
|
Mantzaris G, Rodolakis A, Vlachos G,
Athanasiou S, Theocharis S, Sotiripoulou ChM and Antsaklis A:
Magnifying lenses assisted nerve-sparing radical hysterectomy and
prevention of nerve plexus trauma. Int J Gynecol Cancer.
18:868–875. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Meurs H, Visser O, Buist MR, Ten Kate
FJ and van der Velden J: Frequency of pelvic lymph node metastases
and parametrial involvement in stage IA2 cervical cancer: a
population-based study and literature review. Int J Gynecol Cancer.
19:21–26. 2009.PubMed/NCBI
|
|
20
|
Sakuragi N: Up-to-date management of lymph
node metastasis and the role of tailored lymphadenectomy in
cervical cancer. Int J Clin Oncol. 12:165–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tavares MB, Sousa RB, Oliveirae Silva T,
Moreira LA, Silva LT, Tavares CB and Vieira SC: Prevalence of
prognostic factors for cancer of the uterine cervix after radical
hysterectomy. Sao Paulo Med J. 127:145–149. 2009.PubMed/NCBI
|
|
22
|
Kasamatsu T, Onda T, Sawada M, Kato T and
Ikeda S: Radical hysterectomy for FIGO stage IIB cervical cancer:
clinicopathological characteristics and prognostic evaluation.
Gynecol Oncol. 114:69–74. 2009. View Article : Google Scholar
|
|
23
|
Herr D, Konig J, Heilmann V, Koretz K,
Kreienberg R and Kurzeder C: Prognostic impact of
satellite-lymphovascular space involvement in early-stage cervical
cancer. Ann Surg Oncol. 16:128–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lim CS, Alexander-Sefre F, Allam M, Singh
N, Aleong JC, Al-Rawi H and Jacobs IJ: Clinical value of
immunohistochemically detected lymphovascular space invasion in
early stage cervical carcinoma. Ann Surg Oncol. 15:2581–2588. 2008.
View Article : Google Scholar
|
|
25
|
Strnad P, Robova H, Skapa P, Pluta M,
Hrehorcak M, Halaska M and Rob L: A prospective study of sentinel
lymph node status and parametrial involvement in patients with
small tumour volume cervical cancer. Gynecol Oncol. 109:280–284.
2008.PubMed/NCBI
|
|
26
|
Wright JD, Grigsby PW, Brooks R, Powell
MA, Gibb RK, Gao F, et al: Utility of parametrectomy for early
stage cervical cancer treated with radical hysterectomy. Cancer.
110:1281–1286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Benedetti-Panici P, Maneschi F, Scambia G,
Greggi S, Cutillo G, D'Andrea G, et al: Lymphatic spread of
cervical cancer: an anatomical and pathological study based on 225
radical hysterectomies with systematic pelvic and aortic
lymphadenectomy. Gynecol Oncol. 62:19–24. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Benedetti-Panici P, Maneschi F, D'Andrea
G, Cutillo G, Rabitti C, Congiu M, et al: Early cervical carcinoma:
the natural history of lymph node involvement redefined on the
basis of thorough parametrectomy and giant section study. Cancer.
88:2267–2274. 2000. View Article : Google Scholar
|
|
29
|
Plentl AA and Friedman EA: Lymphatic
system of the female genitalia. The morphologic basis of oncologic
diagnosis and therapy. Major Probl Obstet Gynecol. 2:1–223.
1971.PubMed/NCBI
|
|
30
|
Vieira SC, Sousa RB, Tavares MB, Silva JB,
Abreu BA, Santos LG, et al: Preoperative pelvic lymphoscintigraphy
is of limited usefulness for sentinel lymph node detection in
cervical cancer. Eur J Obstet Gynecol Reprod Biol. 145:96–99. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du XL, Sheng XG, Jiang T, Li QS, Yu H, Pan
CX, et al: Sentinel lymph node biopsy as guidance for radical
trachelectomy in young patients with early stage cervical cancer.
BMC Cancer. 11:1572011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ercoli A, Delmas V, Iannone V, Fagotti A,
Fanfani F, Corrado G, et al: The lymphatic drainage of the uterine
cervix in adult fresh cadavers: anatomy and surgical implications.
Eur J Surg Oncol. 36:298–303. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Plentl AA and Friedman EA: Clinical
significance of cervical lymphatics. Lymphatic System of the Female
Genitalia The Morphological Basis of Oncologic Diagnosis and
Therapy. 1st edition. W.B. Saunders; Philadelphia: pp. 75–115.
1971
|
|
34
|
Park NY, Chong GO, Hong DG, Cho YL, Park
IS and Lee YS: Oncologic results and surgical morbidity of
laparoscopic nerve-sparing radical hysterectomy in the treatment of
FIGO stage IB cervical cance. Int J Gynecol Cancer. 21:355–362.
2011. View Article : Google Scholar : PubMed/NCBI
|