Introduction
Ovarian cancer continues to be the most frequent
cause of cancer mortality among females in Western Europe and the
United States, with the highest mortality rate of all gynecological
malignancies (1). The most common
histological subtype of ovarian cancer is epithelioid cancer
(serous, endometrioid, mucinous and clear cell), accounting for 90%
of ovarian malignancies (2,3).
Although >70% of patients have increased 5-year survival rates
subsequent to surgery followed by chemotherapy plus second-line
therapies, a number of tumor types fail to respond to chemotherapy.
This is due to the fact that in consecutive chemotherapy, these
tumors appear to become less sensitive or resistant to
chemotherapeutic drugs. The intolerable side-effects of the
systemic treatment with chemotherapy means that the development of
novel and effective therapeutic modalities and the identification
of new rational targets for these novel therapies are required
(4).
Organisms living under aerobic conditions are
exposed to reactive oxygen species (ROS), including superoxide
anions (O2−), hydrogen peroxide
(H2O2) and nitric oxide (NO), which are
generated by the redox metabolism mainly in the mitochondria
(5). A number of studies have
demonstrated that ROS in small amounts participate in numerous
physiological processes, including cell proliferation,
differentiation and apoptosis and the modulation of transcription
factors and signal transduction pathways (6,7).
Cancer cells have been demonstrated to possess higher levels of
intracellular ROS due to increased cellular respiration and are
believed to have the potential to oxidize macromolecules, induce
DNA mutation, impair protein function and peroxidize lipids, thus
leading to the development of tumors (8,9).
However, to facilitate the development of tumors, numerous
antioxidative systems have been developed to maintain an
appropriate level of ROS, which include antioxidant enzymes such as
glutathione peroxidases (GPXs), superoxide dismutases (SODs),
catalases and the recently identified rapidly growing family of
peroxiredoxins (Prxs) (10,11).
Prxs have been shown to be involved in diverse
cellular roles, including the control of cell proliferation,
differentiation and apoptosis, the protection of oxidant-sensitive
proteins, the regulation of cellular H2O2 and
redox regulation (12,13). Recent studies have suggested that
Prxs are involved in the development, progression and drug
resistance of cancers (14,15).
As a member of the Prx family, PRDX3 is overexpressed in a number
of diverse tumors, including hepatocellular carcinoma and lung and
prostate cancer (16–18). To the best of our knowledge, there
has only been one study describing the potential role of PRDX3 in
ovarian cancer, in which PRDX3 was determined to be responsible for
resistance to platinum-based chemotherapies (19). As an anti-apoptotic protein for
tumor cell proliferation and survival, therapeutic strategies
targeting PRDX3 may therefore be effective broad-spectrum
anticancer agents.
In the present study, the focus was on the role and
mechanism behind the downregulation of PRDX3 in cisplatin-induced
ovarian cancer cell apoptosis. For the first time we reported that
the overexpression of PRDX3 was observed in ovarian cancer and that
the PRDX3 knockdown resulted in accelerated cisplatin-induced cell
apoptosis through suppression of the NF-κB signaling pathway. This
may provide a new insight into anticancer therapy for ovarian
cancer.
Materials and methods
Tissue samples
In total, 104 paraffin-embedded ovarian cancer
tissues and paired non-cancerous tissues were surgically obtained
from the Maternal and Child Health Hospital of Hubei Province,
Wuhan, Hubei, China, between 2005 and 2011. All the procedures were
approved by the research ethics committee of the Maternal and Child
Health Hospital of Hubei Province. All the patients agreed to the
procedure and signed consent forms. None of the patients had
received chemotherapy or radiation therapy prior to the surgery.
The staging and grading were performed by two experienced
gynecological pathologists according to the criteria of the
International Federation of Gynaecologists and Obstetricians (FIGO)
and the World Health Organization (WHO). The median patient age was
56 years (range, 33–72 years). Detailed patient characteristics are
presented in Table I.
 | Table IAssociations between the expression of
PRDX3 and the clinicopathological parameters in ovarian cancer. |
Table I
Associations between the expression of
PRDX3 and the clinicopathological parameters in ovarian cancer.
| | PRDX3 expression | |
|---|
| |
| |
|---|
| Parameters | Cases (n) | − | + | ++ | +++ | P-value |
|---|
| Age at first
diagnosis |
| <56 years | 45 | 2 | 5 | 22 | 16 | 0.691 |
| ≥56 years | 59 | 6 | 8 | 27 | 18 | |
| Serous |
| I/II | 20 | 3 | 4 | 9 | 4 | 0.045a |
| III/IV | 41 | 1 | 2 | 22 | 16 | |
|
Well-differentiated | 21 | 2 | 2 | 15 | 2 | 0.028b |
|
Moderately-differentiated | 23 | 2 | 3 | 11 | 7 | |
|
Poorly-differentiated | 17 | 0 | 1 | 5 | 11 | |
| Mucinous |
| I/II | 8 | 1 | 2 | 3 | 2 | 0.173a |
| III/IV | 11 | 1 | 1 | 9 | 0 | |
|
Well-differentiated | 9 | 2 | 2 | 3 | 2 | 0.251b |
|
Moderately-differentiated | 6 | 0 | 1 | 5 | 0 | |
|
Poorly-differentiated | 4 | 0 | 0 | 4 | 0 | |
| Endometrioid |
| I/II | 4 | 1 | 2 | 1 | 0 | 0.032a |
| III/IV | 7 | 0 | 0 | 1 | 6 | |
|
Well-differentiated | 4 | 1 | 2 | 0 | 1 | 0.278b |
|
Moderately-differentiated | 4 | 0 | 0 | 1 | 3 | |
|
Poorly-differentiated | 3 | 0 | 0 | 1 | 2 | |
| Clear cell |
| I/II | 4 | 1 | 1 | 2 | 0 | 0.114a |
| III/IV | 9 | 0 | 1 | 2 | 6 | |
|
Well-differentiated | 5 | 0 | 1 | 2 | 2 | 0.71b |
|
Moderately-differentiated | 3 | 0 | 1 | 1 | 1 | |
|
Poorly-differentiated | 5 | 1 | 0 | 1 | 3 | |
| Lymph node
metastasis |
| Negative | 82 | 6 | 8 | 39 | 29 | 0.352 |
| Positive | 22 | 2 | 5 | 10 | 5 | |
Immunohistochemistry and staining score
evaluation
The paraffin slices were successively incubated for
5 min in a series of xylene and ethanol baths of decreasing
concentration. The antigen was retrieved by boiling the slices in a
0.1 M Na citrate buffer (pH 6.0). The endogenous peroxidase was
inhibited by 3% H2O2. The monoclonal mouse
anti-PRDX3 antibodies (Abcam, Cambridge, UK), diluted 100-fold in
1% BSA, were applied and incubated overnight at 4°C. The incubation
with the horseradish peroxidase (HRP)-conjugated goat anti-mouse
antibodies at 37°C for 30 min was followed by the reaction with the
3,3′-diaminobenzidine substrate solution and counterstaining with
Mayer’s hematoxylin. All the steps were performed in a moist
chamber.
The expression of PRDX3 was scored based on the
intensity of the staining and the percentage of positively stained
cells. Staining intensity was scored as follows: lack of staining,
0, weak staining 1, moderate staining, 2 and strong staining, 3.
The percentage of the positively stained cells was scored as 0 if
no staining was observed or if it was present in <5% of the
cells; 1 if positive staining was present in 5–25% of the cells; 2
if positive staining was present in 25–50% of the cells; 3 if
positive staining was present in 50–75% of the cells; and 4 if
positive staining was present in in >75% of cells. The score for
each section was measured as the staining intensity × the
percentage of the positively stained cells. The result was defined
as negative (−, score of 0), weakly positive (+, score of 1–3),
moderately positive (++, score of 4–7) or strongly positive (+++,
score of 8–12). The slides were examined separately by two
independent pathologists who had no prior knowledge of each
patient’s clinical information. Any discrepancies between the two
evaluators were resolved by a re-evaluation and careful discussion
until agreement was reached.
RNA interference experiments
First, the expression of PRDX3 was examined in the
HO-8910, SKOV3, OVCAR-3 ovarian cancer cell lines (ATCC, Manassas,
VA, USA). To knock down the PRDX3 expression, a pGCsi-U6/Neo/GFP
vector was used that encoded a small hairpin RNA directed against
the target gene in the SKOV3 ovarian cancer cells. The target
sequences for PRDX3 were 5′-ACCTTCTGAAAGTACTCTT-3′ [small
interfering (si)RNA-1], 5′-CTTTAGACGAATCAATTCA-3′ (siRNA-2) and
5′-CGTACGACCCACTGTCGTC-3′ (siRNA-3). As a negative control, the
shRNA vector without hairpin oligonucleotides (siRNA-mock) was
used. For the transfection, the ovarian cancer cells were seeded to
achieve 70–80% confluence and then transfected in serum-free medium
for 6 h with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) and
siRNA-PRDX3 or the siRNA-mock. Subsequent to 48 h, the cells were
harvested and a limited dilution was performed in 96-well plates
for the generation of individual cell clones. Three weeks later,
the levels of PRDX3 expression in the cell clones that had been
transfected with siRNA-PRDX3 or the siRNA-mock, were characterized
by a western blot analysis for the PRDX3 protein.
Western blot analysis
The cells were lysed in a lysis buffer (62.5 mM
Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 50 mM dithiothreitol and
0.01% bromophenol blue). The cell extract protein amounts were
quantified using the Bicinchoninic Acid (BCA) Protein Assay kit
(Beyotime, Shanghai, China). Equivalent amounts of protein (50 μg)
were separated using 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and then transferred to a polyvinylidene
fluoride (PVDF) membrane (Millipore Corporation, Billerica, MA,
USA). The blots were probed with specific antibodies for PRDX3
(1:1000, ab16751; Abcam), p-NF-κB (1:1000, sc135769; Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA), Bcl-2 (1:500, ab692,
Abcam), Bcl-XL (1:1000, sc8392; Santa Cruz Biotechnology Inc.), Bax
(1:1000, ab5714; Abcam), caspase-3 (1:3000, ab32351, Abcam),
caspase-9 (1:1000, sc81650; Santa Cruz Biotechnology Inc.) and
β-actin (1:1000, ab3280; Abcam) followed by a secondary detection
step with goat anti-mouse IgG (Abcam). The immunoreactive proteins
were revealed by an Enhanced Chemiluminescence (ECL) kit (Pierce,
Rockford, IL, USA).
Cell proliferation assay
A cell proliferation assay was performed using the
Cell-Counting kit (CCK)-8 (Dojindo, Kumamoto, Japan) to analyze the
proliferation potential of the parental cells, empty vector and
siRNA-PRDX3-transfected cells treated with/without cisplatin. The
cells were harvested and plated in 96-well plates at
1×103 cells/well and maintained at 37°C in a humidified
incubator. At the indicated time-points, 10 μl of the CCK-8
solution were added and incubated for 1.5 h and the number of
viable cells in each well was calculated by measuring the
absorbance at a wavelength of 450 nm.
Colony formation assay
The colony formation assay was performed to measure
the cell growth according to the protocol described previously
(20), with certain modifications.
Identical numbers of parental cells, empty vector and
siRNA-PRDX3-transfected cells treated with/without cisplatin were
seeded in 6-well tissue-culture plates to form colonies. Subsequent
to incubation for 10 days, the number of colonies (≥20 cells)
within a field was counted under a light microscope at a
magnification of ×200. For each test, a total of five fields were
selected at random and the numbers were averaged. For the
calculation of the colony forming ability, the following formula
was used: colony forming efficiency = number of colonies/number of
inoculated cells × 100.
Tumorigenicity assays in athymic
mice
Male, 6–8-week old, BALB/c nude (nu/nu) mice were
purchased from Slac Laboratory Animal Co., Ltd. (Shanghai, China)
and used for the in vivo studies. The mice were randomly
divided into six groups (n=8) and injected with the following: i)
5×106 SKOV3 cells in 100 μl PBS; ii) 5×106
SKOV3/Si-Mock cells in 100 μl PBS; iii) 5×106 SKOV3/Si-1
cells in 100 μl PBS; iv) 5×106 SKOV3 cells plus 0.1 mg
cisplatin in 100 μl PBS; v) 5×106 SKOV3/Si-Mock cells
plus 0.1 mg cisplatin in 100 μl PBS; and vi) 5×106
SKOV3/Si-1 cells plus 0.1 mg cisplatin in 100 μl PBS (5 mg/kg body
weight). The 5×106 cells were subcutaneously injected
into the left back flank of these animals. Cisplatin was
administered by the intraperitoneal route on day 6 subsequent to
the implantation of the tumor cells and again when the tumor
diameter reached ~5 mm (every week, 4 times). The tumor variables
were measured every three days by an electronic caliper and the
tumor volume was calculated using the formula: tumor volume
(mm3) = 0.52 × length (mm) × width2
(mm2). The measurements began in the first week when the
tumor mass was well-established. A third operator evaluated the
morphometric variables in a coded and blinded maner. The tissue
samples were harvested for terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling (TUNEL) analysis.
TUNEL assay
TUNEL analysis was performed with an in situ
Cell Death Detection kit (Roche, Basel, Switzerland). The cell
apoptosis index was quantified by determining the percentage of the
positively stained cells for all of the nuclei in 20 randomly
chosen fields at a magnification of ×200. Slides of the apoptosis
studies were quantified in a blind manner by two independent
reviewers at two differing times.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses were performed by SPSS 13.0 software.
Differences among the groups were determined by an ANOVA analysis
and the comparison between two groups was analyzed by the Student’s
t-test. Nominal variables were compared using a cross-tabulation
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Association between PRDX3 expression and
the clinicopathological characteristics of ovarian cancer
To investigate the role of PRDX3 in the progression
of ovarian cancer, immunohistochemistry was used to assess the
PRDX3 expression levels in the paraffin-embedded tissues. The
association between PRDX3 expression and the clinicopathological
characteristics of the ovarian cancer tissues is shown in Table I. PRDX3 was heavily stained in the
cytoplasm and nuclei of the cancer tissues (12.5% +, 47.1% ++ and
32.7% +++), but little staining was observed in the adjacent
non-cancerous tissues (35.5% − and 64.5% +). The difference between
the cancerous and non-cancerous tissues was significant
(P<0.05). The patients with serous ovarian cancer accounted for
58.7% of the total cases. Serous ovarian cancer mostly presented at
FIGO stage III or IV (FIGO I + II, 32.8% and FIGO III + IV, 67.2%)
and a significant difference was observed between stages I + II and
III + IV (P<0.05) in the various types of ovarian cancer. With
regard to tumor differentiation, significant differences were
identified in the PRDX3 expression among the well-differentiated,
moderately-differentiated and poorly-differentiated serous ovarian
cancer samples (P<0.05). With respect to the mucinous,
endometrioid and clear cell types, there were significant
differences between stages I + II and III + IV in the endometrioid
ovarian cancer samples, but not in the mucinous or clear cell
types. With regard to the tumor differentiation of the mucinous,
endometrioid and clear cell carcinomas, the differences in the
PRDX3 expression among the well-, moderately- and
poorly-differentiated carcinomas were not significant. Only slight
differences in the PRDX3 expression were observed with respect to
the histological subtype, age at first diagnosis and lymph node
metastasis (P>0.05).
Inhibition of PRDX3 expression in the
ovarian cancer cells
To select the optimal cells to be transfected, the
expression of PRDX3 was detected in three cell lines (HO-8910,
SKOV3 and OVCAR-3), as shown in Fig.
1A. The expression level of PRDX3 in the HO-8910, SKOV3 and
OVCAR-3 cell lines showed no significant difference, with the
lowest expression observed in the HO-8910 cells and the highest in
the SKOV3 cells. To investigate the biological significance of
PRDX3 on the ovarian cancer cells, three siRNA expression vectors
(Si-1, Si-2 and Si-3) specific to the PRDX3 transcripts were
constructed and transfected into the ovarian cancer SKOV3 cells
that endogenously expressed a high level of PRDX3 (Fig. 1A). A knockdown effect was observed
by western blot analysis and SKOV3/Si-1 was observed to be the most
effective when compared with the SKOV3/Si-Mock (Fig. 1B). The successful establishment of
the PRDX3 gene silent ovarian cancer cell clone provided a useful
tool for the investigation into the function of PRDX3 in the growth
of the ovarian cancer cells.
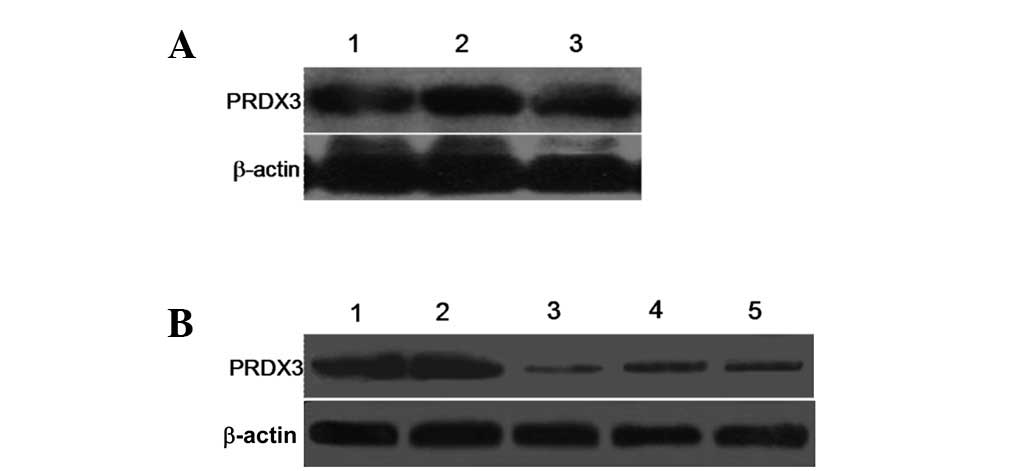 | Figure 1Expression of PRDX3 analyzed by
western blot assay. (A) Expression of PRDX3 in three ovarian cancer
cell lines (HO-8910, SKOV3 and OVCAR-3) was detected. β-actin
served as an internal control. Lanes 1, HO-8910 cells; 2, SKOV3
cells; 3, OVCAR-3 cells. (B) Western blot analysis of PRDX3 in
SKOV3 cells transfected with control (Si-Mock) or small interfering
RNA (Si-1, Si-2 and Si-3). β-actin served as an internal control.
Lanes 1, SKOV3 cells; 2, SKOV3/Si-Mock; 3, SKOV3/Si-1; 4,
SKOV3/Si-2; 5, SKOV3/Si-3. |
Effect of PRDX3-siRNA on the growth of
the ovarian cancer cells
To examine the effect of PRDX3 on the growth of the
ovarian cancer cells, the dynamics of SKOV3, SKOV3/Si-Mock and
SKOV3/Si-1 cell growth were determined by a cell proliferation
assay. Following a 7-day period, the growth of the SKOV3/Si-1 cells
was much slower compared with the SKOV3 or SKOV3/Si-Mock groups
(Fig. 2A). The other pattern of
inhibition for the PRDX3 expression in the ovarian cancer cells was
achieved in the colony formation assay. Consequently, the average
colony number of the SKOV3/Si-1 cells was decreased compared with
the SKOV3 or SKOV3/Si-Mock groups. This difference was significant
(P<0.05; Fig. 2B). To further
explore the reason behind the decrease in cell viability, the
tumorigenicity of the SKOV3/Si-1 cells was examined, as shown in
Fig. 2C. The tumor size in the
nude mice injected with the SKOV3/Si-1 cells was significantly
smaller than that in the mice injected with the SKOV3/Si-Mock cells
(P<0.05). This was associated with the enhanced induction of
apoptosis, as shown by Fig.
4A.
 | Figure 4PRDX3-siRNA enhances cisplatin-induced
ovarian cancer cell apoptosis via the NF-κB signaling pathway. (A)
Apoptotic index of the various ovarian cancer tissues. In the
tumorigenicity assay, tumor tissue samples were collected for the
TUNEL analysis. The apoptotic index of the groups was counted and
calculated as a ratio of the apoptotic cell number to the total
cell number in each field. * vs. SKOV3/Si-Mock or SKOV3
groups, P<0.05 and # vs. No cisplatin-treated group,
P<0.05. (B) Western blot analysis of the expression of
apoptosis-related proteins Bcl-2, Bcl-XL, NF-κB p65, Bax, Caspase-3
and Caspase-9 in the SKOV3/Si-Mock or SKOV3/Si-1 cells with/without
treatment of cisplatin. Lanes 1, SKOV3/Si-Mock; 2,
SKOV3/Si-Mock+Cisplatin; 3, SKOV3/Si-1; 4, SKOV3/Si-1+Cisplatin;
TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick
end-labeling; si, small interfering. |
PRDX3-siRNA enhances cisplatin-induced
ovarian cancer cell apoptosis
To investigate the impact of the PRDX3-siRNA on
cisplatin-induced ovarian cancer cell apoptosis, the effect of
cisplatin on the growth of the SKOV3 cells was firstly examined by
a cell proliferation assay. The SKOV3 cells were treated with 0, 1,
2, 3 and 4 μg/ml cisplatin for 1, 2, 3, 4 and 5 days, respectively
(Fig. 3). The addition of
cisplatin to the culture media inhibited the proliferation of the
SKOV3 cells in a dose-dependent manner. When the cisplatin
concentration was 3 μg/ml, the effect was more evident.
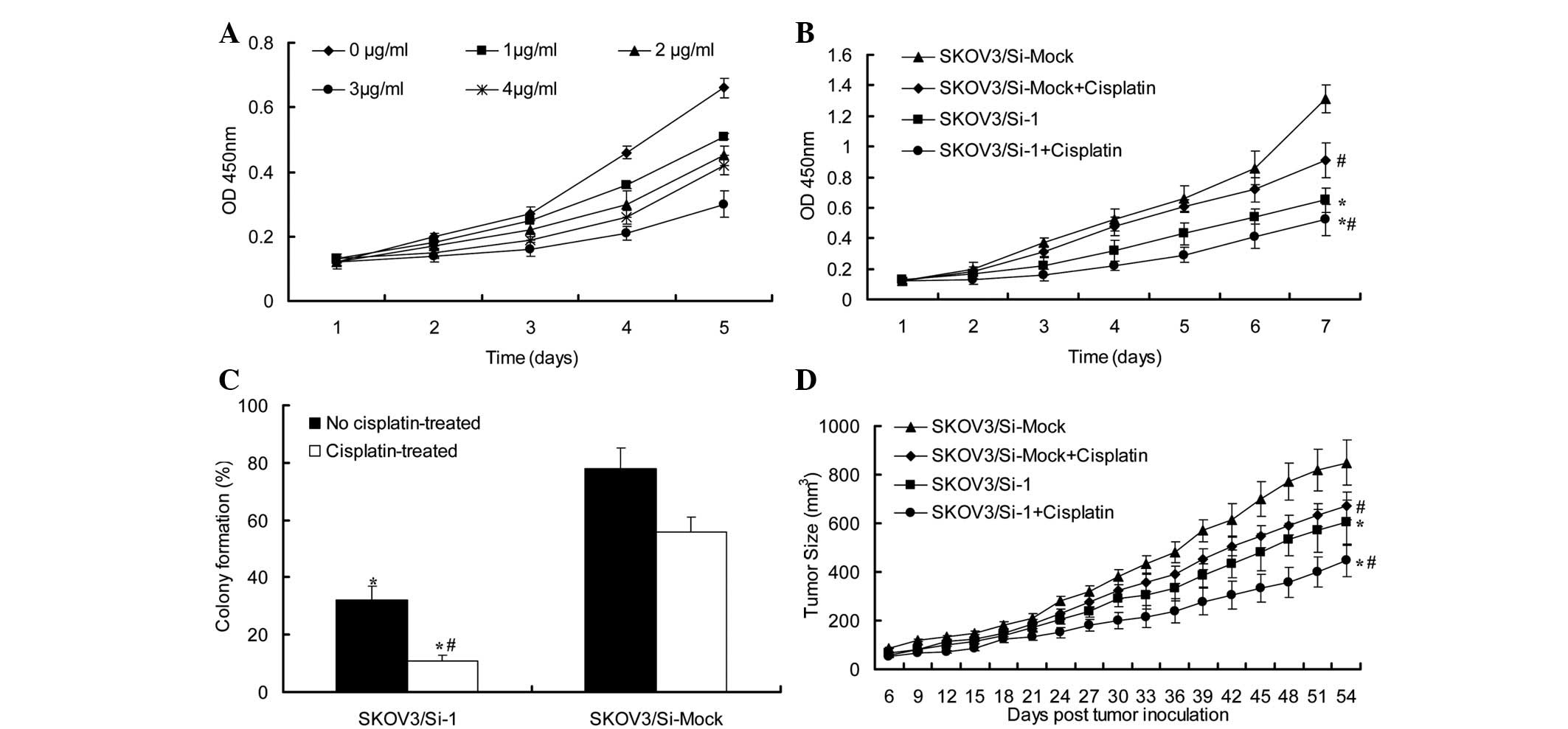 | Figure 3PRDX3-siRNA enhanced cisplatin-induced
ovarian cancer cell apoptosis. (A) Effect of cisplatin on SKOV3
ovarian cancer cells. Under the stimulation of various
concentration of cisplatin (0, 1, 2, 3 and 4 μg/ml) for 1, 2, 3, 4
and 5 days, respectively, the absorbance of SKOV3 cells was
observed by CCK-8 assay. (B) Growth of SKOV3/Si-Mock and SKOV3/Si-1
cells with/without treatment of 3 μg/ml cisplatin was examined by
CCK-8 assay. (C) Growth of SKOV3/Si-Mock and SKOV3/Si-1 cells
with/without treatment of 3 μg/ml cisplatin was examined by colony
formation assay. * vs. SKOV3/Si-Mock group, P<0.05,
# vs. No cisplatin-treated group, P<0.05. (D)
Graphical representation of the tumor size over time in mice
injected with SKOV3/Si-Mock or SKOV3/Si-1 cells with/without
treatment of 3 μg/ml cisplatin (n=8). CCK-8, Cell-Counting kit; si,
small interfering. |
To study whether the PRDX3-siRNA was able to enhance
cisplatin-induced ovarian cancer cell apoptosis, the SKOV3/Si-1 and
SKOV3/Si-Mock cells were treated with or without 3 μg/ml cisplatin.
The cells were then collected and analyzed by cell proliferation
(Fig. 3B) and colony formation
assays (Fig. 3C). These assays
revealed a marked reduction in the cisplatin-treated SKOV3/Si-1
cells compared with the cisplatin-treated SKOV3/Si-Mock cells for
which no knockdown effect was observed. The tumor size of the nude
mice injected with the cisplatin-treated SKOV3/Si-1 cells was
significantly smaller than that of the mice injected with the
cisplatin-treated SKOV3/Si-Mock cells (Fig. 3D). This was associated with the
enhanced induction of apoptosis, as shown by Fig. 4A.
Cisplatin-induced apoptosis may be
enhanced by PRDX3- siRNA via the activation of the caspase,
apoptosis- related proteins
The NF-κB-mediated expression of Bcl-2 and Bcl-XL is
known to protect cancer cells from apoptosis, whereas Bax,
caspase-3 and caspase-9 inhibit cancer cell growth and induce
apoptosis. To validate whether PRDX3-siRNA was able to enhance
cisplatin-induced apoptosis in the ovarian cancer cells via the
activation of the caspase and apoptosis-related proteins, the
expression of these proteins was investigated. The pro-apoptosis
proteins Bax, caspase-3 and caspase-9 were analyzed in the
SKOV3/Si-1 and SKOV3/Si-Mock cells treated with or without
cisplatin (3 μg/ml). The caspase-3 protein was activated and the
Bax expression was increased in the SKOV3/Si-1 or SKOV3/Si-1 cells
treated with cisplatin, but not in the SKOV3/Si-Mock cells treated
with or without cisplatin. Additionally, the activation of the
caspase-9 protein was observed in the SKOV3/Si-1 or SKOV3/Si-1
cells treated with cisplatin and the partial activation of
caspase-9 was observed in the cisplatin-treated SKOV3/Si-Mock
cells, but not in the SKOV3/Si-Mock cells.
Elevated levels of the anti-apoptotic proteins Bcl-2
and Bcl-XL confer chemoresistance, therefore the participation of
Bcl-2 and Bcl-XL was examined by a western blot analysis. As shown
in Fig. 4B, the PRDX3-siRNA was
able to markedly downregulate the expression of the Bcl-2 and
Bcl-XL proteins in the cisplatin-treated SKOV3/Si-1 cells compared
with the SKOV3/Si-Mock cells treated with/without cisplatin. The
phosphorylation of NF-κB p65 was examined and the results showed a
significant inhibition in the cisplatin-treated SKOV3/Si-1
cells.
Discussion
Ovarian cancer remains a leading gynecological
malignancy across the world. As it possesses the highest mortality
rate among the gynecological cancers, ovarian cancer has become the
most lethal malignancy of the female reproductive system (2). To extend the long-term survival rate,
certain improvements have been made, including the modulation of
cellular chemosensitivity, the reversal of tumor resistance and the
increase in the therapeutic effects of chemotherapy. However, due
to the dose-dependent toxicity, eventual tumor recurrence and
emergence of drug-resistance in the disease, the therapeutic
efficacy remains poor (21).
Mounting evidence suggests that cancer cells exhibit increased
intrinsic ROS. In order to protect themselves against oxidative
stress induced by the ROS, cells use several antioxidants or
reductants to maintain the intracellular redox environment in a
highly reduced state. The upregulation of antioxidant enzymes,
particularly in the mitochondria, would provide cancer cells with
certain survival advantages under intrinsically high oxidative
conditions (22). As a significant
cellular antioxidant, PRDX3 may regulate the physiological levels
of H2O2, thus protecting cells against the
apoptosis caused by high levels of H2O2.
Increased mitochondrial ROS generation and the disturbance of prx
production in cancer cells may lead to oxidative stress and hypoxic
microenvironments, subsequently leading to the induction of
apoptosis (17). Increased
expression levels of PRDX3 were therefore assumed to protect tumor
cells against the hypoxia microenvironment and drug-induced
H2O2-dependent apoptosis.
To the best of our knowledge, several studies have
shown that the PRDX3 protein is overexpressed in numerous types of
cancer, including lung, breast and prostate cancer and
hepatocellular carcinoma (16–18),
but few studies have reported the correlation between the
overexpression of PRDX3 and the progress of ovarian cancer. The
present study examined the association between the expression of
PRDX3 and the clinicopathological parameters in various types of
ovarian cancers. The results showed that the high expression of
PRDX3 in serous ovarian cancer was associated with
poorly-differentiated cancer cells. Moreover, FIGO stage
IV/metastatic serous ovarian cancer samples were shown to
overexpress PRDX3 at the highest level, which suggested that the
PRDX3 expression was significantly associated with increasing
cancer progression. A similar tendency was evident in the mucinous,
endometrioid and clear cell types of ovarian cancer, but the
differences between stages I + II and III + IV, among well-,
moderately- and poorly-differentiated ovarian cancer were not
significant. This result should be further verified due to the
limited number of cases. The most common type of ovarian cancer is
serous, therefore, inhibiting the mitochondrial antioxidant enzyme
PRDX3 in the ovarian cancer cells may act as an effective cancer
therapeutic method.
Cisplatin has been shown to induce apoptosis in
various types of cancer cells. Despite the great efficacy at
treating these certain types of cancer, the side-effects or
resistance to cisplatin undermine its curative potential (23). Based on the the previously stated
factors, we assumed that downregulation of the PRDX3 protein would
enhance cisplatin-induced ovarian cancer cell apoptosis. Therefore,
ovarian cancer cells stably transfected with PRDX3-siRNA were first
established by the RNAi approach, in which the target gene was
efficiently knocked down. In the present study, the SKOV3 and
OVCAR-3 cell lines were stably transfected with PRDX3-siRNA
simultaneously, but the transfection efficiency of OVCAR-3 was not
high. This may have been affected by the dose or toxicity of the
transfection reagent or by the transfection time. The gene-silenced
clones of the SKOV3 cells transfected with the PRDX3-siRNA were
screened by a limited dilution method and verified by western blot
analysis (Fig. 1B). Functional
analysis using the siRNA of PRDX3 strongly supported the
involvement of PRDX3 in the development and progression of ovarian
cancer. As shown in Fig. 2, the
proliferation rate of the SKOV3 cells following PRDX3 knockdown was
reduced significantly, whereas the proliferation of the
SKOV3/Si-Mock cells was not inhibited. To further explore whether
the silencing of PRDX3 reversed the resistance of the ovarian
cancer cells to cisplatin, a series of experiments were designed.
The results showed that when comparing the proliferative activity
of the SKOV3/Si-Mock cells treated with cisplatin and the
SKOV3/Si-1 cells, the proliferation of the SKOV3/Si-1 cells treated
with cisplatin significantly decreased (P<0.05). The enhanced
antitumor efficacy in vivo was associated with the enhanced
induction of apoptosis, as verified by the TUNEL analysis (Fig. 4). All the data suggested that the
combination of the PRDX3-siRNA and cisplatin showed a synergistic
anti-cancer effect.
PRDX3 is one of the most significant antioxidant
enzymes that modulates intracellular signaling pathways correlated
to apoptosis and cell proliferation. Understanding the anticancer
mechanism of PRDX3, with the goal of enhancing its efficacy as a
valuable adjunct or single agent in anticancer therapy, is
extremely important. NF-κB has been implicated in carcinogenesis
and the development of drug resistance in cancer cells (24). A previous study indicated that
NF-κB was the most reliable target of the combination
chemotherapeutic treatment to overcome drug resistance (25). It is well-known that the
NF-κB-mediated expression of Bcl-2 and Bcl-XL protects cancer cells
from apoptosis, whereas Bax, caspase-3, and caspase-9 inhibit
cancer cell growth and induce apoptosis. Therefore, whether
PRDX3-siRNA in combination with cisplatin is able to disrupt the
NF-κB pathway or not is extremely significant. The present study
showed that a significant inhibition of the phosphorylation of
NF-κB p65 and a decrease in the levels of the anti-apoptotic
proteins, Bcl-2 and Bcl-XL, were induced in the cisplatin-treated
SKOV3/Si-1 cells. By contrast, an increase in the level of Bax and
the activation of caspase-3 and caspase-9 were observed in the
cisplatin-treated SKOV3/Si-1 cells.
In conclusion, these results suggest that silencing
the expression of PRDX3 may enhance cisplatin-induced ovarian
cancer cell apoptosis through the suppression of the NF-κB
signaling pathway. This provides new evidence for the potential
application of PRDX3-siRNA as a chemosensitizer in ovarian cancer
therapy.
Acknowledgements
The authors would like to thank Junbo Hu for his
pathological assistance and technical support.
References
|
1
|
Wolf JK and Jenkins AD: Gene therapy for
ovarian cancer (Review). Int J Oncol. 21:461–468. 2002.PubMed/NCBI
|
|
2
|
Felix AS, Stone RA, Bowser R, Chivukula M,
Edwards RP, Weissfeld JL and Linkov F: Comparison of survival
outcomes between patients with malignant mixed mullerian tumors and
high-grade endometrioid, clear cell, and papillary serous
endometrial cancers. Int J Gynecol Cancer. 21:877–884. 2011.
View Article : Google Scholar
|
|
3
|
Lalwani N, Prasad SR, Vikram R, Shanbhogue
AK, Huettner PC and Fasih N: Histologic, molecular, and cytogenetic
features of ovarian cancers: implications for diagnosis and
treatment. Radiographics. 31:625–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ledermann J, Harter P, Gourley C, et al:
Olaparib maintenance therapy in platinum-sensitive relapsed ovarian
cancer. N Engl J Med. 366:1382–1392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stańczyk M, Gromadzińska J and Wasowicz W:
Roles of reactive oxygen species and selected antioxidants in
regulation of cellular metabolism. Int J Occup Med Environ Health.
18:15–26. 2005.PubMed/NCBI
|
|
6
|
Okai Y, Sato EF, Higashi-Okai K and Inoue
M: Potentiating effect of an endocrine disruptor, paranonylphenol,
on the generation of reactive oxygen species (ROS) in human venous
blood - association with the activation of signal transduction
pathway. J UOEH. 29:221–233. 2007.
|
|
7
|
Moon HJ, Ko WK, Han SW, Kim DS, Hwang YS,
Park HK and Kwon IK: Antioxidants, like coenzyme Q10, selenite, and
curcumin, inhibited osteoclast differentiation by suppressing
reactive oxygen species generation. Biochem Biophys Res Commun.
418:247–253. 2012. View Article : Google Scholar
|
|
8
|
Avni R, Cohen B and Neeman M: Hypoxic
stress and cancer: imaging the axis of evil in tumor metastasis.
NMR Biomed. 24:569–581. 2011.PubMed/NCBI
|
|
9
|
Morita M, Yano S, Yamaguchi T, Yamauchi M
and Sugimoto T: Phenylacetic acid stimulates reactive oxygen
species generation and tumor necrosis factor-α secretion in
vascular endothelial cells. Ther Apher Dial. 15:147–150.
2011.PubMed/NCBI
|
|
10
|
Pak JH, Choi WH, Lee HM, et al:
Peroxiredoxin 6 overexpression attenuates cisplatin-induced
apoptosis in human ovarian cancer cells. Cancer Invest. 29:21–28.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lowther WT and Haynes AC: Reduction of
cysteine sulfinic acid in eukaryotic, typical 2-Cys peroxiredoxins
by sulfiredoxin. Antioxid Redox Signal. 15:99–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aran M, Ferrero DS, Pagano E and Wolosiuk
RA: Typical 2-Cys peroxiredoxins - modulation by covalent
transformations and noncovalent interactions. FEBS J.
276:2478–2493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rhee SG and Woo HA: Multiple functions of
peroxiredoxins: peroxidases, sensors and regulators of the
intracellular messenger H2O2, and protein
chaperones. Antioxid Redox Signal. 15:781–794. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin H, Lu JP, Laflamme P, et al:
Inter-related in vitro effects of androgens, fatty acids and
oxidative stress in prostate cancer: A mechanistic model supporting
prevention strategies. Int J Oncol. 37:761–766. 2010.
|
|
15
|
Flohé L, Budde H and Hofmann B:
Peroxiredoxins in antioxidant defense and redox regulation.
Biofactors. 19:3–10. 2003.
|
|
16
|
Qiao B, Wang J, Xie J, Niu Y, Ye S, Wan Q
and Ye Q: Detection and identification of peroxiredoxin 3 as a
biomarker in hepatocellular carcinoma by a proteomic approach. Int
J Mol Med. 29:832–840. 2012.PubMed/NCBI
|
|
17
|
Kim YS, Lee HL, Lee KB, et al: Nuclear
factor E2-related factor 2 dependent overexpression of sulfiredoxin
and peroxiredoxin III in human lung cancer. Korean J Intern Med.
26:304–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Basu A, Banerjee H, Rojas H, et al:
Differential expression of peroxiredoxins in prostate cancer:
consistent upregulation of PRDX3 and PRDX4. Prostate. 71:755–765.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai Z, Yin J, He H, et al: Mitochondrial
comparative proteomics of human ovarian cancer cells and their
platinum-resistant sublines. Proteomics. 10:3789–3799. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee S, Kwon H, Jeong K and Pak Y:
Regulation of cancer cell proliferation by caveolin-2
down-regulation and re-expression. Int J Oncol. 38:1395–1402.
2011.PubMed/NCBI
|
|
21
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar
|
|
22
|
Bookman MA: Developmental chemotherapy and
management of recurrent ovarian cancer. J Clin Oncol. 21(10 Suppl):
149s–167s. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Young TW, Mei FC, Yang G, Thompson-Lanza
JA, Liu J and Cheng X: Activation of antioxidant pathways in
ras-mediated oncogenic transformation of human surface ovarian
epithelial cells revealed by functional proteomics and mass
spectrometry. Cancer Res. 64:4577–4584. 2004. View Article : Google Scholar
|
|
24
|
Oiso S, Ikeda R, Nakamura K, Takeda Y,
Akiyama S and Kariyazono H: Involvement of NF-κB activation in the
cisplatin resistance of human epidermoid carcinoma KCP-4 cells.
Oncol Rep. 28:27–32. 2012.
|
|
25
|
Sethi G, Sung B and Aggarwal BB: Nuclear
factor-kappaB activation: from bench to bedside. Exp Biol Med
(Maywood). 233:21–31. 2008. View Article : Google Scholar : PubMed/NCBI
|


















