Introduction
Macrophages comprise an essential part of the innate
immune system and provide a first line of defense against microbial
infections (1). However, due to
their highly phagocytic properties, macrophages are often targeted
by pathogenic bacteria. They may even be exploited as host cells by
some microorganisms such as Mycobacterium tuberculosis
(M. tuberculosis) and Rickettsia akari(2,3). To
avoid becoming a reservoir of infection, a number of defense
mechanisms have evolved to either kill or prevent the growth of
bacteria.
Upon stimulation from bacterial antigens,
macrophages are activated and, in turn, release a wide range of
cytokines, including inferferon-γ (IFN-γ), tumor necrosis factor-α
(TNF-α), as well as interleukin (IL)-1, -6 and -12 (4). IFN-γ is a typical T helper 1 cytokine
that is known as a key cytokine for protective immunity against
M. tuberculosis(5). This
cytokine stimulates nitric oxide (NO) production in macrophages
leading to the clearance of invading pathogens. The impairment of
IFN-γ synthesis by T cells has been found to enhance susceptibility
to tuberculosis in complement C5-deficient mice (6). TNF-α also acts as a protective
cytokine in the resistance to M. tuberculosis(7). It has been shown that IFN-γ, TNF-α
and IL-18 cooperate to control the growth of M. tuberculosis
in human macrophages (8).
The release of reactive nitrogen (RNI) and reactive
oxygen intermediates (ROI) is an additional important mechanism for
macrophages to combat invading pathogens. These mechanisms kill
bacteria by damaging macromolecules such as bacterial DNA.
Inducible NO synthase-deficient mice, which do not produce RNI, and
phagocyte oxidase-deficient mice, which do not produce ROI, are
more susceptible to M. tuberculosis infection compared to
wild-type mice (9,10).
The attenuated M. tuberculosis H37Ra strain
and its virulent counterpart, H37Rv, are derived from the parent
strain H37, which was originally isolated from a 19-year-old male
patient with chronic pulmonary tuberculosis by Edward R. Baldwin in
1905 (11). Over the past decades,
studies on the virulence of M. tuberculosis have frequently
involved comparisons of the H37Rv and H37Ra strains. Comparative
genomic analysis has indicated genetic differences between the 2
strains, providing extensive insight into the basis of the
attenuation of virulence in H37Ra (12). However, there is limited knowledge
available as regards their ability to initiate the macrophage
activation program. Thus, the aim of the present study was to
measure and compare the responses of mouse peritoneal macrophages
following exposure to the live H37Ra or heat-inactivated H37Rv
strains. Since CD40 signaling has been well established to
participate in macrophage activation (13,14),
we investigated whether exposure to the H37Ra and H37Rv strains
affects the surface expression of CD40 ligand (CD40L) in activated
macrophages.
Materials and methods
Animals, bacterial strains and cell
culture
Specific-pathogen-free, 6 to 8-week-old BALB/c mice
(weighing 18±2 g) were purchased from the Laboratory Animal Center,
Chongqing Medical University (Chongqing, China) and housed in a
pathogen-free environment. All the experiments involving animals
were approved by the Institutional Animal Care and Use Committee of
Chongqing Medical University.
The M. tuberculosis H37Ra strain (ATCC 25177)
was obtained from the Department of Immunology (Chongqing Medical
University), and the H37Rv strain (ATCC 27294) was obtained from
the Central Laboratory of Genetic Diagnosis of Tuberculosis of
Chongqing (Chongqing, China). Bacterial cultures were grown in
Middlebrook 7H9 medium (BioMerieux, La Balme-les-Grottes, France)
at 37°C for 2–3 weeks. The mid-log phase cultures were pelleted,
resuspended in sterile saline containing 0.05% Tween-80, and
titered. Heat-killed H37Rv was prepared by heating the bacteria at
80°C for 5 min. Suspensions of bacteria were then supplemented with
5% glycerol and stored at −80°C until use.
Macrophage isolation and infection
Peritoneal macrophages were isolated from 10 normal
BALB/c mice as previously described (15). Briefly, isolated peritoneal cells
were pelleted, resuspended in RPMI-1640 medium (HyClone, Logan, UT,
USA) supplemented with 10% heat-inactivated fetal bovine serum
(FBS) (Gibco, Carlsbad, CA, USA), and plated onto 24-well plates.
After a 4-h culture at 37°C, the non-adherent cells were removed
and adherent cells (macrophage-rich population) were maintained in
fresh culture medium. For phagocytosis assay, macrophages were
seeded onto 24-well plates at a density of 5×105
cells/well. The cells were infected with a multiplicity of
infection of 10:1 (10 bacteria to 1 cell) for 12 h. The ingested
bacteria were detected using acid-fast staining. The percentage of
phagocytosis and the phagocytic index were determined by counting
400 macrophages/glass slide under a light microscope. The
percentage of phagocytosis was defined as the percentage of
macrophages containing ≥1 ingested bacterium. The phagocytic index
was the mean number of bacteria ingested/macrophage. For secretion
assay, macrophages seeded at a density of 5×105
cells/well were added with 5–65 CFU/ml bacteria and incubated for
24 h. The culture supernatants were collected and assessed for the
production of cytokines, NO and hydrogen peroxide
(H2O2).
Enzyme-linked immunosorbent assay
(ELISA)
The levels of IFN-γ, TNF-α and IL-12p40 in the
culture supernatants were measured using ELISA kits (eBioscience,
San Diego, CA, USA) according to the manufacturer’s
instructions.
Measurement of NO and
H2O2
NO levels were determined with the Griess method
using a commercially available kit (Jiancheng Bioengineering
Institute, Nanjing, China) according to the manufacturer’s
instructions. The concentrations of H2O2 were
measured using standard biochemical methods with a commercially
available kit (Jiancheng Bioengineering Institute).
Immunization
BALB/c mice were intraperitoneally injected with an
equal volume of physical saline (used as the control; n=10), H37Ra
(n=10) or H37Rv suspension (n=10). Thirty days following
immunization, the animals were sacrificed and peritoneal
macrophages were collected as described above. Isolated macrophages
were subjected to gene expression analysis by reverse
transcription-polymerase chain reaction (RT-PCR), flow cytometry
and confocal microscopy.
RNA isolation and RT-PCR
Total RNA was isolated from the macrophages using a
total RNA extraction kit (Invitrogen, Carlsbad, CA, USA) according
to the manufacturer’s instructions. Reverse transcription was
performed with the First-Strand cDNA Synthesis kit (Takara, Dalian,
China). PCR amplification was conducted under the following
conditions: initial denaturation at 94°C for 5 min, followed by 28
cycles of denaturation at 94°C for 30 sec, annealing at 55–62°C for
30–45 sec, and elongation at 72°C for 1 min. The PCR primers used
in this study are listed in Table
I. PCR products were separated on a 1.2% agarose gel, stained
with ethidium bromide, and photographed.
 | Table ISequences of PCR primers used in this
study. |
Table I
Sequences of PCR primers used in this
study.
| Gene | Sequence | Product size
(bp) |
|---|
| IL-12p40 | F:
5′-TGCTGGTGTCTCCACTCATG-3′ | 302 |
| R:
5′-CCAAGGCACAGGGTCATCATC-3′ | |
| TNF-α | F:
5′-CTGAGACAGAGCCTGCCTTA-3′ | 449 |
| R:
5′-GTCTGAGAGCCGAAGACTGA-3′ | |
| IFN-γ | F:
5′-AGGCCATCAGCAACAACATAAGTG-3′ | 140 |
| R:
5′-GACAGCTTTGTGCTGGATCTGTG-3′ | |
| GAPDH | F:
5′-AGGGCCGGTGCTGAGTATGTC-3′ | 530 |
| R:
5′-TGCCTGCTTCACCACCTTCT-3′ | |
Flow cytometry
Peritoneal macrophages isolated from immunized mice
were incubated with recombinant mouse IFN-γ (10 ng/ml) for 4 h. The
macrophages were detached, incubated with fluorescein
isothiocyanate (FITC)-labeled anti-mouse CD40L (eBioscinence) at
4°C for 30 min, and immediately examined for the surface expression
of CD40L by flow cytometry (FACSCalibur; Becton-Dickinson, San
Jose, CA, USA).
Immunofluorescent staining and confocal
microscopy
Macrophages were stimulated with IFN-γ (10 ng/ml)
for 4 h as described above, seeded onto coversplips, and cultured
for an additional 24–36 h in fresh culture medium. Following
washing, the cells were fixed with 4% paraformaldehyde for 15 min,
blocked in bovine serum albumin (BSA) for 1 h, and incubated with
goat anti-mouse CD40L (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) at 4°C overnight, followed by incubation with FITC-labeled
secondary antibodies for 1 h. The cells were then mounted in 50%
glycerol and analyzed using a LEICA TCS SP2 confocal microscope
(Leica Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
All statistical analyses were performed using
SPSS.11 software (SPSS, Inc., Chicago, IL, USA). Data are expressed
as the means ± standard deviation (SD). Significant differences
between 2 groups were determined using the Student’s t-test. The
difference among the means of multiple groups was analyzed by
one-way analysis of variance (ANOVA) followed by Tukey’s test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Ingestion of viable H37Ra and
heat-inactivated H37Rv by macrophages
In vitro phagocytosis assay demonstrated that
mouse peritoneal macrophages had a higher capacity to engulf the
viable H37Ra strain compared to the heat-inactivated H37Rv strain,
as evidenced by the increased percentage of phagocytosis and the
phagocytic index (51.7±20.0% vs. 29.6±3.9% and 0.72±0.31 vs.
0.53±0.15, respectively; Table
II).
 | Table IIPhagocytic activity of macrophages
exposed to the viable H37Ra or heat-inactivated H37Rv strain. |
Table II
Phagocytic activity of macrophages
exposed to the viable H37Ra or heat-inactivated H37Rv strain.
| M.
tuberculosis strain |
|---|
|
|
|---|
| Phagocytic
activity | H37Ra | H37Rv |
|---|
| % Phagocytosis | 51.7±20.0 | 29.6±3.9a |
| Phagocytic
index | 0.72±0.31 | 0.53±0.15a |
Cytokine release by H37Ra- and
H37Rv-stimulated macrophages
Cytokine measurements using ELISA indicated that
viable H37Ra-stimulated mouse macrophages produced significantly
increased concentrations of IL-12p40 and TNF-α compared to the
control cells (P<0.05) (Fig.
1). However, there was no significant difference in the release
of the 2 cytokines from the H37Rv-stimulated macrophages and the
control cells. Additionally, stimulation with either the viable
H37Ra strain or the inactivated H37Rv strain yielded a
dose-dependent increase in IFN-γ secretion, with more significant
changes being observed in the macrophages exposed to H37Ra
(Fig. 1).
RT-PCR analysis further demonstrated the viable
H37Ra-induced mRNA expression of IL-12p40, TNF-α and IFN-γ in
macrophages (Fig. 2). There was
also a modest increase in IFN-γ mRNA expression in the macrophages
exposed to the inactivated H37Rv strain compared to the control
cells. However, IL-12p40 and TNF-α mRNA expression remained stable
upon stimulation with inactivated H37Rv.
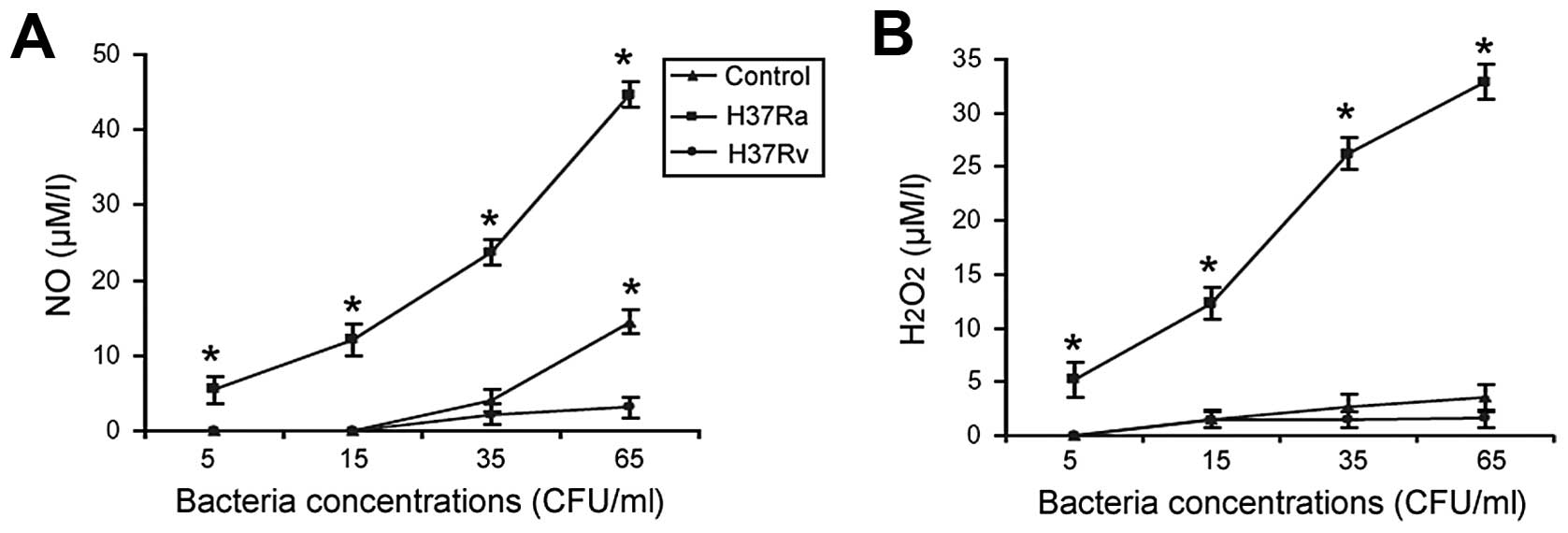
H37Ra- and H37Rv-stimulated release of NO
and H2O2 from macrophages
Exposure to the viable H37Ra strain resulted in a
dose-dependent increase in the release of NO and
H2O2 from the macrophages, with 9- and 7-fold
changes observed between the highest and lowest concentrations,
respectively (Fig. 3). By
contrast, there was little to no increase in NO and
H2O2 production from the macrophages
stimulated with the inactivated H37Rv strain.
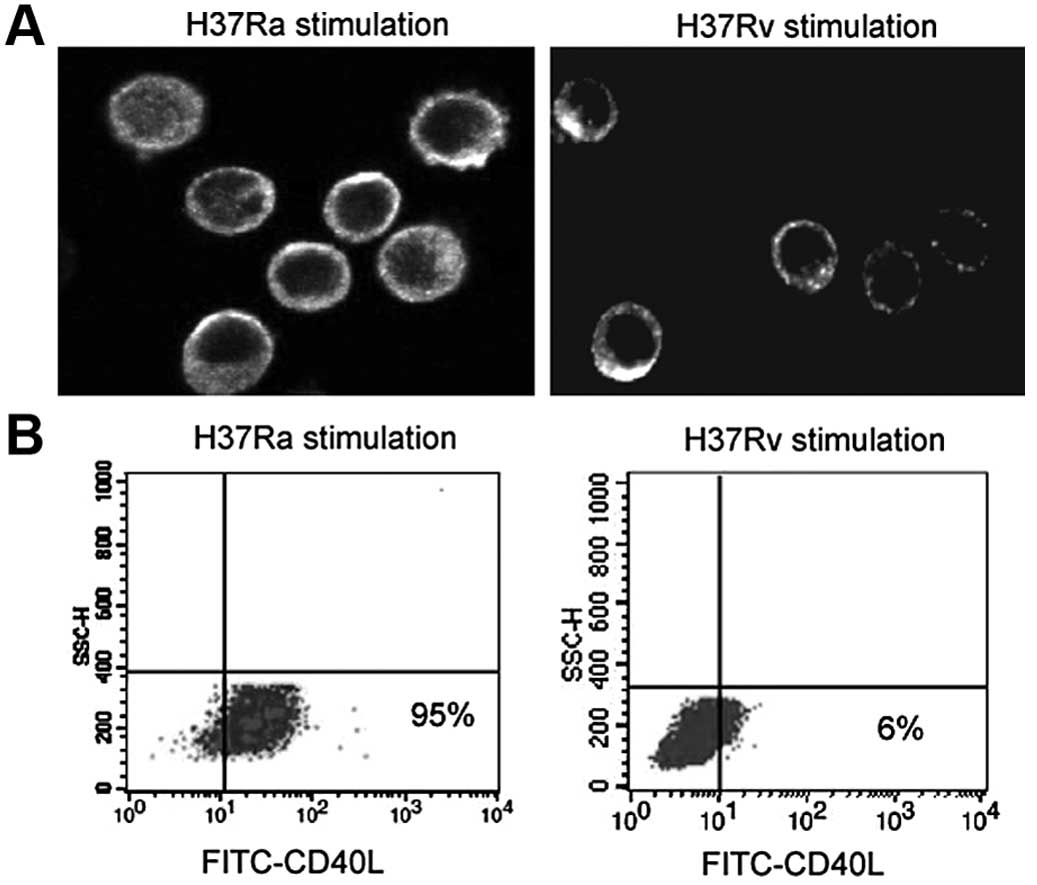
Expression of surface CD40L in
macrophages following exposure to H37Ra and H37Rv
IFN-γ-stimulated peritoneal macrophages from mice
immunized with the viable H37Ra strain showed an enhanced surface
expression of CD40L compared to those from mice exposed to the
inactivated H37Rv strain (Fig.
4A). Flow cytometric analysis further demonstrated that the
majority (95%) of the IFN-γ-stimulated macrophages exposed to the
H37Ra strain displayed a strong surface expression of CD40L, while
only 6% of the IFN-γ-stimulated macrophages exposed to the H37Rv
strain displayed CD40L surface expression (Fig. 4B).
Discussion
Toll-like receptors (TLRs), as a family of
pattern-recognition receptors, are pivotal mediators of the
recognition of pathogens by the innate immune system (16). They discriminate between chemically
diverse classes of microbial components. Our data demonstrated that
the live H37Ra strain was more readily recognized and internalized
by mouse peritoneal macrophages compared to the heat-inactivated
H37Rv strain. This finding may reflect genetic differences between
the H37Ra and H37Rv strains (12).
Alternatively, the specific TLR recognition patterns for M.
tuberculosis may be altered by heating stimuli, thus affecting
the ability of the macrophages to sense and internalize the H37Rv
strain. This is indirectly supported by a previous study according
to which heat-inactivated and live group B streptococcus strains
initiate distinct response pathways in macrophages to activate
antibacterial host defense (17).
During infection with M. tuberculosis, host
macrophages provide the preferred environment for mycobacterial
growth and also serve as pivotal effector cells responsible for the
clearance of the pathogen or killing the bacteria. IFN-γ and TNF-α
represent the key macrophage-activating cytokines in M.
tuberculosis infection. IL-12 plays a key role in cell-mediated
immune responses and stimulates the production of IFN-γ from T
cells (18). Our data demonstrate
that exposure to the viable H37Ra strain markedly promotes the
secretion of IL-12p40, TNF-α and IFN-γ from mouse peritoneal
macrophages. Moreover, the mRNA levels of the 3 cytokines were
increased in the viable H37Ra-stimulated macrophages, indicating
regulation at the transcriptional level. The regulation of gene
expression in response to M. tuberculosis stimulation has
also been described in a previous study (19). By contrast, stimulation with the
heat-inactivated H37Rv strain caused little to no increase in the
levels of these cytokines in the macrophages. The poor cytokine
induction by heat-inactivated H37Rv is consistent with the lower
phagocytic capacity of the macrophages to ingest this M.
tuberculosis strain. These findings demonstrate that the live
H37Ra strain is a stronger inducer of macrophage activation
compared to the inactivated H37Rv strain.
It is noteworthy that unlike the other 2 cytokines
examined, the amount of TNF-α released from macrophages peaked when
15×107 CFU/ml M. tuberculosis was used, followed
by a gradual decline at higher concentrations of mycobacteria. This
may reflect a negative feedback mechanism on TNF-α secretion. TNF-α
is clearly essential in fighting against pathogen infection. TNF-α
blockade is associated with the reduced response to vaccination
(20). However, the excessive
production of TNF-α by macrophages has potentially adverse effects,
since it may initiate cell apoptosis (21,22).
Keane et al(23)
demonstrated that at low multiplicities of infection, attenuated
M. tuberculosis strains induce the apoptosis of human
alveolar macrophages by promoting TNF-α release and activating the
extrinsic apoptotic pathway. Therefore, an optimal TNF-α level
plays an important role in host defense against pathogen
invasion.
NO and H2O2 are 2 primary
antimicrobial effectors produced by activated macrophages. In a
murine model of M. tuberculosis infection, RNI including NO
were established to be responsible for macrophage-mediated killing
and the growth inhibitory effect of virulent M.
tuberculosis(24). Jackett
et al(25) suggested that
H2O2 production by macrophages is involved in
killing M. tuberculosis in vivo. They found that the
exposure of macrophage monolayers to phorbol myristate acetate and
opsonized H37Ra increased the release of
H2O2. In line with these previous studies,
our data demonstrated that exposure to the viable H37Ra strain
stimulated the release of NO and H2O2 from
the peritoneal macrophages in a dose-dependent manner. By contrast,
exposure to the inactivated H37Rv strain caused little to no
production of either NO or H2O2 from
macrophages. These results further confirm the potential induction
of macrophage activation by the viable H37Ra strain as opposed to
the inactivated H37Rv. It has been demonstrated that IFN-γ
stimulates macrophages to produce NO and
H2O2(26,27).
In agreement with these previous studies, we noted that there were
similar alteration patterns for the levels of IFN-γ, NO and
H2O2 following exposure to the H37Ra
strain.
Compelling evidence points toward the importance of
CD40 signaling in the development of protective immunity. It has
been reported that CD40 deficiency predisposes mice to
Mycobacterium avium infection, which is associated with the
impaired production of IL-12p40 and IFN-γ (28). Lazarevic et al(29) demonstrated that CD40-deficient mice
succumbed to low-dose aerosol infection with M. tuberculosis
due to deficient IL-12 production, leading to impaired T cell IFN-γ
responses. Depressed CD40L expression has been found to contribute
to reduced IFN-γ production in human tuberculosis (30). Our data indicated an enhanced
surface expression of CD40L in macrophages exposed to the viable
H37Ra strain, which provided an explanation for the increased
secretion of IL-12p40 and IFN-γ from the activated macrophages. In
addition to the classical IFN-γ-dependent pathway, CD40 ligation
may directly induce the antimicrobial activity of macrophages
against an intracellular pathogen (31,32).
These results suggest that the increased surface expression of
CD40L and the activation of the CD40 pathway are involved in the
live H37Ra-induced macrophage activation. However, the exact
mechanism for the H37Ra-induced upregulation of CD40L remains to be
fully ellucidated in future studies.
In conclusion, our data demonstrate that exposure to
the viable H37Ra strain as opposed to the heat-inactivated H37Rv
strain induces a potent macrophage response, which is associated
with the enhanced surface expression of CD40L in activated
macrophages. These findings warrant further investigation of the
prophylactic potential of a H37Ra-based vaccine in the treatment of
tuberculosis.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (no. 30872261).
References
|
1
|
Rosenberger CM and Finlay BB: Phagocyte
sabotage: disruption of macrophage signalling by bacterial
pathogens. Nat Rev Mol Cell Biol. 4:385–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Radulovic S, Price PW, Beier MS, Gaywee J,
Macaluso JA and Azad A: Rickettsia-macrophage interactions: host
cell responses to Rickettsia akari and Rickettsia
typhi. Infect Immun. 70:2576–2582. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sly LM, Hingley-Wilson SM, Reiner NE and
McMaster WR: Survival of Mycobacterium tuberculosis in host
macrophages involves resistance to apoptosis dependent upon
induction of antiapoptotic Bcl-2 family member Mcl-1. J Immunol.
170:430–437. 2003.PubMed/NCBI
|
|
4
|
Cavaillon JM: Cytokines and macrophages.
Biomed Pharmacother. 48:445–453. 1994. View Article : Google Scholar
|
|
5
|
Flynn JL, Chan J, Triebold KJ, Dalton DK,
Stewart TA and Bloom BR: An essential role for interferon gamma in
resistance to Mycobacterium tuberculosis infection. J Exp
Med. 178:2249–2254. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mashruwala MA, Smith AK, Lindsey DR,
Moczygemba M, Wetsel RA, Klein JR, Actor JK and Jagannath C: A
defect in the synthesis of interferon-γ by the T cells of
complement-C5 deficient mice leads to enhanced susceptibility for
tuberculosis. Tuberculosis (Edinb). 91(Suppl 1): S82–S89. 2011.
|
|
7
|
Appelberg R: Protective role of interferon
gamma, tumor necrosis factor alpha and interleukin-6 in
Mycobacterium tuberculosis and M. avium infections.
Immunobiology. 191:520–525. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Robinson CM, Jung JY and Nau GJ:
Interferon-γ, tumor necrosis factor, and interleukin-18 cooperate
to control growth of Mycobacterium tuberculosis in human
macrophages. Cytokine. 60:233–241. 2012.
|
|
9
|
MacMicking JD, North RJ, LaCourse R,
Mudgett JS, Shah SK and Nathan CF: Identification of nitric oxide
synthase as a protective locus against tuberculosis. Proc Natl Acad
Sci USA. 94:5243–5248. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cooper AM, Segal BH, Frank AA, Holland SM
and Orme IM: Transient loss of resistance to pulmonary tuberculosis
in p47(phox−/−) mice. Infect Immun. 68:1231–1234. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steenken W JR and Gardner LU: History of
H37 strain of tubercle bacillus. Am Rev Tuberc. 54:62–66.
1946.
|
|
12
|
Zheng H, Lu L, Wang B, Pu S, Zhang X, Zhu
G, Shi W, Zhang L, Wang H, Wang S, Zhao G and Zhang Y: Genetic
basis of virulence attenuation revealed by comparative genomic
analysis of Mycobacterium tuberculosis strain H37Ra versus
H37Rv. PLoS One. 3:e23752008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamanaka M, Yu P, Yasui T, Yoshida K,
Kawabe T, Horii T, Kishimoto T and Kikutani H: Protective role of
CD40 in Leishmania major infection at two distinct phases of
cell-mediated immunity. Immunity. 4:275–281. 1996.
|
|
14
|
Buhtoiarov IN, Lum H, Berke G, Paulnock
DM, Sondel PM and Rakhmilevich AL: CD40 ligation activates murine
macrophages via an IFN-gamma-dependent mechanism resulting in tumor
cell destruction in vitro. J Immunol. 174:6013–6022. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao P, Shi L and Zhang RL: Extraction and
identification of peritoneal macrophages form mice. Chin J Med Lab
Technol. 10:400–402. 2004.
|
|
16
|
Ozinsky A, Underhill DM, Fontenot JD,
Hajjar AM, Smith KD, Wilson CB, Schroeder L and Aderem A: The
repertoire for pattern recognition of pathogens by the innate
immune system is defined by cooperation between toll-like
receptors. Proc Natl Acad Sci USA. 97:13766–13771. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Charrel-Dennis M, Latz E, Halmen KA,
Trieu-Cuot P, Fitzgerald KA, Kasper DL and Golenbock DT:
TLR-independent type I interferon induction in response to an
extracellular bacterial pathogen via intracellular recognition of
its DNA. Cell Host Microb. 4:543–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wozniak TM, Ryan AA and Britton WJ:
Interleukin-23 restores immunity to Mycobacterium
tuberculosis infection in IL-12p40-deficient mice and is not
required for the development of IL-17-secreting T cell responses. J
Immunol. 177:8684–8692. 2006.PubMed/NCBI
|
|
19
|
Ehrt S, Schnappinger D, Bekiranov S,
Drenkow J, Shi S, Gingeras TR, Gaasterland T, Schoolnik G and
Nathan C: Reprogramming of the macrophage transcriptome in response
to interferon-gamma and Mycobacterium tuberculosis:
signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J
Exp Med. 194:1123–1140. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Visser LG: TNF-α antagonists and
immunization. Curr Infect Dis Rep. 13:243–247. 2011.
|
|
21
|
Probert L, Eugster HP, Akassoglou K, Bauer
J, Frei K, Lassmann H and Fontana A: TNFR1 signalling is critical
for the development of demyelination and the limitation of T-cell
responses during immune-mediated CNS disease. Brain. 123:2005–2019.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dozmorov M, Wu W, Chakrabarty K, Booth JL,
Hurst RE, Coggeshall KM and Metcalf JP: Gene expression profiling
of human alveolar macrophages infected by B. anthracis
spores demonstrates TNF-alpha and NF-kappab are key components of
the innate immune response to the pathogen. BMC Infect Dis.
9:1522009.PubMed/NCBI
|
|
23
|
Keane J, Balcewicz-Sablinska MK, Remold
HG, Chupp GL, Meek BB, Fenton MJ and Kornfeld H: Infection by
Mycobacterium tuberculosis promotes human alveolar
macrophage apoptosis. Infect Immun. 65:298–304. 1997.
|
|
24
|
Chan J, Xing Y, Magliozzo RS and Bloom BR:
Killing of virulent Mycobacterium tuberculosis by reactive
nitrogen intermediates produced by activated murine macrophages. J
Exp Med. 175:1111–1122. 1992.PubMed/NCBI
|
|
25
|
Jackett PS, Andrew PW, Aber VR and Lowrie
DB: Hydrogen peroxide and superoxide release by alveolar
macrophages from normal and BCG-vaccinated guinea-pigs after
intravenous challenge with Mycobacterium tuberculosis. Br J
Exp Pathol. 62:419–428. 1981.PubMed/NCBI
|
|
26
|
Sharp AK and Banerjee DK: Effect of gamma
interferon on hydrogen peroxide production by cultured mouse
peritoneal macrophages. Infect Immun. 54:597–599. 1986.PubMed/NCBI
|
|
27
|
Herbst S, Schaible UE and Schneider BE:
Interferon gamma activated macrophages kill mycobacteria by nitric
oxide induced apoptosis. PLoS One. 6:e191052011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Flórido M, Gonçalves AS, Gomes MS and
Appelberg R: CD40 is required for the optimal induction of
protective immunity to Mycobacterium avium. Immunology.
111:323–327. 2004.PubMed/NCBI
|
|
29
|
Lazarevic V, Myers AJ, Scanga CA and Flynn
JL: CD40, but not CD40L, is required for the optimal priming of T
cells and control of aerosol M. tuberculosis infection.
Immunity. 19:823–835. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Samten B, Thomas EK, Gong J and Barnes PF:
Depressed CD40 ligand expression contributes to reduced gamma
interferon production in human tuberculosis. Infect Immun.
68:3002–3006. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Andrade RM, Portillo JA, Wessendarp M and
Subauste CS: CD40 signaling in macrophages induces activity against
an intracellular pathogen independently of gamma interferon and
reactive nitrogen intermediates. Infect Immun. 73:3115–3123. 2005.
View Article : Google Scholar
|
|
32
|
Andrade RM, Wessendarp M, Gubbels MJ,
Striepen B and Subauste CS: CD40 induces macrophage
anti-Toxoplasma gondii activity by triggering
autophagy-dependent fusion of pathogen-containing vacuoles and
lysosomes. J Clin Invest. 116:2366–2377. 2006.PubMed/NCBI
|