Introduction
Gastric carcinoma is the third most common
malignancy in China (1) and the
second most common cause of cancer mortality, with 934,000 newly
diagnosed cases and ~700,000 mortalities annually worldwide
(2). Owing to its vague and
non-specific symptoms, gastric cancer is often diagnosed at
advanced stages, with a 5-year survival rate of <30%. In
addition, gastric carcinoma is associated with a high risk of
recurrence even following radical surgery (3). The standard treatment for advanced or
relapsed gastric cancer is chemotherapy. However, the development
of multidrug resistance (MDR) in gastric cancer is a substantial
obstacle for chemotherapy. MDR is considered to be the main cause
of failure in cancer chemotherapy and the mechanism behind it
remains unclear.
Accumulating evidence has suggested that microRNAs
(miRNAs) are key players in MDR. The miRNAs are a large family of
small non-coding RNAs of 19–25 nucleotides that negatively control
gene expression at the mRNA and protein level. Microarray studies
have revealed that individual miRNAs affect the expression of
multiple genes, indicating that miRNAs have pleiotropic effects in
crucial biological processes, including development,
differentiation and apoptosis (4,5).
Since a number of biological processes are relevant to
chemosensitivity and chemoresistance, miRNA is thought to be
associated with drug resistance in cancer, as demonstrated in
several previous studies (6–8). In
addition, treatment with antineoplastic agents alters the
expression of miRNAs in vitro and in vivo(9,10).
However, few studies on MDR-related miRNAs in gastric carcinoma
have been performed.
The aim of the present study was to understand the
molecular mechanism of MDR in human gastric cancer cells following
exposure to 5-fluorouracil (5-Fu). A 5-Fu-induced MDR gastric
cancer cell line was developed and microarray analysis was
performed to directly characterize the molecular basis of MDR.
Following this, the miRNA expression profiles between the MDR and
the parental cell lines were compared.
Materials and methods
Cell lines and cell culture
The human gastric cancer cell line, SGC-790, was
purchased from the Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences (Shanghai, China). SGC-7901 was
maintained in RPMI-1640 medium (Hyclone, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Hyclone). The cells were
removed from the surface of the culture vessels by trypsinization
and then washed with phosphate-buffered saline (PBS). Aliquots (0.5
ml) of the cell suspension were added to 4.5 ml fresh medium in
30-cm2 flasks. This study was approved by the ethics
committee of Huashan Hospital of Fudan University, Shanghai,
China.
Establishment of the drug-resistant cell
line, SGC-7901/5-Fu, in vitro
The resistant cell line was produced by continuous
exposure to 5-Fu (Sigma, St. Louis, MO, USA) of low and gradually
increasing concentrations. When the cells were growing
exponentially, 5 μg/ml 5-Fu was added to the medium. Following 48 h
of incubation, the majority of the cells were dead. The treated
cells were then washed with PBS and cultured in 5-Fu free growth
medium. Following 2–3 days, the dead cells were washed away with
PBS and fresh medium was added. The resistant sub-clones were
isolated by limiting dilution. The cells were passaged at 80%
confluency and then 5μg/ml 5-Fu was added. The concentration of
5-Fu was gradually increased following stable cell growth. Finally,
a cell line resistant to 40 μg/ml 5-Fu was derived from the
SGC-7901 cell line. Prior to further experimentation, these cells
were maintained in a 5-Fu-free culture medium for 1 month and
subcultured at least 3 times.
In vitro drug sensitivity assay
Cisplatin (CDDP), vincristine (VCR) and adriamycin
(ADM) were all purchased from Sigma. The 5-Fu, CDDP, VCR and ADM
were all freshly prepared prior to each experiment. The drug
sensitivity was evaluated by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay. Following this, the cells were cultured in
96-well microculture plates in the presence or absence of various
concentrations of the drugs for 48 h. A 0.05 ml MTT solution (0.1%)
in PBS was added to each well followed by incubation for an
additional 4 h. The medium was removed and the purple formazan
product in the cells was measured at a wavelength of 540 nm and the
number of viable cells was calculated. Measurements were performed
in triplicate and dose-response curves were plotted using data
derived from the MTT assay. The half maximal inhibitory
concentration (IC50) of each anticancer drug was
calculated from this standard curve.
Western blot analysis
Protein extracts of the SGC-7901 and SGC-7901/5-Fu
cells were resolved by 12% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene
difluoride (PVDF) membranes (Millipore, Billerica, MA, USA).
Subsequent to blocking, the PVDF membranes were washed three times
for 15 min with Tris-Buffered Saline with Tween-20 (TBST) at room
temperature and incubated with mouse anti-human P-glycoprotein
(eBioscience, San Diego, CA, USA). P-glycoprotein is also known as
multidrug resistance protein 1 (MDR1). Following extensive washing,
the membranes were incubated with horseradish peroxidase-linked
goat anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA)
for 1 h. Following an additional TBST wash, the immunoreactivity
was visualized using an electrogenerated chemiluminescence kit
(Pierce Biotechnology, Rockford, IL, USA) according to the
manufacturer’s instructions.
miRNA microarray
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) as described
previously (11). RNA labeling and
hybridization to the miRNA microarray chips were performed
according to the manufacturer’s instructions. Briefly, 2.5 μg RNA
from each sample was labeled with the miRNA Universal Linkage
System (ULS)™ Labeling kit (Kreatech Diagnostics,
Amsterdam, Netherlands). Hybridization was performed on the Human
miRNA OneArray® v3 (Phalanx Biotech Group, Belmont, CA,
USA), which contains 1,711 human and 189 experimental control
probes. There were three spot replicates for each probe.
Hybridization images were captured using a GenePix 4000B laser
scanner (Molecular Devices, Sunnyvale, CA, USA) and the data was
analyzed by GenePix Pro 6.0 software (Molecular Devices).
Quantitative real-time RT-PCR of miRNA
and mRNA
The expression of the miRNAs and the drug resistance
gene, MDR1, was assessed using quantitative real-time RT-PCR as
described previously (12). cDNA
synthesis was performed using the miScript Reverse Transcription
kit (Qiagen, Hilden, Germany) according to the manufacturer’s
instructions. Quantitative PCR was performed using the miScript
SYBR Green PCR kit (Qiagen, Hilden, Germany). An expression
analysis was performed in triplicate for each sample. The small
nuclear RNA, U6, was used as the normalization control. The miRNA
and mRNA expression levels were quantified using the ABI 7300
Sequence Detection System (Applied Biosystems, Foster City, CA,
USA). Three independent experiments were performed in
triplicate.
Statistical analysis
All data are presented as the mean ± SD. Statistical
analyses were performed using Stata 8.0 software (Stata, College
Station, TX, USA). The comparison of the IC50 and the
miRNA and MDR1 expression between the MDR and parental cell lines
were tested by the Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference. Data was
normalized and the differentially expressed miRNAs were processed
using GenePix Pro 6.0 software.
Results
Establishment of a 5-Fu-resistant
SGC-7901/5-Fu cell line
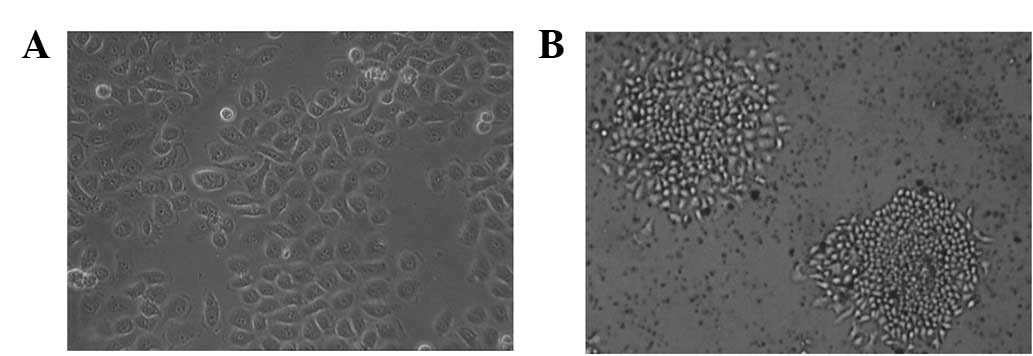
The 5-Fu-resistant SGC-7901 (SGC-7901/5-Fu) cell
line was established by pulse exposure of the SGC-7901 cells to
gradually increasing concentrations of 5-Fu. This led to a marked
alteration in cellular morphology. The parental cells (Fig. 1A) appeared as small, round or
polygonal cells forming a ‘cobblestone’ arrangement, with a
doubling time of 16.4 h. The cells revealed little contact
inhibition and tended to grow in piled-up clusters. Following
acquisition of 5-Fu resistance, the doubling time of the
SGC-7901/5-Fu cells (Fig. 1B) was
slightly longer at 22.1 h and the cells exhibited notable
morphological changes characterized by transition from an
epithelial cobblestone to an elongated fibroblastic phenotype. The
resistant cells became smaller in size and tended to form
well-separated individual colonies.
The sensitivity of the SGC-7901 and SGC-7901/5-Fu
cells to various concentrations of 5-Fu was determined by MTT
assay. As demonstrated in Table I,
the IC50 values for 5-Fu in the SGC-7901 and
SGC-7901/5-Fu cells were 5.0±0.02 and 155.0±3.1 μg/ml,
respectively. The SGC-7901/5-Fu cells were 31-fold more resistant
to 5-Fu than the parent cells. In addition, the cross-resistance to
other anticancer drugs (CDDP, VCR, ADM) was compared between the
parent and 5-Fu-resistant cells and the results indicated that the
SGC-7901/5-Fu cells also had cross-resistance to CDDP, VCR and
ADM.
 | Table IIC50 and RI values of the
SGC-7901 and SGC-7901/5-Fu cells. |
Table I
IC50 and RI values of the
SGC-7901 and SGC-7901/5-Fu cells.
| IC50, mean
± SD | |
|---|
|
| |
|---|
| Drug (μg/ml) | SGC-7901 | SGC-7901/5-Fu | RI |
|---|
| 5-Fu | 5.0±0.02 | 155.0±3.1a | 31.0 |
| CDDP | 3.6±0.12 | 10.7±0.31a | 2.97 |
| VCR | 1.3±0.11 | 3.3±0.12a | 2.54 |
| ADM | 0.57±0.06 | 3.53±0.15a | 6.19 |
Western blot analysis of P-glycoprotein
expression
The P-glycoprotein expression in the SGC-7901/5-Fu
cells was evaluated by western blot analysis. The protein was
detected in the parental SGC-7901 and SGC-7901/5-Fu cells. The
expression levels of the P-glycoprotein were significantly higher
in the SGC-7901/5-Fu-resistant cells than in the SGC-7901 cells
(Fig. 2). Confirmation of the
maintenance of P-glycoprotein expression and its functionality were
important for the culture conditions.
Evaluation of MDR1 mRNA by quantitative
real-time RT-PCR
The expression of the MDR1 mRNA was examined by
quantitative real-time RT-PCR and the results are presented in
Fig. 3. The 5-Fu-resistant cells
demonstrated increased levels of MDR1 mRNA expression when compared
with the SGC-7901 cells (P<0.05).
miRNA expression profiling in the
SGC-7901 vs. SGC-7901/5-Fu cell lines
To investigate the role of miRNAs in the MDR of
human gastric cancer cells following exposure to 5-Fu,
comprehensive miRNA expression profiling of SGC-7901 and
SGC-7901/5-Fu cells was conducted using the Human miRNA
OneArray® v3. The results revealed that 18 miRNAs were
downregulated >2-fold in the SGC-7901/5-Fu cells compared with
the SGC-7901 cells, while 9 miRNAs were upregulated >2-fold in
the SGC-7901/5-Fu cells (Table
II). These results indicate that these 27 miRNAs may play
significant roles in the development of MDR when treating human
gastric cancer cells with 5-Fu. In addition, a hierarchical cluster
analysis based on the expression patterns of these 27 miRNAs
accurately separated the SGC-7901/5-Fu cells from the SGC-7901
cells (Fig. 4).
 | Table IImiRNAs correlated with multidrug
resistance of the SGC-7901/5-Fu cell line. |
Table II
miRNAs correlated with multidrug
resistance of the SGC-7901/5-Fu cell line.
| miRNA | Regulation in
SGC-7901/5-Fu | Chromosomal
location |
|---|
| miR-10b | Up | 2 |
| miR-22 | Up | 17 |
| miR-31 | Up | 9 |
| miR-32 | Down | 9 |
| miR-133b | Up | 6 |
| miR-190 | Up | 15 |
| miR-197 | Down | 1 |
| miR-210 | Down | 11 |
| miR-486-3p | Down | 14 |
| miR-501 | Up | X |
| miR-532 | Down | X |
| miR-615 | Up | 12 |
| miR-501-5p | Up | X |
| miR-615-5p | Up | 12 |
| miR-766 | Down | X |
| miR-877 | Down | 6 |
| miR-1224-3p | Down | 3 |
| miR-1229 | Down | 5 |
| miR-1238 | Down | 19 |
| miR-1273d | Down | 15 |
| miR-3131 | Down | 2 |
| miR-3149 | Down | 8 |
| miR-3162-3p | Down | 11 |
| miR-4701-5p | Down | 12 |
| miR-4728-3p | Down | 17 |
| miR-4763-3p | Down | 22 |
| miR-5096 | Down | 4 |
Validation of microarray data by
real-time RT-PCR
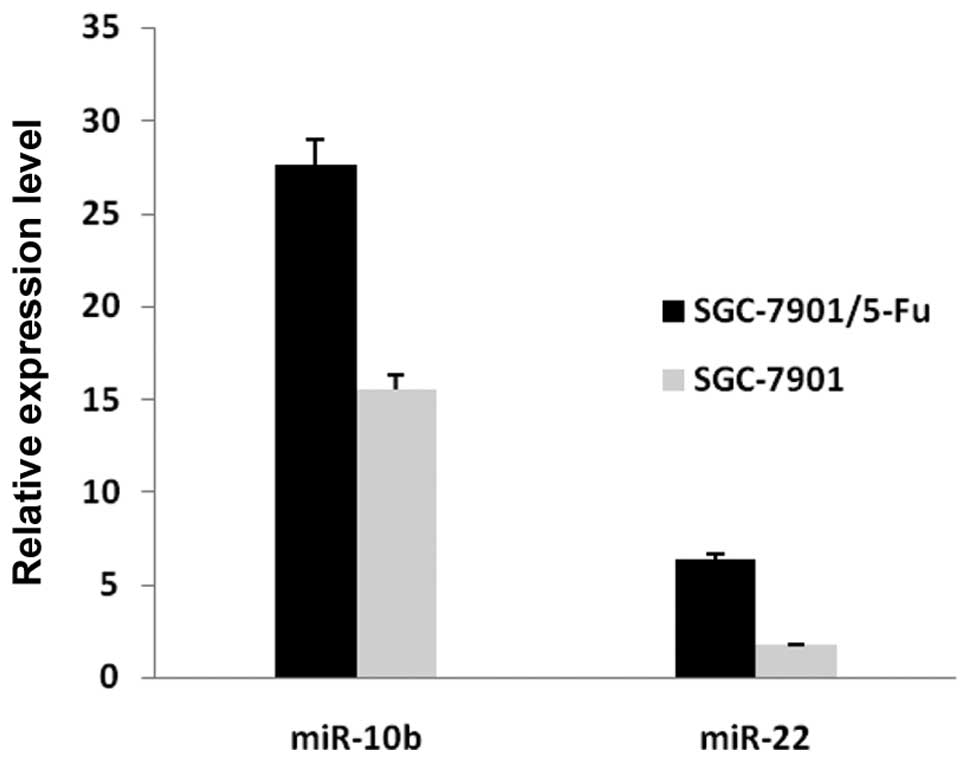
To confirm the results of the miRNA microarray
analysis, miR-10b and miR-22 were selected for analysis by
real-time quantitative RT-PCR. The real-time RT-PCR results were
consistent with the miRNA microarray analysis results (Fig. 5).
Discussion
In the present study, the MDR gastric cell line took
8 months to be successfully established. In addition, the primary
colonies were dissociated into single cells and then cultured. A
single cell formed a secondary colony. The secondary colonies
generated tertiary colonies, indicating that the colony-forming
cells isolated from the existing colonies retained the same
colony-forming potential. These observations indicated that the
resistant cells had a self-renewal capacity resembling that of
cancer stem cells; this was reported for the first time in the
present study.
MDR is defined as the resistance of tumor cells to a
variety of structurally dissimilar and functionally divergent
chemotherapeutic agents. MDR mediates the process of drug
inactivation and removal from the target tumor cells (13) and is a major obstacle for the
treatment of cancer. Although the mechanism of MDR remains unclear,
it has been hypothesized that MDR is a multifactorial process which
includes the following four events: i) the altered expression of
membrane transport proteins to increase drug efflux, ii) enhanced
DNA repair, iii) the amendment of cell cycle regulation to block
apoptosis and iv) detoxification through increasing drug metabolism
and decreasing drug activation (14).
The first mechanism to be associated with MDR was
identified in 1988 (15,16). P-glycoprotein, an active, energy
dependent multidrug transporter in the membrane, was identified and
reported to impede intracellular cytotoxic drug accumulation by
rapid extrusion. In the present study, SGC-7901/5-Fu cells were
also identified to overexpress P-glycoprotein and MDR1 mRNA.
However, a previous study reported that in specific
P-glycoprotein-negative cancer cell lines, the resistance to a wide
variety of chemotherapy drugs was observed (17). These data indicate that there may
be additional factors which are important in MDR (18).
miRNAs are vital to a number of biological
processes, including development, proliferation and apoptosis
(19). A single miRNA may target
dozens of mRNAs and the dysregulation of miRNAs affects the
expression of multiple target mRNAs and therefore multiple
proteins, leading to variations in the chemotherapy
sensitivity/resistance to common cancer treatments via various
cellular processes. Previously, a correlation was identified
between miRNA expression and chemoresistance in several types of
cancer. The expression of miR-34a attenuated the chemoresistance to
camptothecin in prostate cancer cells (20). The dysregulation of let-7i was also
reported to be significantly correlated with chemoresistance in
ovarian and breast cancer (21).
In glioblastoma cells, miR-21 has been reported to contribute to
teniposide resistance (22).
However, few studies have determined the potential
role of miRNAs in the chemoresistance of gastric cancer. The
present study is the first to perform miRNA expression profiling in
the MDR human gastric cancer cell line, SGC-7901, following
exposure to 5-Fu. The results indicate that the expression of 9
miRNAs (miR-10b, -22, -31, -133b, -190, -501, -615, -501-5p and
-615-5p) was upregulated, while 18 miRNAs (miR-32, -197, -210,
-766, -1229, -1238, -3131, -3149, -1224-3p, -3162-3p, -532, -877,
-4701-5p, -5096, -4728-3p, -1273d, -486-3p and -4763-3p) were
downregulated in the SGC-7901/5-Fu cell line compared with its
parental cell line. In comparison to previous studies, the miRNA
expression profile in the SGC-7901/VCR cell line was completely
different to that of the SGC-7901/5-Fu cell line (23), indicating that each antineoplastic
agent is able to alter the expression of relevant miRNAs.
The quantitative RT-PCR analysis of miR-10b and
miR-22 confirmed the expression patterns identified by microarray.
Numerous previous studies (24,25)
have found that miR-10b was highly expressed in metastatic breast
cancer cells and that it regulated cell migration and invasion. In
addition, miR-10b has been identified to positively regulate the
metastasis of nasopharyngeal carcinoma (26). Considering the flexible functions
of miR-10b involved in biological processes closely associated with
chemoresistance, including development, apoptosis and
proliferation, the miRNA molecule is believed to be correlated with
drug resistance in cancer. In addition, a previous study reported
that curcumin and piperine, individually and in combination, were
able to inhibit breast cancer stem cell self-renewal by the
upregulation of miR-22, leading to the restoration of the
susceptibility to chemotherapy (27).
In summary, in the present study, 27 differentially
expressed miRNAs were identified between the parental and resistant
gastric cancer cells. The acquisition of MDR by the cells indicated
that miRNAs are key to the anticancer drug response. These
observations are the first to show that resistant cells have a
self-renewal capability and colony-forming potential resembling
that of cancer stem cells in gastric cancer chemotherapy. The
results provide a novel insight into the mechanisms of MDR in
gastric cancer and may be useful for the future development of a
chemosensitizing strategy through the manipulation of miRNA
expression.
Acknowledgements
The present study was supported by the Shanghai
Young Doctor Training Plan and a grant from the Major Research and
Development Project of Innovative Drugs, Ministry of Science and
Technology (no. 2012ZX09303004-001).
References
|
1
|
Zhao P, Dai M, Chen W and Li N: Cancer
trends in China. Jpn J Clin Oncol. 40:281–285. 2010. View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
3
|
de Bree E, Charalampakis V, Melissas J and
Tsiftsis DD: The extent of lymph node dissection for gastric
cancer: a critical appraisal. J Surg Oncol. 102:552–562. 2010.
|
|
4
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng AM, Byrom MW, Shelton J and Ford LP:
Antisense inhibition of human miRNAs and indications for an
involvement of miRNA in cell growth and apoptosis. Nucleic Acids
Res. 33:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blower PE, Chung JH, Verducci JS, et al:
MicroRNAs modulate the chemosensitivity of tumor cells. Mol Cancer
Ther. 7:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng T, Wang J, Chen X and Liu L: Role of
microRNA in anticancer drug resistance. Int J Cancer. 126:2–10.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarkar FH, Li Y, Wang Z, Kong D and Ali S:
Implication of microRNAs in drug resistance for designing novel
cancer therapy. Drug Resist Updat. 13:57–66. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rossi L, Bonmassar E and Faraoni I:
Modification of miR gene expression pattern in human colon cancer
cells following exposure to 5-fluorouracil in vitro. Pharmacol Res.
56:248–253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakajima G, Hayashi K, Xi Y, et al:
Non-coding microRNAs hsa-let-7g and hsa-miR-181b are associated
with chemoresponse to S-1 in colon cancer. Cancer Genomics
Proteomics. 3:317–324. 2006.PubMed/NCBI
|
|
11
|
Rosenfeld N, Aharonov R, Meiri E, et al:
MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol.
26:462–469. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmittgen TD, Lee EJ, Jiang J, et al:
Real-time PCR quantification of precursor and mature microRNA.
Methods. 44:31–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luqmani YA: Mechanisms of drug resistance
in cancer chemotherapy. Med Princ Pract. 14(Suppl 1): 35–48. 2005.
View Article : Google Scholar
|
|
14
|
Ganguly A, Banerjee K, Chakraborty P, et
al: Overcoming multidrug resistance (MDR) in cancer in vitro and in
vivo by a quinoline derivative. Biomed Pharmacother. 65:387–394.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gottesman MM and Pastan I: The multidrug
transporter, a double-edged sword. J Biol Chem. 263:1263–1266.
1988.PubMed/NCBI
|
|
16
|
Moscow JA and Cowan KH: Multidrug
resistance. J Natl Cancer Inst. 80:14–20. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watson MB, Lind MJ and Cawkwell L:
Establishment of in-vitro models of chemotherapy resistance.
Anticancer Drugs. 18:749–754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh R and Lillard JW Jr:
Nanoparticle-based targeted drug delivery. Exp Mol Pathol.
86:215–223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hammond SM, Bernstein E, Beach D and
Hannon GJ: An RNA-directed nuclease mediates post-transcriptional
gene silencing in Drosophila cells. Nature. 404:293–296.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujita Y, Kojima K, Hamada N, et al:
Effects of miR-34a on cell growth and chemoresistance in prostate
cancer PC3 cells. Biochem Biophys Res Commun. 377:114–119. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Northcott PA, Fernandez-L A, Hagan JP, et
al: The miR-17/92 polycistron is up-regulated in sonic
hedgehog-driven medulloblastomas and induced by N-myc in sonic
hedgehog-treated cerebellar neural precursors. Cancer Res.
69:3249–3255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Li W, Yang Y, et al: MicroRNA-21
targets LRRFIP1 and contributes to VM-26 resistance in glioblastoma
multiforme. Brain Res. 1286:13–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia L, Zhang D, Du R, et al: miR-15b and
miR-16 modulate multidrug resistance by targeting BCL2 in human
gastric cancer cells. Int J Cancer. 123:372–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gee HE, Camps C, Buffa FM, et al:
MicroRNA-10b and breast cancer metastasis. Nature. 455:E8–E9. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li G, Wu Z, Peng Y, et al: MicroRNA-10b
induced by Epstein-Barr virus-encoded latent membrane protein-1
promotes the metastasis of human nasopharyngeal carcinoma cells.
Cancer Lett. 299:29–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kakarala M, Brenner DE, Korkaya H, et al:
Targeting breast stem cells with the cancer preventive compounds
curcumin and piperine. Breast Cancer Res Treat. 122:777–785. 2010.
View Article : Google Scholar : PubMed/NCBI
|