Spandidos Publications style
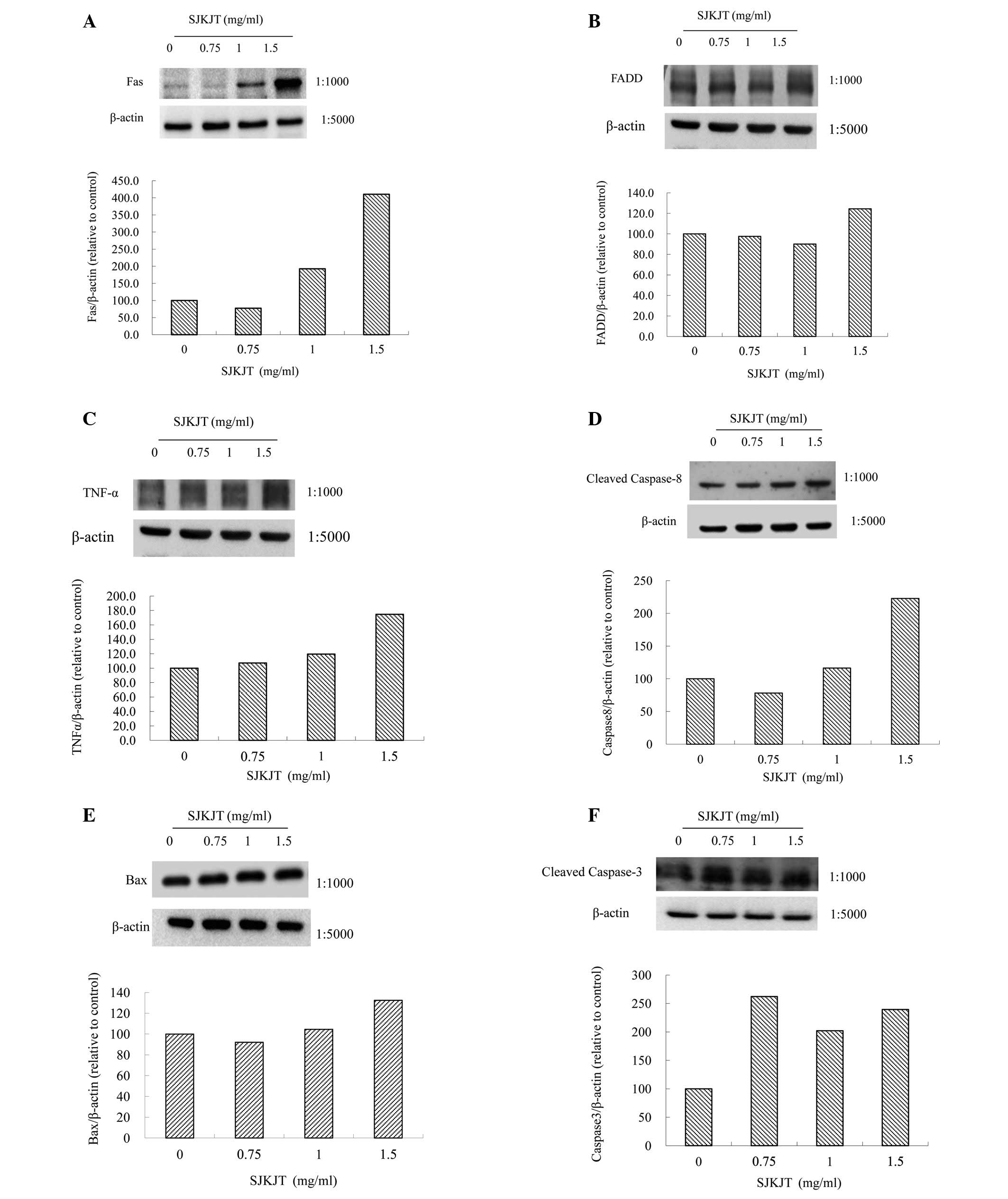
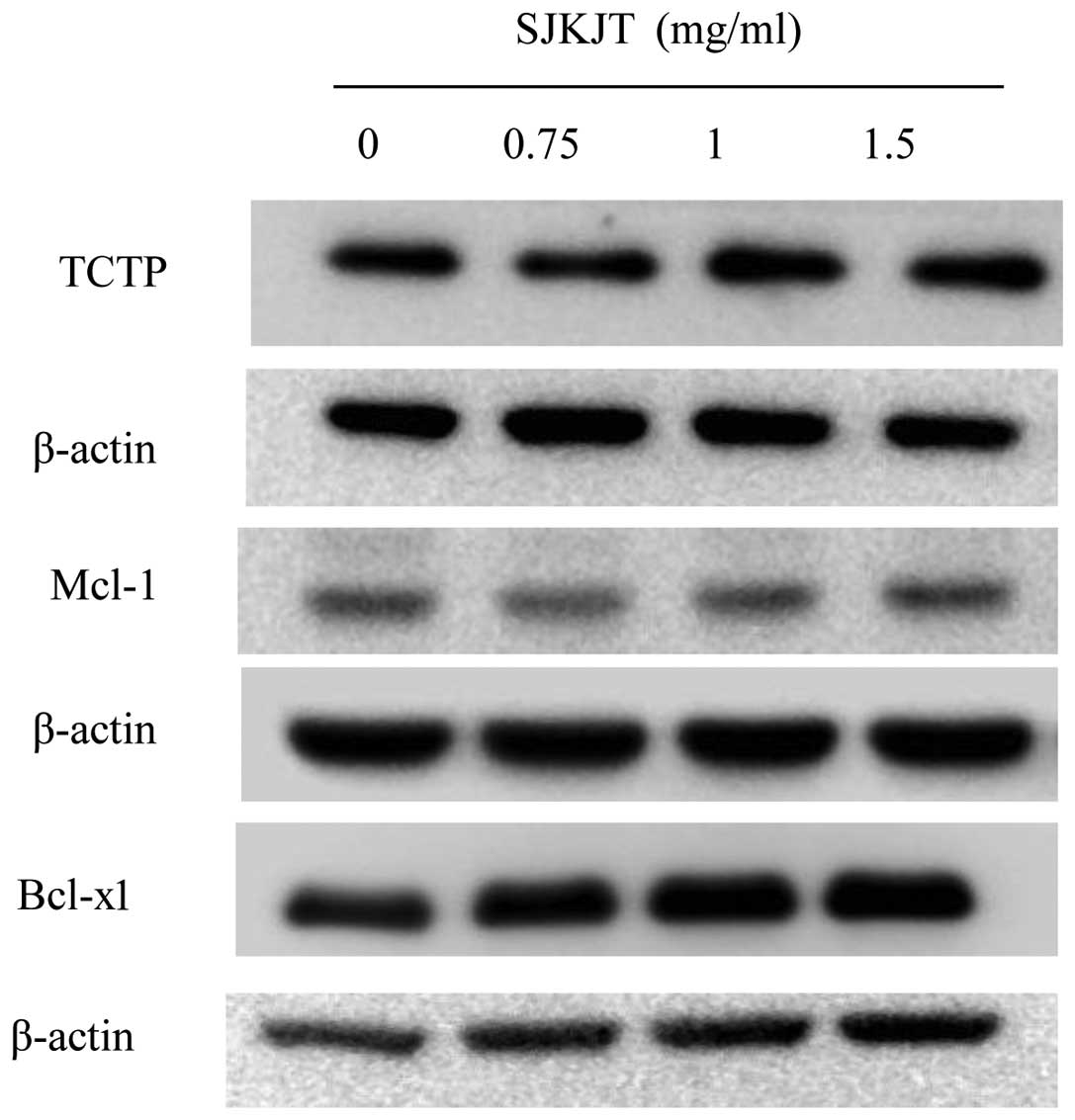
Chen Y, Yan M, Chien S, Kuo S, Chen DR, Cheng C and Su C: Sann-Joong-Kuey-Jian-Tang inhibits hepatocellular carcinoma Hep-G2 cell proliferation by increasing TNF-α, Caspase-8, Caspase- 3 and Bax but by decreasing TCTP and Mcl-1 expression in vitro. Mol Med Rep 7: 1487-1493, 2013.
APA
Chen, Y., Yan, M., Chien, S., Kuo, S., Chen, D., Cheng, C., & Su, C. (2013). Sann-Joong-Kuey-Jian-Tang inhibits hepatocellular carcinoma Hep-G2 cell proliferation by increasing TNF-α, Caspase-8, Caspase- 3 and Bax but by decreasing TCTP and Mcl-1 expression in vitro. Molecular Medicine Reports, 7, 1487-1493. https://doi.org/10.3892/mmr.2013.1381
MLA
Chen, Y., Yan, M., Chien, S., Kuo, S., Chen, D., Cheng, C., Su, C."Sann-Joong-Kuey-Jian-Tang inhibits hepatocellular carcinoma Hep-G2 cell proliferation by increasing TNF-α, Caspase-8, Caspase- 3 and Bax but by decreasing TCTP and Mcl-1 expression in vitro". Molecular Medicine Reports 7.5 (2013): 1487-1493.
Chicago
Chen, Y., Yan, M., Chien, S., Kuo, S., Chen, D., Cheng, C., Su, C."Sann-Joong-Kuey-Jian-Tang inhibits hepatocellular carcinoma Hep-G2 cell proliferation by increasing TNF-α, Caspase-8, Caspase- 3 and Bax but by decreasing TCTP and Mcl-1 expression in vitro". Molecular Medicine Reports 7, no. 5 (2013): 1487-1493. https://doi.org/10.3892/mmr.2013.1381