Introduction
Ursolic acid, a naturally occurring triterpenoid,
induces the apoptosis of human cancer cells through multiple
signaling pathways (1–10). In studies on the pro-apoptotic role
of ursolic acid in urinary system cancer, prostate cancer cells are
usually targeted and the apoptotic signaling pathways have been
shown to be activated by ursolic acid. Kassi et al(11) demonstrated that ursolic acid
downregulates Bcl-2 and promotes apoptosis in PC-3 human hormone
refractory prostate cancer and androgen-sensitive LNCaP cells.
Zhang et al(12) showed
that ursolic acid induces the apoptosis of PC-3 cells, in which
Bcl-2 phosphorylation, Fas overexpression and caspase-8 and -9
activation were detected, through activation of the JNK pathway and
inhibition of the Akt pathway in a dose-dependent manner. Shanmugam
et al(13) revealed that
ursolic acid inhibits the NF-κB and STAT3 cell survival pathways in
the DU145 and LNCaP prostate cancer cell lines, which suppresses
the growth of prostate cancer xenografts in nude mice. Limami et
al(14) demonstrated that the
P2Y2/Src/p38/COX-2 pathway is involved in the resistance to ursolic
acid-induced apoptosis in prostate cancer cells (14).
Bladder cancer is a type of urinary cancer. Two
recent studies demonstrated the pro-apoptotic role of ursolic acid
in bladder cancer cells. Tu et al(15) reported that ursolic acid
derivatives increase the levels of reactive oxygen species (ROS)
and induce apoptosis in NTUB1 human urothelial cancer cells. Zheng
et al(16) showed that
ursolic acid activates AMP-activated protein kinase (AMPK), which
induces the apoptosis of T24 human bladder cancer cells.
Bladder cancer cells also overexpress multiple anti-
apoptotic and drug-resistant signals. Sun et al(17) demonstrated that the PI3K/Akt/mTOR
pathway correlates with tumor progression and poor survival times
in patients with urothelial bladder cancer. Plissonnier et
al(18) reported that
TNF-related apoptosis-inducing ligand (TRAIL) is upregulated by the
antidiabetic drug ciglitazone and induces apoptosis in high-grade
bladder cancer cells. Jayasooriya et al(19) showed that a methanol extract of
Hydroclathrus clathratus downregulates the TNF-α-induced
phosphorylation of PI3K/Akt and mitogen-activated protein kinase
(MAPK) and suppresses matrix metalloproteinase-9 (MMP9) in T24
bladder cancer cells. A study by Kunze et al(20) revealed that bladder cancer cells
overexpress anti-apoptotic Bcl-2, Bcl-xL and XIAP, while survivin
and the use of siRNA knock them down. Chen et al(21) reported that the ERK/JNK-AP1
pathways are activated by 2-aminobiphenyl (21). Yu et al(22) showed that the ROS-modulated
apoptotic pathways in TSGH-8301 human bladder cancer cells are
triggered by norcantharidin and this is accompanied by the
downregulation of FasL, Bax, Bid, cytochrome c and
caspase-3, -8 and -9. Lee et al(23) reported that interleukin-28A
triggers the wound healing migration of bladder cancer cells via
NF-κB-mediated MMP-9 expression, which induces the upregulation of
the MAPK pathway. Takeuchi et al(24) reported that the phosphorylation of
ERK1/2 is involved in chemotherapy-resistance in bladder cancer and
that sunitinib may be used to suppress ERK1/2 phosphorylation to
enhance the antitumor effects. According to Huang et
al(25), the downregulation of
cyclin D, CDK4, cyclin E, CDK2, phospho-Rb, phospho-Akt and Bcl-2
and the simultaneous upregulation of cytochrome c, Apaf-1,
AIF, caspase-3, -7 and -9 and Bax protein expression and caspase
activity occurs in T24 cells following bufalin treatment.
Therefore, we hypothesized that ursolic acid is
important in promoting the apoptosis of bladder cancer cells via
the suppression of the Akt and NF-κB signaling pathways. In the
present study, ursolic acid was used to treat bladder cancer cells
to investigate its role in the apoptotic signaling pathways.
Materials and methods
Materials
Ursolic acid (purity, >90%) was purchased from
Sigma-Aldrich (Aldrich U6753; Shanghai, China). The total protein
extraction and TRIzol total RNA extraction kits were purchased from
Invitrogen (Carlsbad, CA, USA). Anti-phospho-IκBα (anti-pIκBα;
phospho-S32/S36; sc-8404), anti-NF-κBp65 (sc-8008), anti-Bcl-2
(sc-509) and anti-caspase-3 (sc-7272) antibodies were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Anti-phospho-Akt1 (anti-pAkt1; phospho-T308; ab105731) and
anti-glyceraldehyde 3-phosphate dehydrogenase (anti-GAPDH; ab8245)
monoclonal mouse antibodies were obtained from Abcam (Beijing,
China). Horseradish peroxidase (HRP)-labeled goat anti-mouse
secondary antibody was purchased from Abcam.
3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT)
was purchased from Sigma (St. Louis, MO, USA). The Moloney Murine
Leukemia Virus Reverse Transcriptase (M-MLV RTase) kit was
purchased from Promega (Beijing, China). The 2X SYBR real-time PCR
kit was obtained from Roche (Shanghai, China). The bicinchoninic
acid (BCA) protein detection kit and the enhanced chemiluminescence
(ECL) detection kit were purchased from Pierce Chemicals, Thermo
Fisher Scientific Inc. (Rockford, IL, USA).
Cell line
The T24 human urinary bladder cancer (transitional
cell carcinoma) cell line was purchased from the American Type
Culture Collection (ATCC; no. HTB-4; Manassas, VA, USA). The cells
were cultured in Dulbecco’s modified Eagle’s medium (DMEM)
containing 10% fetal bovine serum (FBS) (Invitrogen, Gibco,
Carlsbad, CA, USA) in a 5% CO2 incubator and were
passaged with a 0.25% trypsin (Sigma)/0.03% EDTA
solution.
Treatment
T24 cells were digested, suspended and seeded in
each well of 6-well plates at a density of 1.0×106/ml in
2 ml of complete culture medium. The cells were cultured for 24 h
and exposed to ursolic acid for 48 h. Ursolic acid was dissolved in
anhydrous ethanol, then added to the cells at final concentrations
of 12.5, 25 or 50 μmol/l. An equivalent amount of ethanol was added
to the cells as a control.
Quantitative PCR (qPCR)
T24 cells were harvested and total RNA was extracted
with the total RNA extraction kit using the TRIzol method.
First-strand cDNA was synthesized using M-MLV RTase according to
the manufacturer’s instructions and real-time PCR was performed
using the cDNA template according to the manufacturer’s
instructions. The amplification of GAPDH was used as an internal
control in each reaction system. The reaction conditions were as
follows: 40 cycles of 95°C for 30 sec, 58°C for 60 sec and 72°C for
60 sec. The primers were designed based on the GenBank sequence
using Beacon Designer 7 (Premier Biosoft, Palo Alto, CA, USA) and
the primer sequences were verified using Blast (26). Primer synthesis and DNA sequencing
were performed by Shanghai Sangon Biotechnology (Shanghai, China).
The primer sequences were as follows: NF-κBp65 sense,
5′-GCAAAGGAAACGCCAGAAGC-3′ and antisense,
5′-CACTACCGAACATGCCTCCAC-3′; Bcl-2 sense,
5′-ATGACTTCTCTCGTCGCTACT-3′ and antisense,
5′-CCCATCCCTGAAGAGTTCCGA-3′; caspase-3 sense,
5′-CATGGCCTGTCAGAAAATAC-3′ and antisense,
5′-TAACCCGAGTAAGAATGTGC-3′; and GAPDH (housekeeping gene) sense,
5′-AATGTGTCCGTCGTGGATCTG-3′ and antisense,
5′-CAACCTGGTCCTCAGTGTAGC-3′.
Western blotting
Western blotting was used to detect the protein
expression levels of pAkt1, pIκBα, NF-κBp65, Bcl-2 and caspase-3.
The T24 cells were harvested and cell lysis was performed using the
eukaryotic cell lysis buffer according to the manufacturer’s
instructions, followed by extraction of the total protein. Protein
quantity was determined using the BCA method. For each sample (30
μg), proteins were separated by 12% SDS-PAGE and blotted with a wet
transfer device (BioRad Laboratories, Inc., Shanghai, China) onto
nitrocellulose membranes. The membranes were then immersed in a
blocking solution containing 10% skimmed milk in PBS Tween-20
(PBST), followed by agitation for 1 h. After washing three times
with Tris-buffered saline Tween-20 (TBST) for 5 min each time, the
membranes were immersed in the primary antibody at a dilution of
1:1,000 with the blocking solution at room temperature and then
agitated for 1 h. After washing, the membranes were incubated in
the HRP-labeled secondary antibody at a dilution of 1:10,000 with
the blocking solution at room temperature and then agitated for 1
h. After an additional rinse, the membranes underwent color
development using the ECL method, followed by X-film photography.
GAPDH protein was used as an internal control. The grayscale values
(total raw density) of blots were measured using the VisionWorksLS
analysis software available in the UVP EC3 (600) Imaging System
(Ultra-Violet Products, Upland, CA, USA).
MTT assay
The medium was refreshed to discard the ursolic
acid. The cells were supplemented with 20 μl MTT solution (5
mg/ml), followed by incubation in a CO2 incubator for 4
h. The supernatant was discarded and 100 μl dimethylsulfoxide
(DMSO; Sigma) was applied to each well. When the purple crystals at
the bottom of the well were completely dissolved, the absorbance
value was measured with a Thermo Multiskan MK3 microplate reader
(Thermo Fisher Scientific Inc., Waltham, MA, USA) at a wavelength
of λ=490 nm. Cell viability (%) was calculated as experimental
absorbance/normal absorbance × 100.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). The statistical software SPSS10.0 was used for statistical
analysis. Paired comparisons were performed using the Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Detection of mRNA levels using qPCR
The expression of cell signaling molecules detected
using qPCR are shown in Fig. 1.
Prior to ursolic acid treatment, the control cells expressed high
mRNA levels of anti-apoptotic NF-κBp65 and Bcl-2 and a low level of
pro-apoptotic caspase-3 mRNA. As increasing concentrations of
ursolic acid were applied (12.5, 25.0 and 50.0 μmol/l), the
anti-apoptotic signaling was inhibited and pro-apoptotic signaling
was activated. Anti-apoptotic NF-κBp65 levels decreased 0.74
(38.9/52.6), 0.35 (18.6/52.6) and 0.17 (8.9/52.6)-fold,
respectively; and Bcl-2 levels decreased 0.77 (32.6/42.3), 0.50
(21.3/42.3) and 0.22 (9.5/42.3)-fold, respectively. Pro-apoptotic
caspase-3 levels increased 1.63 (13.2/8.1), 2.53 (20.5/8.1), 4.78
(38.7/8.1)-fold, respectively. The pro-apoptotic induction
triggered by ursolic acid occurred in a dose-dependent manner.
Detection of protein levels using western
blotting
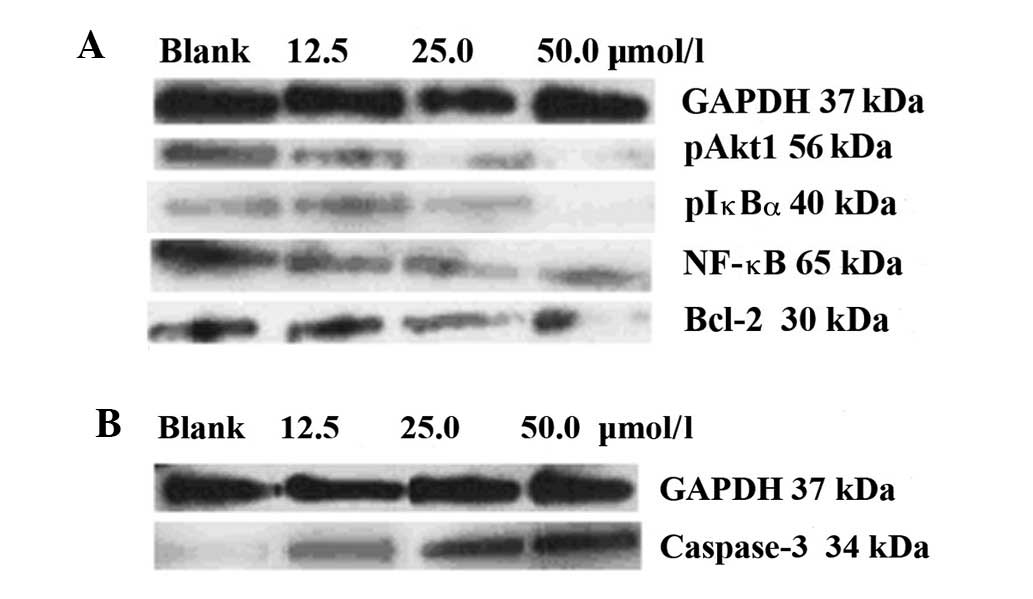
Fig. 2 shows the
expression of the cell signaling molecules, detected using western
blotting. Prior to treatment with ursolic acid, high levels of
anti-apoptotic pAkt1, pIκBα, NF-κBp65 and Bcl-2 and low levels of
pro-apoptotic caspase-3 were expressed in the control cells. With
the application of increasing concentrations of ursolic acid (12.5,
25.0 and 50.0 μmol/l), all the anti-apoptotic signaling was
inhibited (Fig. 2A), while the
pro-apoptotic signaling was upregulated (Fig. 2B).
Table I shows the
complete grayscales of the blots presented in Fig. 2, demonstrating the total levels of
the proteins detected. The blot grayscales for the anti-apoptotic
pAkt1 protein were 26.6, 10.4 and 5.1 vs. 32.3; for pIκBα were
17.3, 8.8 and 3.2 vs. 24.2; for pNF-κBp65 were 32.2, 21.2 and 8.5
vs. 45.1; for Bcl-2 were 33.6, 19.7 and 9.2 vs. 40.3; and for
pro-apoptotic caspase-3 protein were 6.1, 11.6 and 20.7 vs. 4.7,
respectively (12.5, 25.0 and 50.0 μmol/l ursolic acid vs. control).
The pro-apoptotic induction triggered by ursolic acid treatment
occurred in a dose-dependent manner.
 | Table IRelative grayscales of blots (48 h,
%/GAPDH). |
Table I
Relative grayscales of blots (48 h,
%/GAPDH).
| | Ursolic acid doses
(μmol/l) |
|---|
| |
|
|---|
| Protein blots | Control | 12.5 | 25.0 | 50.0 |
|---|
| GAPDH (37 kDa) | 104.5 | 100.0 | 99.3 | 100.2 |
| pAkt1 (56 kDa) | 32.3 | 26.6 | 10.4 | 5.1 |
| pIκBα (40 kDa) | 24.2 | 17.3 | 8.8 | 3.2 |
| NF-κBp65 (65
kDa) | 45.1 | 32.2 | 21.2 | 8.5 |
| Bcl-2 (30 kDa) | 40.3 | 33.6 | 19.7 | 9.2 |
| Caspase-3 (34
kDa) | 4.7 | 6.1 | 11.6 | 20.7 |
Cell proliferation
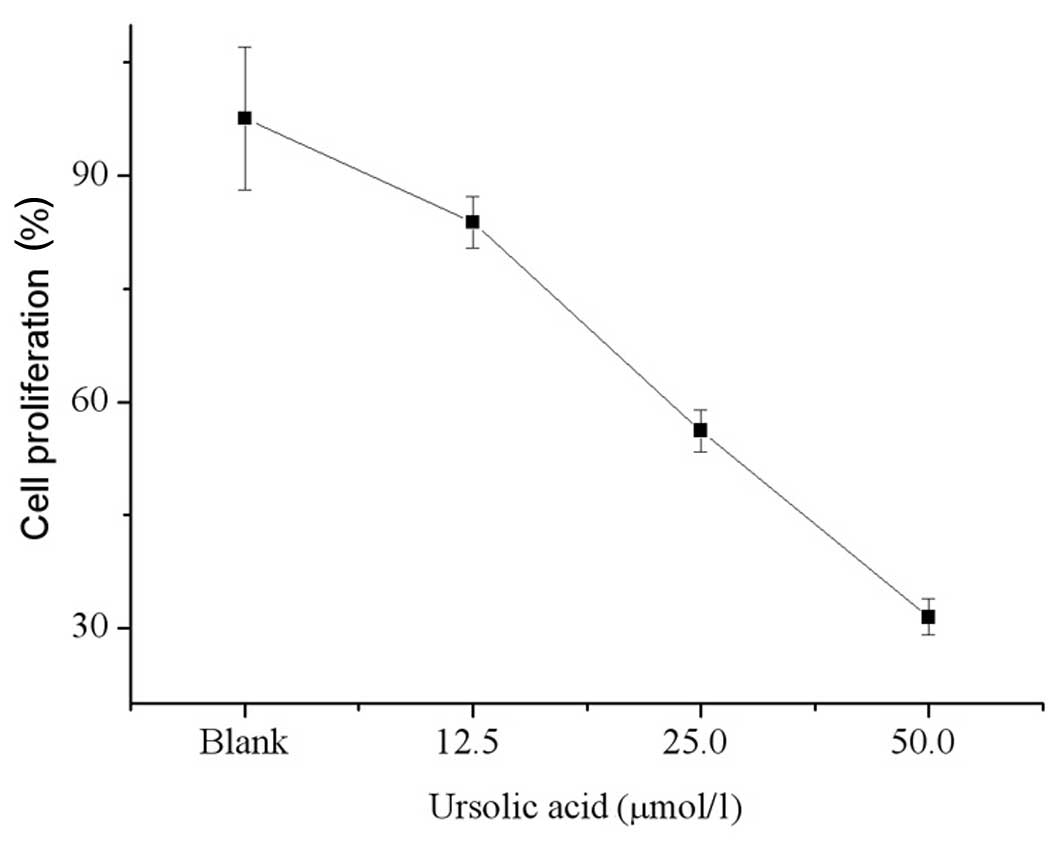
Fig. 3 shows the
cell viability after 48 h of treatment with ursolic acid. The
proliferative activity of T24 cells treated with 12.5, 25.0 and
50.0 μmol/l ursolic acid decreased and was significantly lower
compared with that of the control cells (83.8, 56.2, 31.5 vs.
97.6%, respectively; P<0.05 for each). The antitumor effect of
ursolic acid treatment occurred in a dose-dependant manner.
Discussion
The Akt/NF-κB pathways are involved in numerous
anti-apoptotic and drug-resistant events, which occur in various
types of bladder cancer (17,19,23,25).
Inhibition of the Akt/NF-κB pathways results in the downregulation
of Bcl-2 with a simultaneous upregulation of caspase-3 (20,22,25).
In the present study, ursolic acid was used to treat T24 bladder
cancer cells. qPCR and western blotting were performed to
investigate the role of ursolic acid in altering the levels of
anti-apoptotic pAkt, pIκBα, pNF-κBp65 and Bcl-2 and pro-apoptotic
caspase-3.
Prior to the treatment with ursolic acid, Akt1
phosphorylation at threonine 308 was overexpressed in the control
cells (26). The hyperactivated
pAkt1 exhibited a serine-threonine protein kinase activity and
triggered the cascade enzymes, resulting in an increased
phosphorylation of IκBα at serines 32 and 36. pIκBα was
disassociated from NF-κB, resulting in increased NF-κBp65 at the
mRNA and protein levels. The hyperactivated pAkt1 also triggered an
overexpression of anti-apoptotic Bcl-2 at the mRNA and protein
levels, which contributed to the sustained proliferation of the
control cells.
By contrast, the use of ursolic acid led to a
significant decrease in pAkt1 and pIκBα and in the NF-κBp65 mRNA
and protein levels. The downregulation of pAkt1 indicates that the
serine-threonine protein kinase activity of Akt was weakened.
Subsequently, the phosphorylation of IκBα was downregulated to a
level that caused the the release of NF-κB to be repressed,
resulting in a decrease in NF-κBp65 levels. The decreased
serine-threonine protein kinase activity of Akt also resulted in
the downregulation of anti-apoptotic Bcl-2; thus, suppression of
T24 cell apoptosis was reduced.
The downregulation of pAkt1 and NF-κBp65 indicates
that the signal amplification and transduction pathways were
efficiently inhibited. Accordingly, the pro-apoptotic caspase-3
mRNA and protein levels were significantly upregulated. As
previously reported, the upregulated caspase-3 decreases IKK2
levels (27,28) in necrotized or apoptotic cancer
cells, which decreases IκBα phosphorylation and leads to a reduced
NF-κBp65 level. The upregulated caspase-3 also directly decreases
the NF-κBp65 protein level (27,29),
resulting in a secondary downregulation of NF-κBp65 in apoptotic
cancer cells. The present study demonstrated that NF-κBp65
signaling was markedly downregulated in T24 cells. The apoptotic
T24 cells showed a decrease in proliferation. The MTT assay results
revealed that the proliferation of T24 cells was significantly
inhibited by ursolic acid. Additionally, the pro-apoptotic
induction triggered by ursolic acid occured in a dose-dependent
manner.
In conclusion, ursolic acid is important in the
induction of apoptosis via AKT/NF-κB signaling suppression in T24
human bladder cancer cells and this occurs in a dose-dependent
manner. Thus, Akt and NF-κB are potential targets for bladder
cancer therapy and ursolic acid may serve as a naturally-occurring
candidate drug for the prevention and treatment of bladder
cancer.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (no. 81172270/H1617).
References
|
1
|
Achiwa Y, Hasegawa K, Komiya T and Udagawa
Y: Ursolic acid induces Bax-dependent apoptosis through the
caspase-3 pathway in endometrial cancer SNG-II cells. Oncol Rep.
13:51–57. 2005.PubMed/NCBI
|
|
2
|
Kim KH, Seo HS, Choi HS, Choi I, Shin YC
and Ko SG: Induction of apoptotic cell death by ursolic acid
through mitochondrial death pathway and extrinsic death receptor
pathway in MDA-MB-231 cells. Arch Pharm Res. 34:1363–1372. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manu KA and Kuttan G: Ursolic acid induces
apoptosis by activating p53 and caspase-3 gene expressions and
suppressing NF-kappaB mediated activation of bcl-2 in B16F-10
melanoma cells. Int Immunopharmacol. 8:974–981. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu JJ, Liu WD, Yang HZ, Zhang Y, Fang ZG,
Liu PQ, Lin DJ, Xiao RZ, Hu Y, Wang CZ, Li XD, He Y and Huang RW:
Inactivation of PI3k/Akt signaling pathway and activation of
caspase-3 are involved in tanshinone I-induced apoptosis in myeloid
leukemia cells in vitro. Ann Hematol. 89:1089–1097. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Liang X and Yang X: Ursolic acid
inhibits growth and induces apoptosis in gemcitabine-resistant
human pancreatic cancer via the JNK and PI3K/Akt/NF-κB pathways.
Oncol Rep. 28:501–510. 2012.PubMed/NCBI
|
|
6
|
Shishodia S, Majumdar S, Banerjee S and
Aggarwal BB: Ursolic acid inhibits nuclear factor-kappaB activation
induced by carcinogenic agents through suppression of IkappaBalpha
kinase and p65 phosphorylation: correlation with down-regulation of
cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer
Res. 63:4375–4383. 2003.
|
|
7
|
Tang C, Lu YH, Xie JH, Wang F, Zou JN,
Yang JS, Xing YY and Xi T: Downregulation of survivin and
activation of caspase-3 through the PI3K/Akt pathway in ursolic
acid-induced HepG2 cell apoptosis. Anticancer Drugs. 20:249–258.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu B, Wang X, Chi ZF, Hu R, Zhang R, Yang
W and Liu ZG: Ursolic acid-induced apoptosis in K562 cells
involving upregulation of PTEN gene expression and inactivation of
the PI3K/Akt pathway. Arch Pharm Res. 35:543–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Li Y, Wang X and Jiang C: Ursolic
acid inhibits proliferation and induces apoptosis in human
glioblastoma cell lines U251 by suppressing TGF-β1/miR-21/PDCD4
pathway. Basic Clin Pharmacol Toxicol. 111:106–112. 2012.PubMed/NCBI
|
|
10
|
Wang JS, Ren TN and Xi T: Ursolic acid
induces apoptosis by suppressing the expression of FoxM1 in MCF-7
human breast cancer cells. Med Oncol. 29:10–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kassi E, Papoutsi Z, Pratsinis H,
Aligiannis N, Manoussakis M and Moutsatsou P: Ursolic acid, a
naturally occurring triterpenoid, demonstrates anticancer activity
on human prostate cancer cells. J Cancer Res Clin Oncol.
133:493–500. 2007. View Article : Google Scholar
|
|
12
|
Zhang Y, Kong C, Zeng Y, Wang L, Li Z,
Wang H, Xu C and Sun Y: Ursolic acid induces PC-3 cell apoptosis
via activation of JNK and inhibition of Akt pathways in vitro. Mol
Carcinog. 49:374–385. 2010.PubMed/NCBI
|
|
13
|
Shanmugam MK, Rajendran P, Li F, Nema T,
Vali S, Abbasi T, Kapoor S, Sharma A, Kumar AP, Ho PC, Hui KM and
Sethi G: Ursolic acid inhibits multiple cell survival pathways
leading to suppression of growth of prostate cancer xenograft in
nude mice. J Mol Med (Berl). 89:713–727. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Limami Y, Pinon A, Leger DY, Pinault E,
Delage C, Beneytout JL, Simon A and Liagre B: The
P2Y2/Src/p38/COX-2 pathway is involved in the resistance to ursolic
acid-induced apoptosis in colorectal and prostate cancer cells.
Biochimie. 94:1754–1763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tu HY, Huang AM, Wei BL, Gan KH, Hour TC,
Yang SC, Pu YS and Lin CN: Ursolic acid derivatives induce cell
cycle arrest and apoptosis in NTUB1 cells associated with reactive
oxygen species. Bioorg Med Chem. 17:7265–7274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng QY, Jin FS, Yao C, Zhang T, Zhang GH
and Ai X: Ursolic acid-induced AMP-activated protein kinase (AMPK)
activation contributes to growth inhibition and apoptosis in human
bladder cancer T24 cells. Biochem Biophys Res Commun. 419:741–747.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun CH, Chang YH and Pan CC: Activation of
the PI3K/Akt/mTOR pathway correlates with tumour progression and
reduced survival in patients with urothelial carcinoma of the
urinary bladder. Histopathology. 58:1054–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plissonnier ML, Fauconnet S, Bittard H and
Lascombe I: The antidiabetic drug ciglitazone induces high grade
bladder cancer cells apoptosis through the up-regulation of TRAIL.
PLoS One. 6:e283542011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jayasooriya RG, Choi YH, Moon SK, Kim WJ
and Kim GY: Methanol extract of Hydroclathrus clathratus
suppresses matrix metalloproteinase-9 in T24 bladder carcinoma
cells by suppressing the NF-κB and MAPK pathways. Oncol Rep.
27:541–546. 2012.
|
|
20
|
Kunze D, Kraemer K, Erdmann K, Froehner M,
Wirth MP and Fuessel S: Simultaneous siRNA-mediated knockdown of
antiapoptotic BCL2, Bcl-xL, XIAP and survivin in bladder cancer
cells. Int J Oncol. Jul 6–2012.(Epub ahead of print). View Article : Google Scholar
|
|
21
|
Chen CC, Cheng YY, Chen SC, Tuan YF, Chen
YJ, Chen CY and Chen LC: Cyclooxygenase-2 expression is
up-regulated by 2-aminobiphenyl in a ROS and MAPK-dependent
signaling pathway in a bladder cancer cell line. Chem Res Toxicol.
25:695–705. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu CC, Ko FY, Yu CS, Lin CC, Huang YP,
Yang JS, Lin JP and Chung JG: Norcantharidin triggers cell death
and DNA damage through S-phase arrest and ROS-modulated apoptotic
pathways in TSGH 8301 human urinary bladder carcinoma cells. Int J
Oncol. 41:1050–1060. 2012.
|
|
23
|
Lee SJ, Lim JH, Choi YH, Kim WJ and Moon
SK: Interleukin-28A triggers wound healing migration of bladder
cancer cells via NF-κB-mediated MMP-9 expression inducing the MAPK
pathway. Cell Signal. 24:1734–1742. 2012.PubMed/NCBI
|
|
24
|
Takeuchi A, Eto M, Shiota M, Tatsugami K,
Yokomizo A, Kuroiwa K, Itsumi M and Naito S: Sunitinib enhances
antitumor effects against chemotherapy-resistant bladder cancer
through suppression of ERK1/2 phosphorylation. Int J Oncol.
40:1691–1696. 2012.
|
|
25
|
Huang WW, Yang JS, Pai SJ, Wu PP, Chang
SJ, Chueh FS, Fan MJ, Chiou SM, Kuo HM, Yeh CC, Chen PY, Tsuzuki M
and Chung JG: Bufalin induces G(0)/G(1) phase arrest through
inhibiting the levels of cyclin D, cyclin E, CDK2 and CDK4, and
triggers apoptosis via mitochondrial signaling pathway in T24 human
bladder cancer cells. Mutat Res. 732:26–33. 2012. View Article : Google Scholar
|
|
26
|
Weizhong Z, Shuohui G, Hanjiao Q, Yuhong
M, Xiaohua Y, Jian C and Lisen L: Inhibition of cytohesin-1 by
siRNA leads to reduced IGFR signaling in prostate cancer. Braz J
Med Biol Res. 44:642–646. 2011.PubMed/NCBI
|
|
27
|
Barkett M and Gilmore TD: Control of
apoptosis by Rel/NF-κB transcription factors. Oncogene.
18:6910–6924. 1999.
|
|
28
|
Tang G, Yang J, Minemoto Y and Lin A:
Blocking caspase-3-mediated proteolysis of IKKbeta suppresses
TNF-alpha-induced apoptosis. Mol Cell. 8:1005–1016. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ravi R, Mookerjee B, van Hensbergen Y,
Bedi GC, Giordano A, El-Deiry WS, Fuchs EJ and Bedi A: p53-mediated
repression of nuclear factor-kappaB RelA via the transcriptional
integrator p300. Cancer Res. 58:4531–4536. 1998.PubMed/NCBI
|