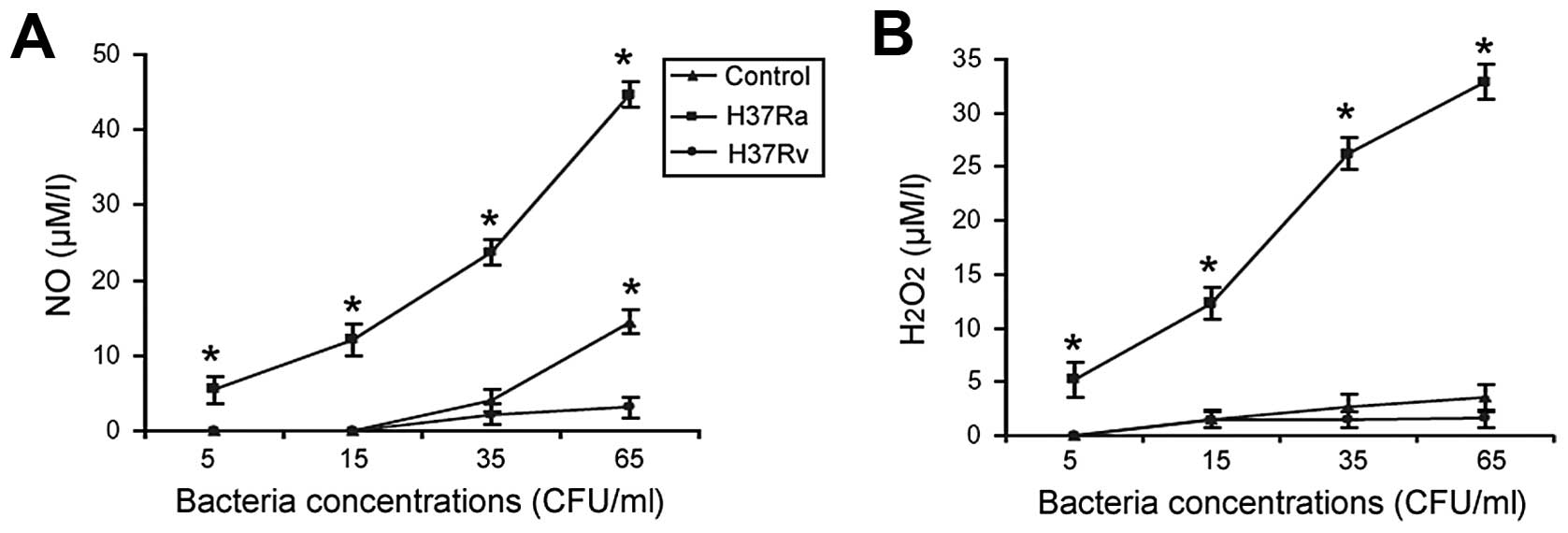
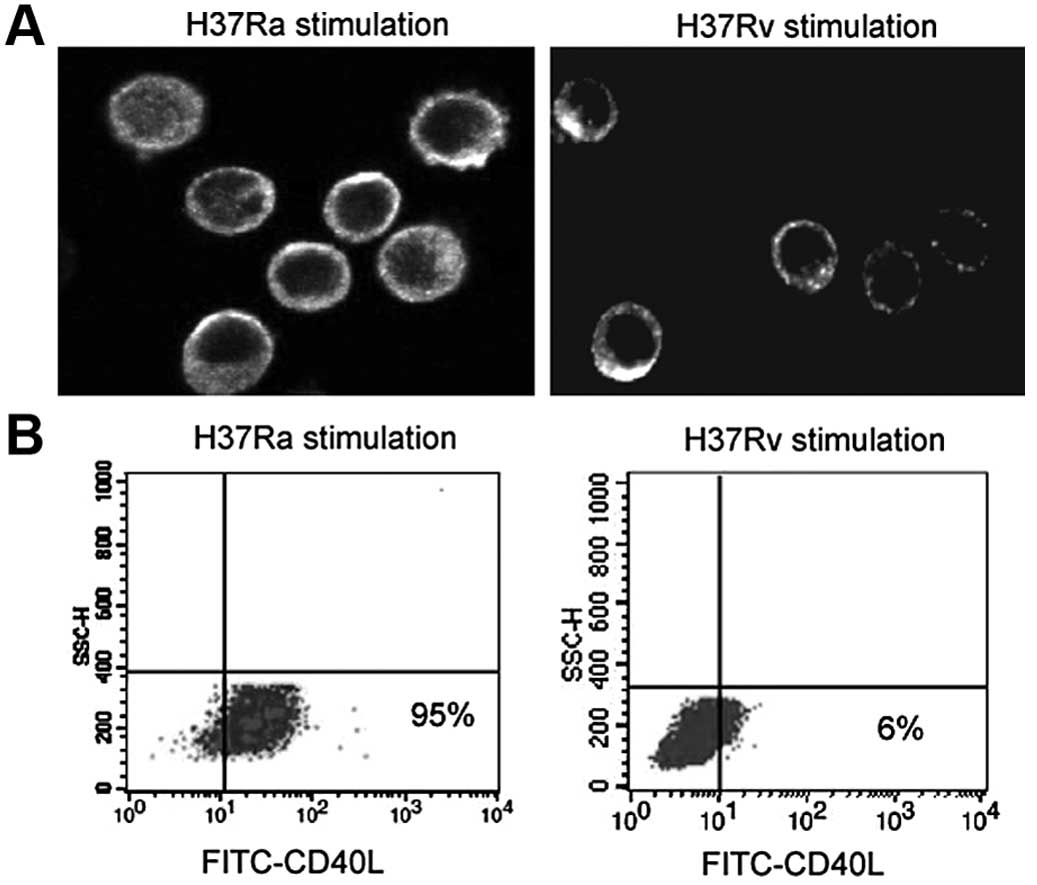
|
1
|
Rosenberger CM and Finlay BB: Phagocyte
sabotage: disruption of macrophage signalling by bacterial
pathogens. Nat Rev Mol Cell Biol. 4:385–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Radulovic S, Price PW, Beier MS, Gaywee J,
Macaluso JA and Azad A: Rickettsia-macrophage interactions: host
cell responses to Rickettsia akari and Rickettsia
typhi. Infect Immun. 70:2576–2582. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sly LM, Hingley-Wilson SM, Reiner NE and
McMaster WR: Survival of Mycobacterium tuberculosis in host
macrophages involves resistance to apoptosis dependent upon
induction of antiapoptotic Bcl-2 family member Mcl-1. J Immunol.
170:430–437. 2003.PubMed/NCBI
|
|
4
|
Cavaillon JM: Cytokines and macrophages.
Biomed Pharmacother. 48:445–453. 1994. View Article : Google Scholar
|
|
5
|
Flynn JL, Chan J, Triebold KJ, Dalton DK,
Stewart TA and Bloom BR: An essential role for interferon gamma in
resistance to Mycobacterium tuberculosis infection. J Exp
Med. 178:2249–2254. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mashruwala MA, Smith AK, Lindsey DR,
Moczygemba M, Wetsel RA, Klein JR, Actor JK and Jagannath C: A
defect in the synthesis of interferon-γ by the T cells of
complement-C5 deficient mice leads to enhanced susceptibility for
tuberculosis. Tuberculosis (Edinb). 91(Suppl 1): S82–S89. 2011.
|
|
7
|
Appelberg R: Protective role of interferon
gamma, tumor necrosis factor alpha and interleukin-6 in
Mycobacterium tuberculosis and M. avium infections.
Immunobiology. 191:520–525. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Robinson CM, Jung JY and Nau GJ:
Interferon-γ, tumor necrosis factor, and interleukin-18 cooperate
to control growth of Mycobacterium tuberculosis in human
macrophages. Cytokine. 60:233–241. 2012.
|
|
9
|
MacMicking JD, North RJ, LaCourse R,
Mudgett JS, Shah SK and Nathan CF: Identification of nitric oxide
synthase as a protective locus against tuberculosis. Proc Natl Acad
Sci USA. 94:5243–5248. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cooper AM, Segal BH, Frank AA, Holland SM
and Orme IM: Transient loss of resistance to pulmonary tuberculosis
in p47(phox−/−) mice. Infect Immun. 68:1231–1234. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steenken W JR and Gardner LU: History of
H37 strain of tubercle bacillus. Am Rev Tuberc. 54:62–66.
1946.
|
|
12
|
Zheng H, Lu L, Wang B, Pu S, Zhang X, Zhu
G, Shi W, Zhang L, Wang H, Wang S, Zhao G and Zhang Y: Genetic
basis of virulence attenuation revealed by comparative genomic
analysis of Mycobacterium tuberculosis strain H37Ra versus
H37Rv. PLoS One. 3:e23752008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamanaka M, Yu P, Yasui T, Yoshida K,
Kawabe T, Horii T, Kishimoto T and Kikutani H: Protective role of
CD40 in Leishmania major infection at two distinct phases of
cell-mediated immunity. Immunity. 4:275–281. 1996.
|
|
14
|
Buhtoiarov IN, Lum H, Berke G, Paulnock
DM, Sondel PM and Rakhmilevich AL: CD40 ligation activates murine
macrophages via an IFN-gamma-dependent mechanism resulting in tumor
cell destruction in vitro. J Immunol. 174:6013–6022. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao P, Shi L and Zhang RL: Extraction and
identification of peritoneal macrophages form mice. Chin J Med Lab
Technol. 10:400–402. 2004.
|
|
16
|
Ozinsky A, Underhill DM, Fontenot JD,
Hajjar AM, Smith KD, Wilson CB, Schroeder L and Aderem A: The
repertoire for pattern recognition of pathogens by the innate
immune system is defined by cooperation between toll-like
receptors. Proc Natl Acad Sci USA. 97:13766–13771. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Charrel-Dennis M, Latz E, Halmen KA,
Trieu-Cuot P, Fitzgerald KA, Kasper DL and Golenbock DT:
TLR-independent type I interferon induction in response to an
extracellular bacterial pathogen via intracellular recognition of
its DNA. Cell Host Microb. 4:543–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wozniak TM, Ryan AA and Britton WJ:
Interleukin-23 restores immunity to Mycobacterium
tuberculosis infection in IL-12p40-deficient mice and is not
required for the development of IL-17-secreting T cell responses. J
Immunol. 177:8684–8692. 2006.PubMed/NCBI
|
|
19
|
Ehrt S, Schnappinger D, Bekiranov S,
Drenkow J, Shi S, Gingeras TR, Gaasterland T, Schoolnik G and
Nathan C: Reprogramming of the macrophage transcriptome in response
to interferon-gamma and Mycobacterium tuberculosis:
signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J
Exp Med. 194:1123–1140. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Visser LG: TNF-α antagonists and
immunization. Curr Infect Dis Rep. 13:243–247. 2011.
|
|
21
|
Probert L, Eugster HP, Akassoglou K, Bauer
J, Frei K, Lassmann H and Fontana A: TNFR1 signalling is critical
for the development of demyelination and the limitation of T-cell
responses during immune-mediated CNS disease. Brain. 123:2005–2019.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dozmorov M, Wu W, Chakrabarty K, Booth JL,
Hurst RE, Coggeshall KM and Metcalf JP: Gene expression profiling
of human alveolar macrophages infected by B. anthracis
spores demonstrates TNF-alpha and NF-kappab are key components of
the innate immune response to the pathogen. BMC Infect Dis.
9:1522009.PubMed/NCBI
|
|
23
|
Keane J, Balcewicz-Sablinska MK, Remold
HG, Chupp GL, Meek BB, Fenton MJ and Kornfeld H: Infection by
Mycobacterium tuberculosis promotes human alveolar
macrophage apoptosis. Infect Immun. 65:298–304. 1997.
|
|
24
|
Chan J, Xing Y, Magliozzo RS and Bloom BR:
Killing of virulent Mycobacterium tuberculosis by reactive
nitrogen intermediates produced by activated murine macrophages. J
Exp Med. 175:1111–1122. 1992.PubMed/NCBI
|
|
25
|
Jackett PS, Andrew PW, Aber VR and Lowrie
DB: Hydrogen peroxide and superoxide release by alveolar
macrophages from normal and BCG-vaccinated guinea-pigs after
intravenous challenge with Mycobacterium tuberculosis. Br J
Exp Pathol. 62:419–428. 1981.PubMed/NCBI
|
|
26
|
Sharp AK and Banerjee DK: Effect of gamma
interferon on hydrogen peroxide production by cultured mouse
peritoneal macrophages. Infect Immun. 54:597–599. 1986.PubMed/NCBI
|
|
27
|
Herbst S, Schaible UE and Schneider BE:
Interferon gamma activated macrophages kill mycobacteria by nitric
oxide induced apoptosis. PLoS One. 6:e191052011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Flórido M, Gonçalves AS, Gomes MS and
Appelberg R: CD40 is required for the optimal induction of
protective immunity to Mycobacterium avium. Immunology.
111:323–327. 2004.PubMed/NCBI
|
|
29
|
Lazarevic V, Myers AJ, Scanga CA and Flynn
JL: CD40, but not CD40L, is required for the optimal priming of T
cells and control of aerosol M. tuberculosis infection.
Immunity. 19:823–835. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Samten B, Thomas EK, Gong J and Barnes PF:
Depressed CD40 ligand expression contributes to reduced gamma
interferon production in human tuberculosis. Infect Immun.
68:3002–3006. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Andrade RM, Portillo JA, Wessendarp M and
Subauste CS: CD40 signaling in macrophages induces activity against
an intracellular pathogen independently of gamma interferon and
reactive nitrogen intermediates. Infect Immun. 73:3115–3123. 2005.
View Article : Google Scholar
|
|
32
|
Andrade RM, Wessendarp M, Gubbels MJ,
Striepen B and Subauste CS: CD40 induces macrophage
anti-Toxoplasma gondii activity by triggering
autophagy-dependent fusion of pathogen-containing vacuoles and
lysosomes. J Clin Invest. 116:2366–2377. 2006.PubMed/NCBI
|