Introduction
Acute myocardial infarction is a major cause of
sudden cardiac death (SCD) in patients with coronary artery disease
and ischemic heart disease (1–3). The
quantity of ischemic myocardium and extent of infarct expansion are
crucial factors determining the occurrence of SCD during
experimental acute myocardial ischemia (AMI), which may lead to
congestive heart failure, aneurysm formation and myocardial
rupture. Traditionally, the early myocardial damage has been
identified using routine hematoxylin and eosin (H&E) staining,
following the occurrence of myocardial ischemic injury for ≥6 h
(4). Certain studies have shown
that myocardial apoptosis, histochemical techniques (including
tetrazolium salts, phosphotungstic acid hematoxylin, trichrome,
periodic acid-Schiff and hematoxylin-basic fuchsin-picric acid) and
immunohistochemical staining may also be used to identify markers
of myocardial damage 2–4 h following the onset of ischemia
(1,5–8).
However, these methods are not suitable for assessing the early
phase of acute myocardial damage, particularly in cases where the
patient succumbed within 1 h. Their sensitivity and specificity
remain controversial, due to the fact that positive results may
sometimes be related to agonal factors and/or post-mortem changes
(4). It may currently be
challenging for forensic pathologists to make a post-mortem
diagnosis of AMI when the ischemic insult occurred within minutes
to 1 h, as it is commonly asymptomatic or unrecognized. We have
previously demonstrated that basigin may be associated with AMI
within 1 h in rats by suppression subtractive hybridization, which
revealed differentially expressed genes (9).
Basigin is also known as extracellular matrix
metalloproteinase inducer (EMMPRIN), tumor cell-derived collagenase
stimulatory factor (TCSF) and CD147. It was first identified on the
surface of tumor cells, where it stimulated adjacent fibroblasts
and endothelial or tumor cells to produce matrix metalloproteinases
(MMPs), and facilitated the invasion of cancer cells (10–12).
Basigin is also involved in tissue remodeling in numerous
physiological and pathological conditions. Previously, certain
studies have demonstrated that basigin is expressed in cardiac
myocytes, but not in cardiac microvascular endothelial cells or
cardiac fibroblasts (13,14), and basigin protein expression
levels are significantly elevated in acute infarcted myocardium
(15,16), destabilizing atheroma (17), human heart failure myocardium
(13) and the ventricle of
ischemic cardiomyopathy (18). A
limited number of studies had investigated the theory that basigin
mRNA and protein were expressed in AMI within 1 h. In the present
study, we investigated the temporal and spatial expression patterns
of basigin in the early phase of AMI during different ischemic
periods, as basigin is a known differentially expressed gene
potentially related to the early phase of AMI.
Materials and methods
Animal model and tissue preparation
Adult healthy male Sprague-Dawley rats (weight,
200–250 g) were provided by the West China Medical Animal Center of
Sichuan University (Chengdu, China). Experiments were performed
with the approval of the Sichuan University Animal Research
Committee), and were in accordance with animal protection
guidelines (Guide for the Care and Use of Laboratory Animals, 8th
edition). The animal models were created as previously described in
detail (19). In brief,
thoracotomy was performed in rats in the surgery group; the left
anterior descending (LAD) coronary artery was ligated with a 6-0
silk suture by piercing the pericardial membrane, and
electrocardiograms (ECGs) were recorded to confirm the successful
ligation. Cervical dislocation of all experimental rats was
performed 0, 15, 30, 60, 120 and 240 min following ligation of the
LAD coronary artery (n=6 for each time interval), respectively.
Early ischemic myocardium (EIM) was assessed by 1% Evans blue.
Additionally, EIM and proximate non-ischemic myocardium (NIM) were
collected from each rat. The sham-operated rats (n=36) were treated
similarly to the rats receiving surgery, except they did not
receive LAD coronary artery ligation, and the corresponding
myocardium with EIM was collected and regarded as sham operation
myocardium (SOM). Subsequently, the samples were fixed in 4%
freshly made paraformaldehyde and embedded in optimal cutting
temperature (OCT) medium for in situ hybridization (ISH) and
immunohistochemistry (IHC), or were stored in liquid nitrogen for
real-time quantitative PCR and western blot analysis.
Real-time quantitative PCR
Total RNA was extracted using the RNAiso Plus
Isolation kit according to the manufacturer's instructions (Takara
Bio Inc., Otsu, Japan). cDNA was synthesized from 500 ng total RNA
using oligo dT primers and a PrimeScript™ RT Enzyme mix (Takara Bio
Inc.).
Real-time quantitative PCR was conducted using the
SYBR Premix Ex Taq™ II kit (Takara Bio Inc.) in the ABI 7500 Fast
system (Applied Biosystems, Foster City, CA, USA). According to
Genbank (AY 120888.1), oligonucleotide primers for basigin were
designed as follows: forward: 5′-GGCACCATCGTAACCTCTGT-3′ and
reverse: 5′-CAGGCTCAGGAAGGAAGATG-3′ (size, 211 bp). The primers for
the internal control, β-actin, were as follows: forward:
5′-TCACCCACACTGTGCCCATCTATGA-3′ and reverse:
5′-CATCGGAACCGCTCATTGCCGATAG-3′ (size, 300 bp). Samples without a
template and samples processed without the reverse transcriptase
step served as blank and negative controls, respectively. PCR
amplification of cDNA was performed in a 20 μl reaction volume
containing 10 μl SYBR Premix Ex Taq (X2) with 0.4 μl each primer,
0.4 μl ROX Reference Dye and 2 μl template cDNA. Cycling parameters
were 95°C for 10 sec, followed by annealing and extension during 40
cycles of 95°C for 5 sec, 60°C for 20 sec and 72°C for 34 sec. The
standard curves for each gene were generated by serial dilution of
a plasmid containing basigin and β-actin cDNA, respectively, in
order to quantify the mRNA concentrations. We confirmed the melting
curve for detecting the SYBR-Green-based objective amplicon, as
SYBR-Green also detected double-stranded DNA including primer
dimers, contaminating DNA and PCR products from misannealed
primers. Contaminating DNA or primer dimers appeared as peaks
separated from the desired amplicon peak.
Each cDNA sample was run in triplicate, and the
amplification efficiencies of primer pairs were determined by
serial dilutions of the input template. The comparative cycle
threshold method was used to analyze the data by generating
relative values of the quantity of target cDNA. All data were
normalized to correspond with β-actin mRNA, and were expressed as
basigin mRNA in ischemic and non-ischemic myocardium during
different ischemic periods. The relative difference in the initial
amount of each mRNA species (or cDNA) was determined by comparing
the CT values. The ratios of basigin/β-actin were calculated to
adjust for any variations in the real-time quantitative PCR
reaction.
ISH
The oligonucleotide primers used for basigin reverse
transcription-PCR were as follows: forward:
5′-GCTGTCTGTTGATGGGCTCG-3′ and reverse: 5′-GGCTTCCGCCTCTTCTCGTA-3′
(size, 786 bp) for the rat myocardium basigin cDNA (GenBank; AY
120888.1). PCR amplification was performed with AmpliTaq
Gold® DNA Polymerase (Applied Biosystems, Foster City,
CA, USA), and the PCR products were directly inserted into a
pEASY-T3 Cloning vector (Beijing TransGen Biotech Co., Ltd.,
China). Plasmids were isolated and analyzed by restriction mapping
and DNA sequencing. Digoxin (DIG)-labeled antisense and sense
complementary RNA probes were prepared using DIG-labeled RNA kits
(Sp6/T7; Roche Diagnostics GmbH, Mannheim, Germany), and plasmid
cDNA was cloned as described in previous studies (20,21).
The hearts of two rats were evaluated by ISH per each ischemic time
point, and at least six sections per heart were analyzed with the
antisense basigin probe, including ischemic and non-ischemic
myocardium, respectively (≥18 tissue sections were analyzed per
time point). Briefly, frozen sections (12 mm) were fixed in 4%
paraformaldehyde and phosphate-buffered saline (PBS) solution. The
sections were then acetylated and dehydrated in preparation for
hybridization with 2-40 μg/ml RNase-free proteinase K, for 10–30
min, and with/without 0.2 M HCl for 10 min at room temperature
(RT), respectively. Quantification and efficiency of probe labeling
were analyzed by serial dilution of test strips. Probes were used
at a concentration of 2 μg/ml. The slides were hybridized overnight
with DIG-labeled antisense rat basigin probe per slide in a
humidified chamber at 65°C, and unbound probe was removed by RNase
treatment and stringent washes. Nitro blue tetrazolium and
5-bromo-4-chloro-3-indolylphosphate were used for color detection.
Tissues were examined with a Nikon phase contrast microscope
(Nikon, Melville, NY, USA). Negative and positive controls were
conducted with sense basigin RNA probe and 4-day-old rat brain,
respectively.
Western blot analysis
Basigin protein levels in AMI were analyzed by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE). The tissue specimens were lysed in
radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris, pH 8.0;
150 mM NaCl; 1% Triton X-100; 1 mM EDTA, pH 8.0; and 0.1% SDS),
supplemented with protease inhibitor cocktail (50 μM), lactacystin
(20 μM), β-glycerophosphate (25 mM) and sodium orthovanadate (1
mM). Tissue proteins (20 μg/lane) were analyzed by continuous 10%
SDS-PAGE, and the electrophoresed proteins were blotted onto a
polyvinylidene difluoride (PVDF) membrane (BioRad, Hercules, CA,
USA).
In order to reveal the basigin antigen, the membrane
was blocked with 5% bovine serum albumin (BSA) in 5% Tween-20
balanced salt Tris solution (TBST) for 1 h at RT, and subsequently
incubated with rabbit anti-rat basigin antibody (1 mg/ml, Abcam,
Cambridge, MA, USA) overnight at 4°C. Following primary antibody
incubation, the membrane was washed with TBST and incubated for 1 h
with secondary goat anti-rabbit IgG conjugated with horseradish
peroxidase (Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd., China). Finally, the signal was visualized with an enhanced
chemiluminescence (ECL) kit (Amersham Biosciences Corp.,
Piscataway, NJ, USA) according to the manufacturer's instructions.
The relative quantity of basigin was determined based on the signal
intensity of the bands normalized to the β-actin signal in the same
lane.
IHC
The frozen myocardial sections (12 mm) were fixed in
4% paraformaldehyde solution and blocked with 5% BSA prior to
incubation at 4°C overnight with mouse anti-rat basigin (1:150; BD
Biosciences, Franklin Lakes, NJ, USA). Bound antibody was labeled
with goat anti-mouse secondary antibody conjugated with horseradish
peroxidase (1:1000; 30 min at RT; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.), and detected using a
3,3′-diaminobenzidine (DAB) kit and light microscopy. Irrelevant
rabbit IgG was used for the primary layer as a negative control.
The intensity of staining was semi-quantitatively assigned a score
from 0–3, with the two observers blind to the source of the
biopsy.
Statistical analysis
Data were presented as the mean ± standard deviation
(SD). The EIM was compared to the NIM for each group of rats by a
paired Student's t-test. An unpaired t-test and an analysis of
variance (ANOVA) test were utilized among the different ischemic
time points. P≤0.05 was considered to indicate a statistically
significant difference.
Results
Initial findings
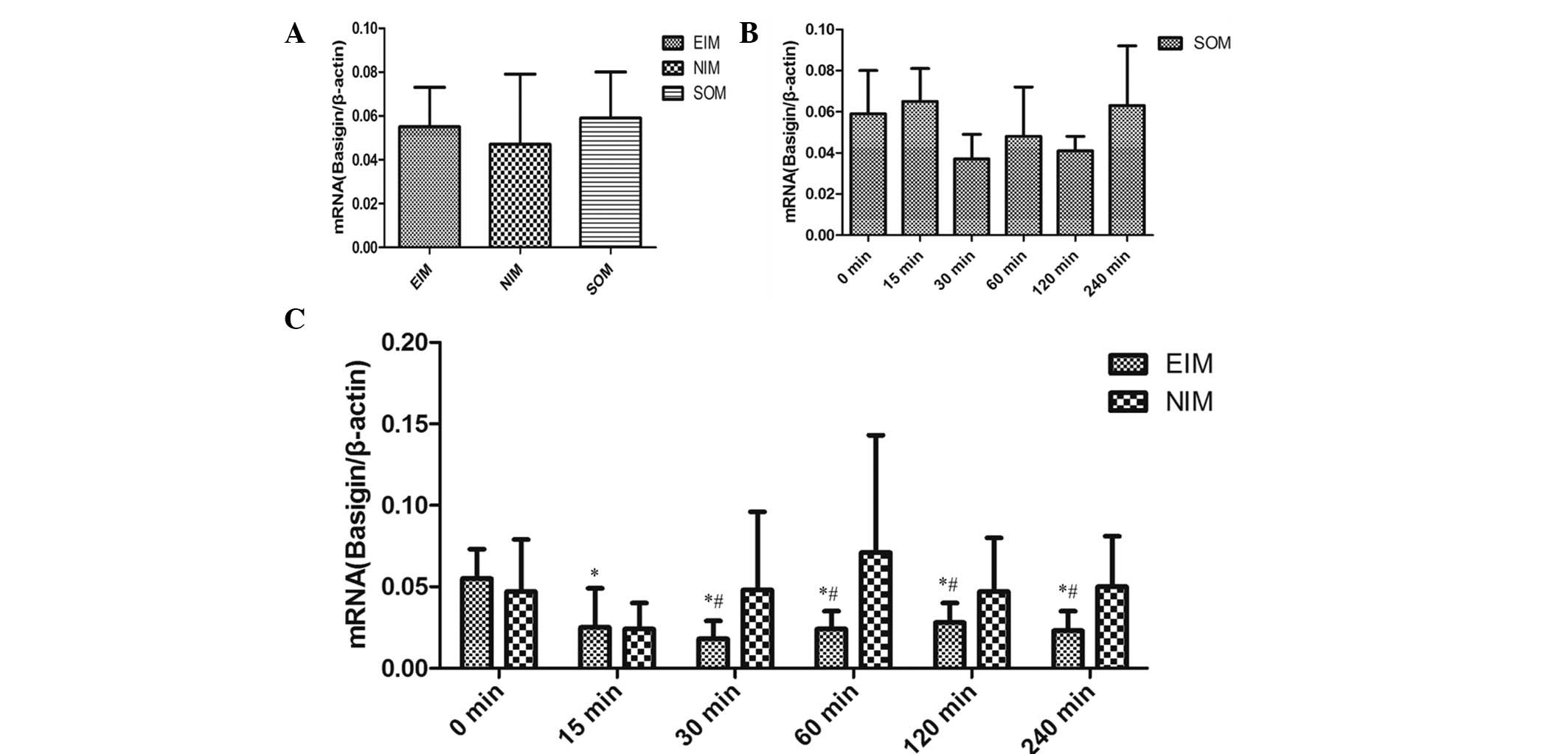
No significant differences were observed in the
expression levels of basigin mRNA among the EIM, NIM and SOM at 0
min (P>0.05; Fig. 1A). In
addition, no significant differences were identified in either the
basigin mRNA or protein expression levels in the SOM between
different time points, by real-time quantitative PCR and western
blot analysis, respectively (Fig.
1B and Fig. 2A). It was
demonstrated that the AMI model in rats achieved by ligating the
LAD coronary artery was mature, and that the result was stable as
well as reliable for further experiments.
Expression of basigin mRNA
Basigin mRNA expression levels significantly
decreased in the EIM following ischemia for 15–240 min compared
with that at 0 min, respectively (P<0.001; Fig. 1C). However, no significant
difference was observed in the NIM from 0–240 min (P>0.05).
Basigin mRNA expression levels decreased significantly in the EIM
compared with that in the corresponding NIM following myocardial
ischemia for 30 min (P<0.05; Fig.
1C).
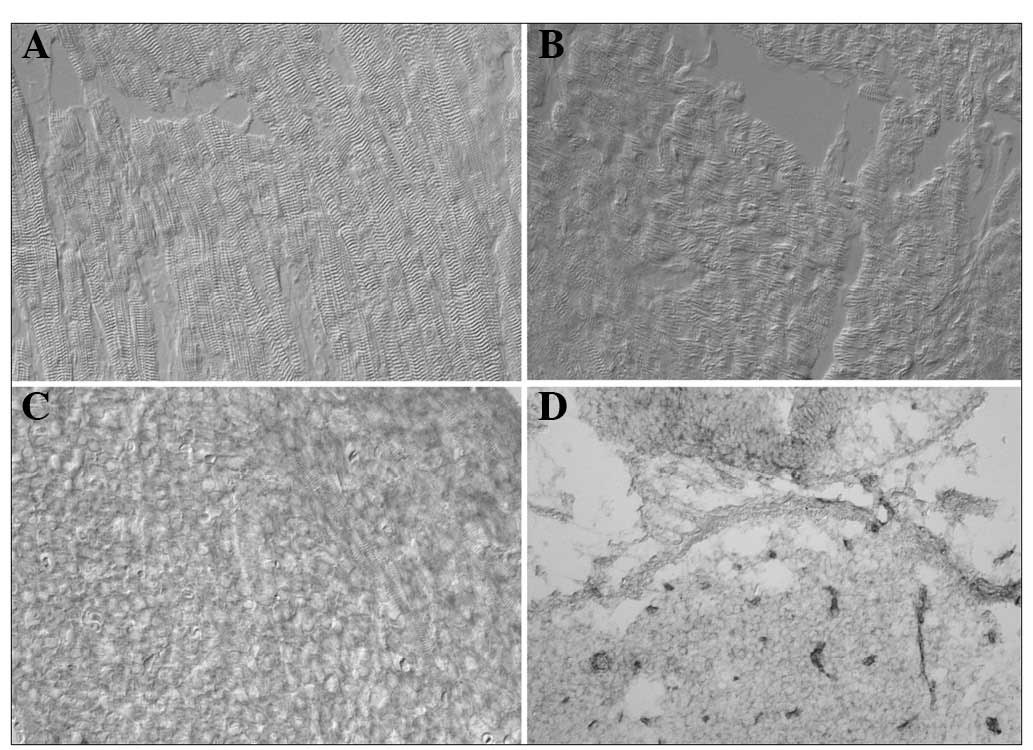
We failed to detect an expression signal for basigin
mRNA in the myocardium, including in the EIM, NIM and 4-day-old rat
myocardium, using the DIG-labeled cRNA probe (Fig. 3A-C). However, normal neurons in the
4-day-old rat brain (the positive control) emitted a blue signal,
which indicated the presence of basigin mRNA (Fig. 3D).
Basigin protein expression
Basigin was demonstrated to exist in the myocardium
as a highly glycosylated (HG) form migrating at 58 kDa, as opposed
to as a less-glycosylated (LG) form migrating at 32–44 kDa
(Fig. 2A and B). The western blot
analysis results revealed that basigin protein expression levels in
the EIM following ischemia for 30–240 min significantly increased
to approximately double that at 0 min in the EIM (P<0.05). We
observed that basigin protein expression levels in the EIM were
significantly different compared with those in the corresponding
NIM following myocardial ischemia for 30 min (P<0.05; Fig. 2B).
Basigin protein was observed to be mainly expressed
on the membranes of normal cardiac myocytes, and occasionally
expressed in the cytoplasm and nucleoli of cardiac myocytes by IHC.
Positive basigin protein expression was found in the NIM and SOM at
different ischemic periods, and in the EIM within 15 min following
myocardial ischemia (Fig. 4A-D).
Furthermore, its expression strongly increased in the EIM following
myocardial ischemia for >30 min (Fig. 4E-H). Additionally, the degree of
positively stained cells was uniform in the EIM following
myocardial ischemia for 30–240 min (Fig. 4E-H). The IHC results were
concordant with the western blot analysis results.
Discussion
Basigin was initially characterized as a tumor cell
surface glycoprotein, composed of two extracellular immunoglobulin
domains, a transmembrane domain and a cytoplasmic domain (10,22),
which is distributed broadly in normal and tumor cells (23). Basigin has been most extensively
investigated with respect to tumor invasion and metastasis, and it
has been implicated as a factor contributing to the induction of
MMP expression by autocrine or paracrine mechanisms (10,24–26).
It has been demonstrated that basigin is expressed on cardiac
myocytes (13,14), and is involved with the human left
ventricle, following acute myocardial infarction, heart failure
myocardium and the ventricle of ischemic cardiomyopathies (13,15,18).
These results strongly suggested that basigin may be involved in
myocardial injury.
The current study demonstrated that basigin mRNA and
protein expression levels were significantly different from control
levels as early as 30 min after the initiation of ischemia in the
EIM, and the changes continued to be present throughout the
ischemic period (240 min). The results were consistent with our
previous study showing that basigin mRNA expression in EIM
significantly decreased in the 15, 30 and 60 min groups compared
with that of 0 min group (9), and
certain signal pathway alterations of basigin in the
ischemia/reperfusion myocardium, and irreversible myocardial cell
injury 20–30 min following the occlusion of a coronary vessel
(27). Similarly, phosphorylated
MAPK family members, including ERK 1/2, SAPKs and p38 MAPK,
demonstrated biphasic maximum levels at 5–10 min and 30 min through
basigin (28). Foda et al
demonstrated that basigin mRNA and protein expression levels
increased in alveolar macrophages and airway epithelial cells
within 1 h in ventilator-induced lung injury of rats (29). To the best of our knowledge, the
present study is the first to demonstrate the accumulation feature
of basigin at the early phase of AMI.
We demonstrated that basigin protein expression
levels significantly increased in the EIM following ischemia for
>30 min, and the protein was predominantly expressed on myocyte
sarcolemma. This was concordant with other studies (15,30–32)
in which basigin expression significantly increased around the zone
of necrosis and in monocytes in the acute myocardial infarction.
Upregulation of monocarboxylate transporters (MCTs) and basigin
expression in cardiac and neuronal cells under ischemic conditions
has been observed (33–35); as well as the release of
cyclophilin A (CyPA) from cardiac myocytes upon
hypoxia/reoxygenation in vitro or ischemia/reperfusion in
vivo, accompanied by the upregulation of basigin (16,28).
The correlation between basigin/CyPA/MCTs and related signaling may
provide protection for cardiac myocytes and neurons under
conditions of ischemic damage. However, basigin has the potential
to induce extracellular matrix degradation by itself or by
activating neighboring cells to induce certain MMPs [such as MMP-1,
−2, −3 and −9, and membrane type 1(MT1)-MMP] in the myocardium, and
by interactions with other proteins, which in turn may contribute
to the progression of myocardial pathology (10,36–38).
It has been suggested that basigin may be involved in the
pathological processes during the early phase of AMI (ischemia
>30 min). However, this issue has remained speculative and
warrants further study.
Notably, we observed that the expression level of
basigin mRNA was significantly reduced in the EIM. The correlation
between basigin mRNA and protein expression was not consistent with
certain studies (29,39,40).
For example, soluble basigin protein was significantly increased in
proximal tubular epithelial cells by epidermal growth factor (EGF)
and phorbol myristate acetate (PMA), although its mRNA level was
not altered by these agents (40).
In bleomycin-treated lungs, basigin mRNA was not present in areas
of fibrosis, while coding protein levels were prominently increased
in the fibro-inflammatory lesions (39). However, basigin mRNA and protein
responses were correlated with increases in ventilator-induced lung
injury of rats (29). Several
theories have been proposed for the molecular basis of the
differences of basigin mRNA and protein expression in the EIM. For
example, Sp1 and Sp3 elements in the promoter of basigin may be
critical for the regulation of basigin gene expression in
macrophages, and a variety of stimuli affect their levels,
activation and binding (39,41).
It has been demonstrated that the expression levels of basigin mRNA
and protein are affected by different factors and so they may be
involved in different pathological processes (39), or negative regulatory factors may
be present (42). Another reason
may be post-transcriptional mechanisms. We observed that only the
HG form of basigin existed in the EIM, and not the LG form,
indicating that basigin upregulation in the EIM may not be the
cause of the ischemic myocardium. This finding was consistent with
a study in which basigin potentially underwent significant
translocation under certain circumstances (39). In addition, Joghetaei et al
revealed that the migration of monocytes into aortic valve tissue
may be affected by a basigin-based interaction of monocytes and
endothelial cells (43).
In the present study, basigin mRNA levels were
demonstrated to be significantly reduced in the EIM by real-time
quantitative PCR; however, we were not able to detect an expression
signal of basigin mRNA in either the EIM or the NIM by ISH. This
was likely to be due to the low sensitivity of ISH compared with
real-time quantitative PCR, and the basigin mRNA may have been of
low abundance or low gene copy number in the myocardium.
In conclusion, the basigin gene may be involved in
the myocardial pathophysiology process following continual
myocardial ischemia for >30 min. We suggest that basigin may be
identified as a predictor of acute myocardial ischemia in forensic
medicine. Additionally, basigin may be a novel target for the
clinical diagnosis and therapeutic intervention of acute myocardial
ischemia.
Acknowledgements
This study was financially supported by a grant from
the National Natural Science Foundation of China (grant no.
30973368/C1709).
References
|
1
|
Xu XH, Chen JG and Zhu JZ: Primary study
of vascular endothelial growth factor immunohistochemical staining
in the diagnosis of early acute myocardial ischemia. Forensic Sci
Int. 118:11–14. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ostadal B, Netuka I, Maly J, Besik J and
Ostadalova I: Gender differences in cardiac ischemic injury and
protection - experimental aspects. Exp Biol Med (Maywood).
234:1011–1019. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mehta D, Curwin J, Gomes JA and Fuster V:
Sudden death in coronary artery disease: acute ischemia versus
myocardial substrate. Circulation. 96:3215–3223. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Campobasso CP, Dell'Erba AS, Addante A,
Zotti F, Marzullo A and Colonna MF: Sudden cardiac death and
myocardial ischemia indicators: a comparative study of four
immunohistochemical markers. Am J Forensic Med Pathol. 29:154–161.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vargas SO, Sampson BA and Schoen FJ:
Pathologic detection of early myocardial infarction: a critical
review of the evolution and usefulness of modern techniques. Mod
Pathol. 12:635–645. 1999.PubMed/NCBI
|
|
6
|
Bardales RH, Hailey LS, Xie SS, Schaefer
RF and Hsu SM: In situ apoptosis assay for the detection of early
acute myocardial infarction. Am J Pathol. 149:821–829.
1996.PubMed/NCBI
|
|
7
|
Xiaohong Z, Xiaorui C, Jun H and Qisheng
Q: The contrast of immunohistochemical studies of myocardial
fibrinogen and myoglobin in early myocardial ischemia in rats. Leg
Med (Tokyo). 4:47–51. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lie JT, Holley KE, Kampa WR and Titus JL:
New histochemical method for morphologic diagnosis of early stages
of myocardial ischemia. Mayo Clin Proc. 46:319–327. 1971.PubMed/NCBI
|
|
9
|
Liu Y, Gao LB, Liang WB, Pan XM, Wang YY,
Xue H, Chen TY, Zhang LS, Zhu Y and Zhang L: The expression of
Basigin mRNA in early ischemic myocardium and non-ischemic
myocardium. Sichuan Da Xue Xue Bao Yi Xue Ban. 41:81–84. 2010.(In
Chinese).
|
|
10
|
Biswas C, Zhang Y, DeCastro R, et al: The
human tumor cell-derived collagenase stimulatory factor (renamed
EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res.
55:434–439. 1995.PubMed/NCBI
|
|
11
|
Biswas C: Tumor cell stimulation of
collagenase production by fibroblasts. Biochem Biophys Res Commun.
109:1026–1034. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caudroy S, Polette M, Nawrocki-Raby B, et
al: EMMPRIN-mediated MMP regulation in tumor and endothelial cells.
Clin Exp Metastasis. 19:697–702. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spinale FG, Coker ML, Heung LJ, et al: A
matrix metalloproteinase induction/activation system exists in the
human left ventricular myocardium and is upregulated in heart
failure. Circulation. 102:1944–1949. 2000. View Article : Google Scholar
|
|
14
|
Siwik DA, Kuster GM, Brahmbhatt JV, et al:
EMMPRIN mediates beta-adrenergic receptor-stimulated matrix
metalloproteinase activity in cardiac myocytes. J Mol Cell Cardiol.
44:210–217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nie R, Xie S, Du B, Liu X, Deng B and Wang
J: Extracellular matrix metalloproteinase inducer (EMMPRIN) is
increased in human left ventricle after acute myocardial
infarction. Arch Med Res. 40:605–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seizer P, Ochmann C, Schönberger T, et al:
Disrupting the EMMPRIN (CD147)-cyclophilin A interaction reduces
infarct size and preserves systolic function after myocardial
ischemia and reperfusion. Arterioscler Thromb Vasc Biol.
31:1377–1386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon YW, Kwon HM, Hwang KC, et al:
Upstream regulation of matrix metalloproteinase by EMMPRIN;
extracellular matrix metalloproteinase inducer in advanced
atherosclerotic plaque. Atherosclerosis. 180:37–44. 2005.
View Article : Google Scholar
|
|
18
|
Spinale FG, Coker ML, Bond BR and Zellner
JL: Myocardial matrix degradation and metalloproteinase activation
in the failing heart: a potential therapeutic target. Cardiovasc
Res. 46:225–238. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang G, Zhou B, Zheng Y, et al: Time
course proteomic profile of rat acute myocardial infarction by
SELDI-TOF MS analysis. Int J Cardiol. 131:225–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ushizawa K, Takahashi T, Hosoe M, et al:
Gene expression profiles of novel caprine placental
prolactin-related proteins similar to bovine placental
prolactin-related proteins. BMC Dev Biol. 7:162007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calmels TP and Mazurais D: In situ
hybridization: a technique to study localization of cardiac gene
expression. Methods Mol Biol. 366:159–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo H, Majmudar G, Jensen TC, Biswas C,
Toole BP and Gordon MK: Characterization of the gene for human
EMMPRIN, a tumor cell surface inducer of matrix metalloproteinases.
Gene. 220:99–108. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng HC, Wang W, Xu XY, et al:
Up-regulated EMMPRIN/CD147 protein expression might play a role in
colorectal carcinogenesis and its subsequent progression without an
alteration of its glycosylation and mRNA level. J Cancer Res Clin
Oncol. 137:585–596. 2011. View Article : Google Scholar
|
|
24
|
Suzuki S, Sato M, Senoo H and Ishikawa K:
Direct cell-cell interaction enhances pro-MMP-2 production and
activation in co-culture of laryngeal cancer cells and fibroblasts:
involvement of EMMPRIN and MT1-MMP. Exp Cell Res. 293:259–266.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanata K, Yamaguchi N, Yoshikawa K, et al:
Soluble EMMPRIN (extra-cellular matrix metalloproteinase inducer)
stimulates the migration of HEp-2 human laryngeal carcinoma cells,
accompanied by increased MMP-2 production in fibroblasts. Arch
Histol Cytol. 70:267–277. 2007. View Article : Google Scholar
|
|
26
|
Kanekura T, Chen X and Kanzaki T: Basigin
(CD147) is expressed on melanoma cells and induces tumor cell
invasion by stimulating production of matrix metalloproteinases by
fibroblasts. Int J Cancer. 99:520–528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sommers HM and Jennings RB: Experimental
acute myocardial infarction; histologic and histochemical studies
of early myocardial infarcts induced by temporary or permanent
occlusion of a coronary artery. Lab Invest. 13:1491–1503. 1964.
|
|
28
|
Seko Y, Fujimura T, Taka H, Mineki R,
Murayama K and Nagai R: Hypoxia followed by reoxygenation induces
secretion of cyclophilin A from cultured rat cardiac myocytes.
Biochem Biophys Res Commun. 317:162–168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Foda HD, Rollo EE, Drews M, et al:
Ventilator-induced lung injury upregulates and activates
gelatinases and EMMPRIN: attenuation by the synthetic matrix
metalloproteinase inhibitor, Prinomastat (AG3340). Am J Respir Cell
Mol Biol. 25:717–724. 2001. View Article : Google Scholar
|
|
30
|
Schmidt R, Redecke V, Breitfeld Y, et al:
EMMPRIN (CD 147) is a central activator of extracellular matrix
degradation by Chlamydia pneumoniae-infected monocytes.
Implications for plaque rupture. Thromb Haemost. 95:151–158.
2006.PubMed/NCBI
|
|
31
|
May AE, Schmidt R, Bülbül BO, et al:
Plasminogen and matrix metalloproteinase activation by
enzymatically modified low density lipoproteins in monocytes and
smooth muscle cells. Thromb Haemost. 93:710–715. 2005.PubMed/NCBI
|
|
32
|
Schmidt R, Bültmann A, Fischel S, et al:
Extracellular matrix metalloproteinase inducer (CD147) is a novel
receptor on platelets, activates platelets, and augments nuclear
factor kappaB-dependent inflammation in monocytes. Circ Res.
102:302–309. 2008. View Article : Google Scholar
|
|
33
|
Zhang F, Vannucci SJ, Philp NJ and Simpson
IA: Monocarboxylate transporter expression in the spontaneous
hypertensive rat: effect of stroke. J Neurosci Res. 79:139–145.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han M, Trotta P, Coleman C and Linask KK:
MCT-4, A511/Basigin and EF5 expression patterns during early chick
cardiomyogenesis indicate cardiac cell differentiation occurs in a
hypoxic environment. Dev Dyn. 235:124–131. 2006. View Article : Google Scholar
|
|
35
|
Kirk P, Wilson MC, Heddle C, Brown MH,
Barclay AN and Halestrap AP: CD147 is tightly associated with
lactate transporters MCT1 and MCT4 and facilitates their cell
surface expression. EMBO J. 19:3896–3904. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gabison EE, Mourah S, Steinfels E, et al:
Differential expression of extracellular matrix metalloproteinase
inducer (CD147) in normal and ulcerated corneas: role in
epithelio-stromal interactions and matrix metalloproteinase
induction. Am J Pathol. 166:209–219. 2005. View Article : Google Scholar
|
|
37
|
Zavadzkas JA, Plyler RA, Bouges S, et al:
Cardiac-restricted overexpression of extracellular matrix
metalloproteinase inducer causes myocardial remodeling and
dysfunction in aging mice. Am J Physiol Heart Circ Physiol.
295:H1394–H1402. 2008. View Article : Google Scholar
|
|
38
|
Burggraf D, Liebetrau M, Martens HK, et
al: Matrix metalloproteinase induction by EMMPRIN in experimental
focal cerebral ischemia. Eur J Neurosci. 22:273–277. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Betsuyaku T, Kadomatsu K, Griffin GL,
Muramatsu T and Senior RM: Increased basigin in bleomycin-induced
lung injury. Am J Respir Cell Mol Biol. 28:600–606. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shimada M, Yamabe H, Osawa H, et al:
Extracellular matrix metalloproteinase inducer is expressed in the
proximal tubular epithelial cells of the human kidney. Nephrology
(Carlton). 14:171–178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liang L, Major T and Bocan T:
Characterization of the promoter of human extracellular matrix
metalloproteinase inducer (EMMPRIN). Gene. 282:75–86. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ruiz S, Castro-Castro A and Bustelo XR:
CD147 inhibits the nuclear factor of activated T-cells by impairing
Vav1 and Rac1 downstream signaling. J Biol Chem. 283:5554–5566.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Joghetaei N, Akhyari P, Rauch BH, et al:
Extracellular matrix metalloproteinase inducer (CD147) and membrane
type 1-matrix metalloproteinase are expressed on tissue macrophages
in calcific aortic stenosis and induce transmigration in an
artificial valve model. J Thorac Cardiovasc Surg. 142:191–198.
2010. View Article : Google Scholar
|