Introduction
Hepatic fibrosis is a healing response to all causes
of chronic hepatic injury. However, it also leads to numerous
clinically significant problems that are correlated with the
progression of portal hypertension and liver failure (1,2).
Hepatic stellate cells (HSCs) are a key source of
extracellular matrix (ECM). The activation of HSCs is one of the
key steps in the development of liver fibrosis (3,4),
which is a common pathological change characterized by an excessive
deposition of ECM that occurs in the majority of types of chronic
liver diseases. In vitro and in vivo studies have
suggested that HSCs are also involved in the regulation of the
liver microcirculation and the pathogenesis of portal hypertension
(5). HSCs comprise 15% of the
total number of resident liver cells and reside in the space of
Disse in close contact with sinusoidal endothelial cells and
hepatocytes (6). HSCs activated by
liver injury lose retinoids and increase the level of ECM. HSCs
also express α-smooth muscle actin (α-SMA) and possess contractile
abilities (7).
Somatostatin, a significant peptide for inhibiting
cell proliferation and differentiation, is able to retard the
growth of various types of cells by blocking the synthesis and/or
secretion of numerous key cytokines and hormones (8–12).
At a low dose, somatostatin may exert antiproliferative and
proapoptotic activities on activated HSCs. This result may provide
a basis for utilizing somatostatin and potentially its analogue,
octreotide, to protect and treat individuals suffering from hepatic
fibrosis. However, the underlying mechanisms require further
investigation.
The primary objective of the present study was to
evaluate the effects of octreotide on hepatic fibrosis in the
immortalized HSC cell line, LX-2. The mechanism of octreotide in
LX-2 was also investigated.
Materials and methods
Cell culture
The human immortalized HSC line, LX-2, was obtained
from the Shanghai Fuxing Biotechnology Co., China. Cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 g/ml streptomycin, and incubated in a 37°C humidified
atmosphere with 5% CO2. The cultured cells were then
used in the subsequent experiments. The study was approved by the
ethics committee of the Medical School of Shandong University,
Jinan, China.
mRNA expression of specificity protein 1
(sp-1), c-Jun, α-SMA, smad-4, vascular endothelial growth factor
(VEGF) and transforming growth factor β (TGF-β) in cultured
cells
Cultured cells were homogenized in TRIzol
(Invitrogen Life Technologies, Carlsbad, CA, USA) and centrifuged
at 10,000 × g, at 4°C for 15 min, in the presence of chloroform.
The upper aqueous phase was collected, and total RNA was
precipitated by the addition of isopropanol and centrifugation at
7,500 × g at 4°C for 5 min. RNA pellets were washed with 75%
ethanol, dried and reconstituted with sterile water. The
concentration was determined by measuring the absorbance at 490 nm
with a spectrophotometer.
Total RNA was reverse transcribed in a final volume
of 10 μl using an RNA polymerase chain reaction (PCR) kit (Takara
Bio, Inc., Katsushika, Tokyo, Japan). Primers were designed using
Primer 5.0 software and the sequences were as follows: Human c-Jun
forward, 5′-AGGAGGAGCCTC AGACAGTG-3′ and reverse,
5′-TGTTTAAGCTGTGCC ACCTG-3′; α-SMA forward, 5′-AGGGAGTAATGGTTG
GAATGGG-3′ and reverse, 5′-GGAGTACGGTACGCAGA-3′; smad-4 forward,
5′-GGCAGCCATAGTGAAGGACTG-3′ and reverse, 5′-GGC
GGGTGGTGCTGAAGATGG-3′; sp-1 forward, 5′-AAG
AAATGACCTTAGGAACATACCC-3′ and reverse,
5′-CCGTATATGTCTACACACAGATGAC-3′; VEGF forward,
5′-CGGGAACCAGATCTCTCACC-3′ and reverse, 5′-AAAATGGCGAATCCAATTCC-3′;
TGF-β forward, 5′-AAGGCCAAATATCCCAAACA-3′ and reverse, 5′-CCA
ACATTCTCTCATAATTTTAGCC-3′; glyceraldehyde 3-phosphate dehydrogenase
(GAPDH; used as an endogenous standard) forward,
5′-ACATGTTCCAATATGATTCC-3′ and reverse,
5′-TGGACTCCACGACGTACTCAG-3′. Real-time PCR reactions were conducted
using the LightCycler 480 instrument (Roche Molecular Biochemicals,
Mannheim, Germany) and performed according to the manufacturer′s
instructions. Reactions were conducted in a tube with a total
volume of 10 μl, which consisted of 1 μl cDNA, 5 μl SYBR-Green
real-time PCR master mix (Toyobo Co., Ltd., Osaka, Japan) and 1 μl
each primer. Fluorescent signals were captured during each of the
40 cycles (denaturizing for 10 sec at 95°C, annealing for 15 sec at
60°C and extension for 30 sec at 72°C). GAPDH was used as a
reference gene for normalization and water was used as the negative
control. Relative quantification was calculated using the
comparative threshold cycle (CT), which was inversely related to
the abundance of mRNA transcripts in the initial sample. The mean
CT of the duplicate measurements was used to calculate ΔCT as the
difference in CT for the target and reference. The relative
quantity of the product was expressed as the fold-induction of the
target gene compared with the reference gene, according to the
formula 2−ΔΔCT, where ΔΔCT represented ΔCT values
normalized with the mean ΔCT of the control samples.
Western blot analysis of c-Jun, sp-1,
VEGF and GAPDH in cultured cells
Cultured cells were individually homogenized in a
buffer solution with 20 mm Tris-HCl (pH 7.4) containing 1% Triton
X-100, 0.1% SDS, 50 mm NaCl, 2.5 mm EDTA, 1 mm
Na4P2O7, 10 mm H2O, 20
mm NaF, 1 mm Na3VO4, 2 mm Pefabloc and a
cocktail of protease inhibitors (Complete Mini; Roche Applied
Science, Indianapolis, IN, USA), then centrifuged at 16,000 × g for
5 min at 4°C. Supernatants were collected following centrifugation,
and the protein concentrations were determined using the BCA
Protein Assay kit (Thermo Fisher Scientific, Rockford, IL, USA).
Total protein was separated by sodium dodecyl sulphate
polyacrylamide gel electrophoresis (SDS-PAGE) and transblotted onto
nitrocellulose membranes (GE Healthcare, Piscataway, NJ, USA).
Western blot analysis was conducted using antibodies against c-Jun,
sp-1 and VEGF (Abcam, Cambridge, UK) at a 1,000-fold dilution. All
primary and secondary antibodies were diluted in 5% dry skimmed
milk in Tris-buffered saline with Tween [TBST; 0.02 M Tris base and
0.137 M NaCl in distilled water (pH 7.6) containing 0.1% Tween-20].
Immunosignals were visualized with the Protein Detector BCIP/NBT
Western Blot kit (Beyotime Institute of Biotechnology, Shanghai,
China) according to the manufacturer’s instructions. Quantification
was conducted using ImageQuant 5.2 software. An additional membrane
prepared following the same protocol was probed with anti-GADPH
antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) to
normalize the sample loading.
MTT assay
LX-2 was plated into 96-well plates at a density of
5×105 cells/well in 100 μM growth medium, and allowed to
grow overnight to reach ~85% confluence. Three different
concentrations of octreotide in 100 ml growth medium
(10−5, 10−6 and 10−7 nM) were
added to the wells. Following incubation for 48 h, the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was used to measure cell proliferation. In brief, 20 ml of 5
mg/ml MTT was added to each well and the cells were incubated at
37°C for 4 h. The MTT was then removed and replaced with 100 ml
dimethyl sulfoxide (Sigma, St. Louis, MO, USA) for further
incubation for 10 min at 37°C, until the crystals had dissolved.
The optical density (OD) value of each well was measured using a
microplate reader (ELx800; BioTek Instruments, Winooski, VT, USA)
with a test wavelength of 570 nm. For each concentration or
control, triplicate determinations were made.
Measurement of soluble secreted ET-1,
collagen I and VEGF
Cells were seeded in high-glucose Dulbecco’s
Modified Eagle’s Medium (DMEM) containing 1% FBS 24 h prior to
octreotide treatment. The total soluble secreted ET-1 (R&D
Systems, Minneapolis, MN, USA), VEGF (R&D Systems) and collagen
I (Cosmo Bio, Co., Ltd., Tokyo, Japan) in the culture supernatants
in the absence or presence of octreotide for 48 h was measured
using a sandwich ELISA, according to the manufacturer’s
instructions for each kit. Briefly, microtiter wells were
pre-coated for overnight 1:20 dilutions of LX-2 cell conditioned
medium supernatant. The plates were then developed by the addition
of biotinylated antibodies against ET-1, VEGF or collagen I,
followed by avidin-conjugated horseradish peroxidase. The color
reaction was developed using a tetramethylbenzidine substrate
solution and the absorbance was measured at 450 nm using a Model
550 plate reader (BioRad, Hercules, CA, USA). The assay was
performed in triplicate and the mean values of each sample were
calculated.
Statistical analysis
The statistical significance of the differences
between the means was assessed by a one-way analysis of variance
(ANOVA) for multiple comparisons and an independent-sample
Student’s t-test. Differences between two or more groups were
analyzed using the Mann-Whitney U test or the Kruskal-Wallis test.
Statistical analyses were performed with SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
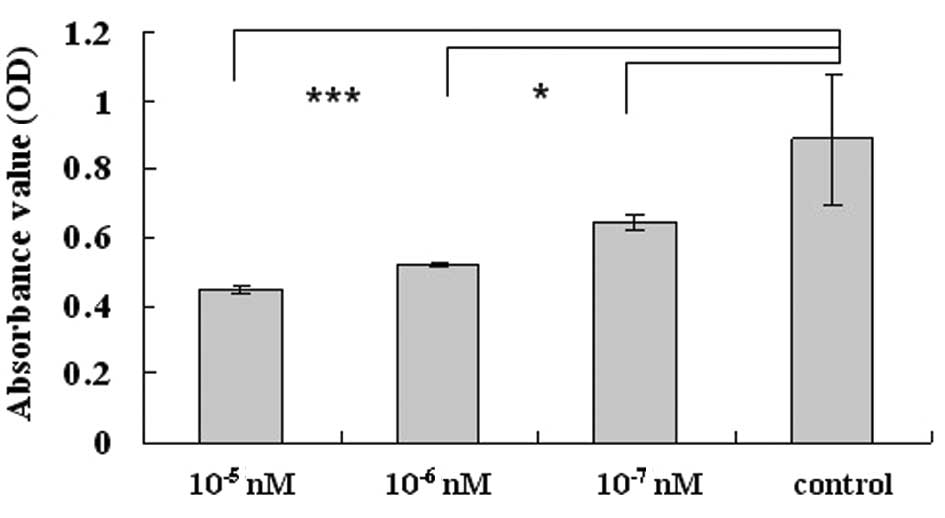
Octreotide inhibits LX-2
proliferation
To investigate the function of the analogue of
somatostatin, octreotide, we analyzed the rate of cell growth in
LX-2 cells following treatment at concentrations of
10−5, 10−6 and 10−7 nM. LX-2 cells
without any treatment were selected as the controls. The results
showed a significant decrease in cell proliferation, in a
concentration- dependent manner, compared with the controls, when
examined by the MTT assay (P<0.05). In addition, the inhibitory
rates of the octreotide treatment groups increased to 27.5 and
49.8% when the concentration was 10−7 and
10−5 nM, respectively (Fig.
1).
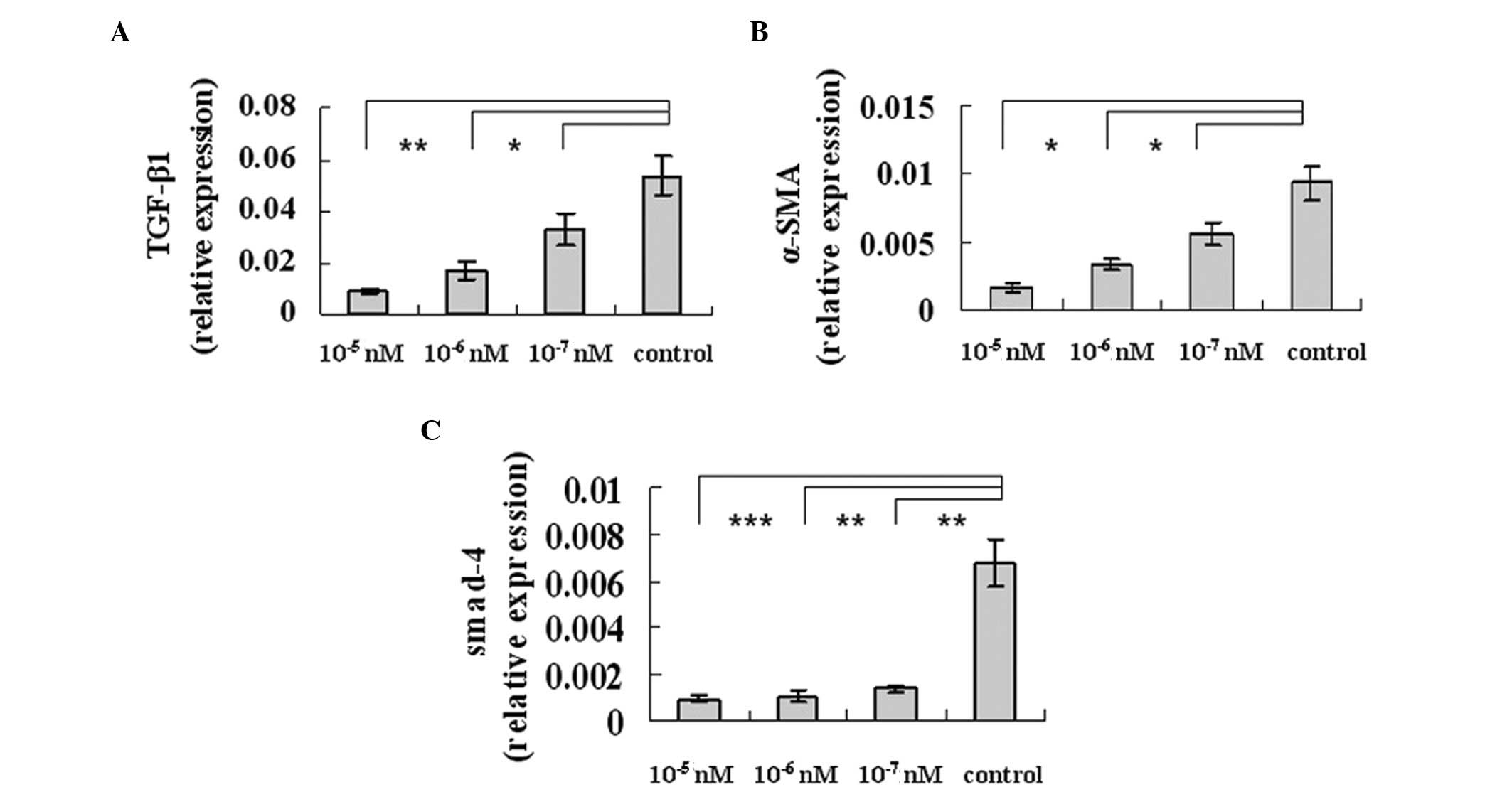
Octreotide alleviates the fibrogenesis
function of HSCs
In order to determine whether octreotide was able to
alleviate fibrosis in LX-2 cells, we selected TGF-β (a potent
profibrogenic cytokine proposed to play a central role in
regulating tissue fibrosis), α-SMA and smad-4α (two major
profibrogenic cytokines implicated in the development of liver
fibrosis). Real-time PCR analysis of extracted mRNA samples of the
cultured cells revealed decreased levels of TGF-β1 (Fig. 2A), α-SMA (Fig. 2B) and smad-4α (Fig. 2C) expression in the octreotide
group, thus illustrating its inhibitory effects on profibrogenic
gene expression.
ET-1 and collagen I expression levels
decrease following treatment with octreotide
To investigate whether octreotide was directly
involved in collagen I and ET-1 production in activated HSCs, we
analyzed the effect of octreotide on secreted collagen I and ET-1
production using the LX-2 cell line. As indicated, the level of
soluble secreted ET-1 (Fig. 3A)
and collagen I (Fig. 3B) in the
supernatant of the octreotide-treated LX-2 cells was lower than
that produced by the control cells.
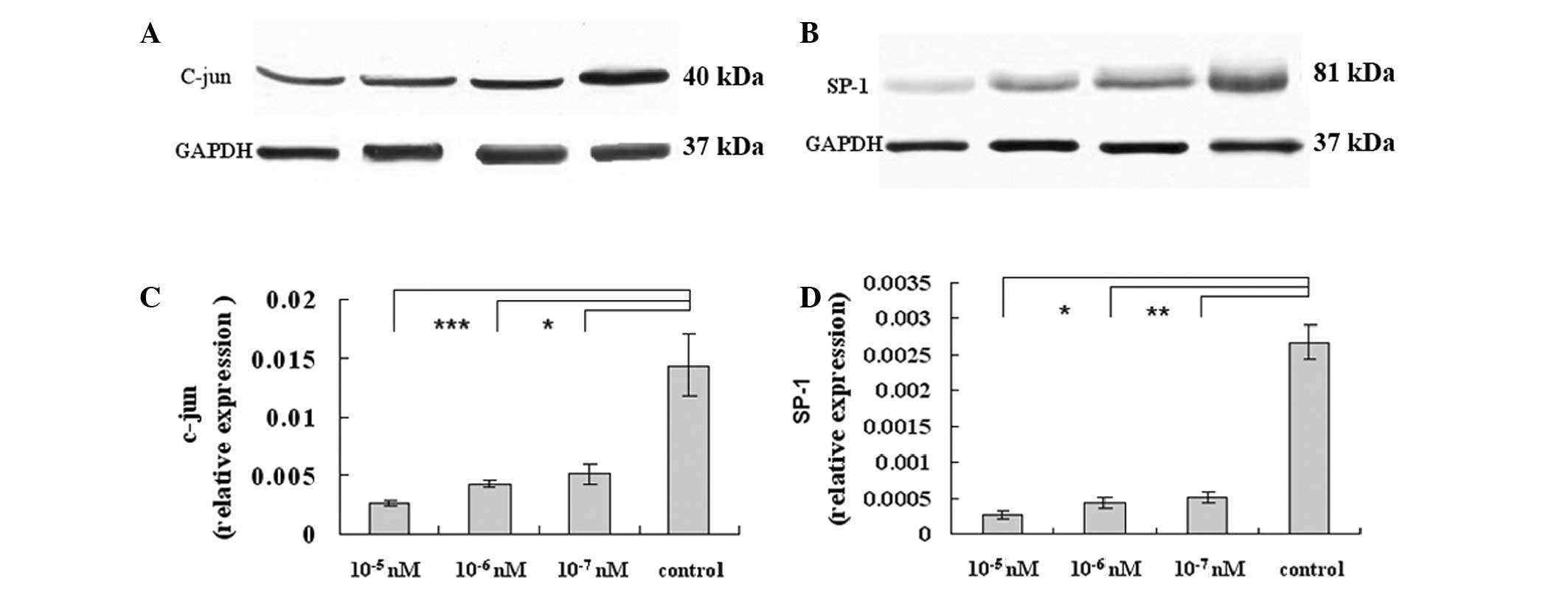
Octreotide attenuates hypertension and
fibrosis through the inhibition of c-Jun and sp-1 expression
Previous studies have demonstrated that IFN-γ
signals to c-Jun to negatively regulate preproET-1 transcription.
Combined with our results, it may be proposed that octreotide
decreases ET-1 expression. We hypothesized that octreotide may
target c-Jun or other transcription factors, either directly or
indirectly, which in turn may mediate the inhibitory effect of
octreotide on the expression of fibrosis-related genes. Sp-1 also
demonstrated a protective function against apoptosis and injury. As
demonstrated in Fig. 4A, the c-Jun
protein levels significantly decreased compared with the control,
following octreotide exposure for 48 h (Fig. 4A). We also examined c-Jun mRNA
expression following octreotide treatment. Notably, the c-Jun
protein levels were markedly reduced at 48 h, while octreotide
maintained its inhibitory effect throughout this time (Fig. 4C). In addition, the level of sp-1
was observed to decrease at the transcriptional and translational
levels (Fig. 4B and D,
respectively). In conclusion, octreotide may ameliorate fibrosis by
downregulating c-Jun and sp-1.
Octreotide increases VEGF expression in
LX-2 cells
To analyze the effects of octreotide on VEGF
activity, LX-2 cells were treated with octreotide for 48 h at
various concentrations. The VEGF mRNA transcription, protein and
secreted soluble protein levels were quantified by real-time
RT-PCR, western blot analysis and ELISA, respectively. Octreotide
increased the expression of VEGF mRNA when at a higher
concentration (10−5 nM), but this declined to the normal
level as the concentration was decreased. The ELISA results
revealed the same tendency for VEGF at a high octreotide
concentration (10−5 nM; Fig. 5A-C).
Discussion
Activated hepatic stellate cells (HSCs) are key
participants in hepatic fibrosis. In vivo and in
vitro studies have suggested that HSCs are also involved in the
regulation of the liver microcirculation and the pathogenesis of
portal hypertension. Thus, the induction of HSC apoptosis has been
proposed as an antifibrotic treatment strategy. Consistent with
other studies (13), at a low
dose, octreotide, the analogue of somatostatin, was demonstrated to
exert antiproliferative actions on LX-2 cells and to inhibit the
expression of α-SMA, smad-4 and TGF-β, which are all markers of
fibrosis. The aforementioned LX-2 cell line makes it feasible to
clarify the mechanism whereby octreotide exerts its effects on
HSCs.
ET-1, a powerful vasoconstrictor peptide, is
produced by activated HSCs and promotes cell proliferation,
fibrogenesis and contraction; the latter of which has been proposed
to be mechanistically linked to portal hypertension in cirrhosis.
In the present study, we found that octreotide downregulated ET-1
expression in LX-2 cells, which suggested an additional plausible
pathway that ET-1 may use during the attenuation of portal
hypertension. In a study by Li et al, it was demonstrated
that the IFNγ-induced inhibition of preproET-1 mRNA expression was
closely linked to the AP-1 and Smad3 signaling pathways (14). IFNγ reduced JNK phosphorylation,
which was correlated with the decrease in phosphorylation of
downstream factors, c-Jun and Smad3, and the decrease in the
binding activity of c-Jun and Smad3 in the preproET-1 promoter.
Notably, IFN-γ reduced c-Jun mRNA and protein levels (14). To investigate whether transcription
factors participated in octreotide-induced effects, we screened
susceptible factors, including cox-2, c-fos, c-Jun, sp-1, c-myc,
fra-1, vhl and Jun-B, and found that the c-Jun and sp-1 levels
significantly decreased (the data with regard to the remaining
transcription factors are not shown), suggesting that the two
transcription factors may be accountable for the effects of
octreotide.
It has been demonstrated that c-Jun is able to
promote pulmonary artery endothelial cell (PAEC) growth and
angiogenesis during pulmonary arterial hypertension (15). Furthermore, c-Jun also functions as
a downstream mediator of the pro-fibrotic effects of TGF-β and
platelet-derived growth factor (PDGF) in systemic sclerosis (SSc)
fibroblasts (16). Drugs that
block the p38/AP-1 pathway may inhibit liver extracellular matrix
synthesis and suppress liver fibrosis (17). Certain studies have found that
all-trans retinoic acid (ATRA) was capable of inhibiting the
proliferation and collagen production of HSCs via the suppression
of active protein-1 and the c-Jun N-terminal kinase signal, and
subsequently by decreasing the mRNA expression of the profibrogenic
genes (18–20). The aforementioned findings suggest
that c-Jun has important roles in the octreotide-induced ease of
portal hypertension and in decreased fibrosis.
An increased activation of sp-1 was identified under
conditions in which estrodial enhanced growth and reduced
TNFα-induced apoptosis in human umbilical vein endothelial cells
(HUVEC) (21). Evans et al
demonstrated that signaling through CD31 in endothelial cells leads
to protection from apoptosis in association with activation of the
transcription factor sp-1 (22).
Kang and Chen demonstrated that curcumin inhibits srebp-2
expression in cultured HSCs by reducing sp-1 activity (23). Furthermore, the transient
overexpression of integrin αvβ5 in normal fibroblasts has been
demonstrated to enhance human α2(I) collagen promoter activity
through sp-1 (24). Although the
targeting genes of c-Jun and sp-1 require further study, the fact
that these genes are involved in octreotide-induced effects
suggests that it may be possible to develop medicines that target
these transcription factors, in order to treat fibrosis and portal
hypertension in the liver.
In the present study, we also found that the
expression of VEGF increased with 10−5 nM octreotide,
but decreased to normal levels when the octreotide concentration
declined. As determined previously, VEGF promoted angiogenesis and
fibrosis, which affect portal hypertention, under abnormal
conditions. However, the in vitro antiangiogenic effects of
somatostatin and its analogues are likely to be efficiently
counterbalanced in the tumor microenvironment by the concomitant
release of proangiogenic factors, including VEGF (25), which may account for the elevated
level of VEGF in our study. Experimental and clinical studies have
clearly demonstrated that hepatic angiogenesis, irrespective of
aetiology, occurs in conditions of chronic liver diseases (CLDs),
and is characterized by perpetuation of cell injury and death, the
inflammatory response and progressive fibrogenesis. Angiogenesis
and associated changes in the liver vascular architecture, which in
turn increase vascular resistance and portal hypertension and
decrease parenchymal perfusion, have been proposed to favour the
fibrogenic progression of the disease towards the endpoint of
cirrhosis. Therefore, upregulated VEGF may be accountable for the
side-effects of octreotide treatment at high doses (26). Rosmorduc demonstrated that
antiangiogenic therapies may therefore, by limiting liver fibrosis
and inflammation in cirrhosis, prevent the occurrence of severe
complications, including portal hypertension and potentially, liver
cancer (27). Therefore, the
combination of octreotide and antiangiogenic therapy has potential
if long-term administration at a high concentration is
necessary.
There were certain limitations to the present study;
the transcription factors were not screened systematically and
their exact roles remained unknown. Therefore, further study is
required.
In the present study, we have demonstrated that the
effects of octreotide on HSCs were elicited to alleviate fibrosis
and hypertension by downregulating c-Jun and sp-1, and that the
downstream targets require further investigation. Furthermore, VEGF
levels increased at a high octreotide concentration, which may
explain the side-effects of a long-term administration of
octreotide at high concentrations.
Acknowledgements
This study was supported by a grant from the
Shandong Science and Technology Research Program (grant no.
2010G0020223).
References
|
1
|
Arroyo V, Terra C and Ginès P: Advances in
the pathogenesis and treatment of type-1 and type-2 hepatorenal
syndrome. J Hepatol. 46:935–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman SL: Liver fibrosis - from bench
to bedside. J Hepatol. 38(Suppl 1): S38–S53. 2003. View Article : Google Scholar
|
|
3
|
Bartlett ST, Kuo PC, Johnson LB, Lim JW
and Schweitzer EJ: Pancreas transplantation at the University of
Maryland. Clin Transpl. 271–280. 1996.
|
|
4
|
Sutherland DE, Gruessner R, Gillingham K,
et al: A single institution’s experience with solitary pancreas
transplantation: a multivariate analysis of factors leading to
improved outcome. Clin Transpl. 141–152. 1991.
|
|
5
|
Watts SW, Yang P, Banes AK and Baez M:
Activation of Erk mitogen-activated protein kinase proteins by
vascular serotonin receptors. J Cardiovasc Pharmacol. 38:539–551.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li T, Weng SG, Leng XS, et al: Effects of
5-hydroxytamine and its antagonists on hepatic stellate cells.
Hepatobiliary Pancreat Dis Int. 5:96–100. 2006.PubMed/NCBI
|
|
7
|
Friedman SL: Molecular regulation of
hepatic fibrosis, an integrated cellular response to tissue injury.
J Biol Chem. 275:2247–2250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia D, Zhao RQ, Wei XH, Xu QF and Chen J:
Developmental patterns of GHr and SS mRNA expression in porcine
gastric tissue. World J Gastroenterol. 9:1058–1062. 2003.PubMed/NCBI
|
|
9
|
Vindeløv SD, Hartoft-Nielsen ML, Rasmussen
AK, et al: Interleukin-8 production from human somatotroph adenoma
cells is stimulated by interleukin-1β and inhibited by growth
hormone releasing hormone and somatostatin. Growth Horm IGF Res.
21:134–139. 2011.PubMed/NCBI
|
|
10
|
Yao YL, Xu B, Zhang WD and Song YG:
Gastrin, somatostatin, and experimental disturbance of the
gastrointestinal tract in rats. World J Gastroenterol. 7:399–402.
2001.PubMed/NCBI
|
|
11
|
Sun FP, Song YG, Cheng W, Zhao T and Yao
YL: Gastrin, somatostatin, G and D cells of gastric ulcer in rats.
World J Gastroenterol. 8:375–378. 2002.PubMed/NCBI
|
|
12
|
Li YY: Mechanisms for regulation of
gastrin and somatostatin release from isolated rat stomach during
gastric distention. World J Gastroenterol. 9:129–133.
2003.PubMed/NCBI
|
|
13
|
Reynaert H, Thompson MG, Thomas T and
Geerts A: Hepatic stellate cells: role in microcirculation and
pathophysiology of portal hypertension. Gut. 50:571–581. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li T, Shi Z and Rockey DC:
Preproendothelin-1 expression is negatively regulated by IFNγ
during hepatic stellate cell activation. Am J Physiol Gastrointest
Liver Physiol. 302:G948–G957. 2012.
|
|
15
|
Ma J, Zhang L, Han W, et al: Activation of
JNK/c-Jun is required for the proliferation, survival, and
angiogenesis induced by EET in pulmonary artery endothelial cells.
J Lipid Res. 53:1093–1105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fuest M, Willim K, MacNelly S, et al: The
transcription factor c-Jun protects against sustained hepatic
endoplasmic reticulum stress thereby promoting hepatocyte survival.
Hepatology. 55:408–418. 2012. View Article : Google Scholar
|
|
17
|
Reich N, Tomcik M, Zerr P, et al: Jun
N-terminal kinase as a potential molecular target for prevention
and treatment of dermal fibrosis. Ann Rheum Dis. 71:737–745. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y and Yao X: Role of c-Jun
N-terminal kinase and p38/activation protein-1 in
interleukin-1β-mediated type I collagen synthesis in rat hepatic
stellate cells. APMIS. 120:101–107. 2012.PubMed/NCBI
|
|
19
|
Avouac J, Palumbo K, Tomcik M, et al:
Inhibition of activator protein 1 signaling abrogates transforming
growth factor β-mediated activation of fibroblasts and prevents
experimental fibrosis. Arthritis Rheum. 64:1642–1652. 2012.
|
|
20
|
Ye Y and Dan Z: All-trans retinoic acid
diminishes collagen production in a hepatic stellate cell line via
suppression of active protein-1 and c-Jun N-terminal kinase signal.
J Huazhong Univ Sci Technolog Med Sci. 30:726–733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ling S, Zhou L, Li H, et al: Effects of
17beta-estradiol on growth and apoptosis in human vascular
endothelial cells: influence of mechanical strain and tumor
necrosis factor-alpha. Steroids. 71:799–808. 2006. View Article : Google Scholar
|
|
22
|
Evans PC, Taylor ER and Kilshaw PJ:
Signaling through CD31 protects endothelial cells from apoptosis.
Transplantation. 71:457–460. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang Q and Chen A: Curcumin inhibits
srebp-2 expression in activated hepatic stellate cells in vitro by
reducing the activity of specificity protein-1. Endocrinology.
150:5384–5394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asano Y, Ihn H, Yamane K, Kubo M and
Tamaki K: Increased expression levels of integrin alphavbeta5 on
scleroderma fibroblasts. Am J Pathol. 164:1275–1292. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Walter T, Hommell-Fontaine J, Gouysse G,
et al: Effects of somatostatin and octreotide on the interactions
between neoplastic gastroenteropancreatic endocrine cells and
endothelial cells: a comparison between in vitro and in vivo
properties. Neuroendocrinology. 94:200–208. 2011. View Article : Google Scholar
|
|
26
|
Paternostro C, David E, Novo E and Parola
M: Hypoxia, angiogenesis and liver fibrogenesis in the progression
of chronic liver diseases. World J Gastroenterol. 16:281–288. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rosmorduc O: Antiangiogenic therapies in
portal hypertension: a breakthrough in hepatology. Gastroenterol
Clin Biol. 34:446–449. 2010. View Article : Google Scholar : PubMed/NCBI
|