Introduction
Peripheral nerve injury is common and previous
studies have investigated numerous approaches to accelerate neural
recovery. Low-intensity electrical stimulation (ES) has been shown
to improve nerve regeneration (1–4) by
increasing the expression of brain-derived neurotrophic factor
(BDNF) (5,6), which is known to enhance myelin
formation during the early stages of development (7). ES has also been shown to promote the
expression of growth-associated genes (8,9) and
signaling by neurotrophins (10).
Alrashdan et al(11)
reported that low-intensity ES for 30 min promoted nerve
regeneration, while Yeh et al(12) observed that the timing of ES
application affected the maturity of regenerating rat sciatic
nerves, which indicates that early intervention after severe
peripheral nerve injury may be important for recovery. Additional
studies have demonstrated that ES for 1 h facilitates nerve
regeneration (7,13,14).
Brief pulses (duration, 0.1 ms) of suprathreshold ES (3 V, 20 Hz)
have been shown to enhance remyelination and functional recovery
(2,14,15),
promoting the speed and accuracy of motor axonal regeneration
(1,16) and sensory neuron regeneration
(9). ES has also been shown to
facilitate regeneration in a diabetic model (17). Thus, ES has been proposed as a
therapeutic method to repair nerve lesions in a clinical
setting.
However, there have also been studies which
contraindicate ES therapy for peripheral nerve injury. For example,
Baptista et al(18)
demonstrated that high- and low-frequency transcutaneous electrical
nerve stimulation delayed sciatic nerve regeneration following
crush injury. Gigo-Benato et al(19) showed that ES impaired early
functional recovery and exacerbated skeletal muscle atrophy after
sciatic nerve crush injury in rats. Hamilton et al(20) revealed that ES promoted axon
regeneration at the expense of decreasing the fidelity of muscle
reinnervation, thus the functional recovery was unchanged. Lu et
al(21) determined that ES was
able to have a positive or negative impact on peripheral nerve
regeneration and recommended that clinical trials which combine
stimulation with rehabilitation should identify safe and effective
parameters. Thus, whether ES therapy is beneficial after peripheral
nerve trauma remains controversial and requires further
investigation.
The aim of the present study was to determine
whether brief ES improves functional recovery after a crush injury
by promoting remyelination, which is the main process underlying
the restoration of injured peripheral nerves. Myelin protein zero
(P0) is a marker of axon regeneration and remyelination (22) and its mRNA and protein levels were
determined using RT-PCR and western blotting, respectively, to
assess recovery.
Materials and methods
Animals
Wistar rats (200 g; n=54) were obtained from the
Experimental Animal Center of China Medical University (Shenyang,
China; certification no. SCXK Liao 2003-0009). This study was
approved by the Institutional Animal Care and Use Committee and the
Experimental Animal Administration Committee of China Medical
University; it was determined that the number of animals used in
the present study and their distress was appropriately
minimized.
Surgical procedure and electrical
stimulation
Rats were anesthetized with 10% chloral hydrate (0.3
ml/100 g; i.p.). The right sciatic nerve was exposed and crushed
for 3 min using a non-serrated clamp with a force of 54 N (23) to induce an axonotmesic lesion. The
crush site was ~6 mm long, located 5 mm above the bifurcation and
was sutured with 8-0 nylon as a marker. The proximal nerve trunk
was electrically stimulated as described by Al-Majed et
al(1). The rats were randomly
divided into 3 groups (n=18/group); the control, crush or crush +
ES group. Rats of the control group had sciatic nerve exposure with
no crush procedures, while rats of the crush group had electrodes
implanted with the stimulator turned off. Rats of the crush + ES
group had electrodes implanted proximal to the injury site to
deliver a continuous train of 20 Hz square pulses of 3 V at 0.1 ms
for 1 h. The wounds were kept warm and moist with sterile saline
gauze during the ES. Following completion of the ES procedure was
completed, the skin was sutured with 4-0 stitches and all the rats
were housed in cages with food and water ad libitum for 4
weeks.
Motor function evaluation using walking
track analysis
An assay of motor nerve functional recovery was
performed weekly for 4 weeks following surgery. The sciatic
functional index (SFI) was calculated, as described by Bain et
al(24). The hind paws of rats
trained on the procedure prior to surgery were dipped in blue ink
and these rats were allowed to walk down a plastic corridor (60 cm
long, 10 cm wide) lined with white paper. The SFI was calculated
according to the following equation: SFI = −38.3(EPL-NPL)/NPL +
109.5(ETS-NTS)/NTS + 13.3(EIT-NIT)/NIT - 8.8. PL was defined as the
distance between the heel and the third toe, TS as the distance
between the first and fifth toes and IT as the distance between the
second and fourth toes. E and N represented the experimental and
normal sides.
Electrophysiological assessment
Four weeks after surgery, all the rats were
anesthetized and the right sciatic nerve was exposed. A bipolar
stimulating electrode was placed around the sciatic nerve, proximal
to the injury site and a bipolar recording electrode was placed in
the gastrocnemius muscle. Compound muscle action potential (CMAP)
and amplitude were recorded using an RM6240 physiological signal
processing apparatus (Chengdu Instrument Factory, Chengdu, China).
The distance between the two electrodes was measured and used to
calculate the CMAP conduction velocity.
Nerve histomorphometry
The crush sites of sciatic nerves (n=6/group) were
removed and fixed in 2.5% glutaraldehyde solution, dehydrated in
graded acetone, which was then replaced with acetone, and embedded
in epoxyresin for sectioning. Semi-thin cross sections (2 μm) were
obtained using a microtome (UltraCut E; Leica Microsystems, Vienna,
Austria) and stained with 1% toluidine blue solution for light
microscopy, while ultra-thin cross sections (70 nm) were analyzed
using a transmission electron microscope (TEM, JEM-1200EX; Jeol
Ltd., Tokyo, Japan). Myelin sheath thickness and axonal diameter
were analyzed using the MetaMorph/DP10/BX41 image analysis
system.
RT-PCR
The crush sites of sciatic nerves (n=6/group) were
excised and homogenized for RT-PCR. The total RNA was extracted
using the TRIzol method and cDNA was synthesized using an
oligo(dT)-adaptor primer. PCR was performed using a kit (Takara
Biomedical Technology, Dalian, China), with the following 35
cycles: 94°C for 30 sec, 60°C (P0 and GAPDH) for 30 sec and 72°C
for 45 sec. GAPDH was used as an internal control. The following
gene-specific primers were used: P0 forward,
5′-CTCTTCTCTTCTTTGGTGCT-3′ and reverse, 5′-TTCTTATCCTTGCGAGACTC-3′
(692-bp amplification fragment); and GAPDH forward,
5′-GGTGAAGGTCGGT GTGAACG-3′ and reverse, 5′-CAAAGTTCTCATGGAT
GACC-3′ (497-bp amplification fragment). The amplification product
was visualized using 1.5% agarose gel electrophoresis and analyzed
with Image J software. Data were expressed as the ratio between the
amplification products of P0 and GAPDH.
Western blotting
Four weeks after surgery, the crush sites of sciatic
nerves (n=6/group) were excised and lysed in ice-cold RIPA buffer
containing protease inhibitor (PMSF) using an ultrasonic wave
disintegrator. The samples of total protein lysate (20 μg) were
resolved on 10% SDS-PAGE gels by electrophoresis and transferred
onto PVDF membranes. The membranes were blocked at room temperature
with 20% bovine serine albumin (BSA) dissolved in Tris-buffered
saline containing 1% Tween-20 (TBST) for 2 h. The membranes were
then incubated with goat polyclonal antibody to P0 (1:1,000; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and mouse monoclonal
antibody to GAPDH (1:5,000; Santa Cruz Biotechnology, Inc.)
overnight at 4°C. After rinsing with TBST three times, the
membranes were incubated at room temperature for 2 h with rabbit
anti-goat IgG (1:5,000; Santa Cruz Biotechnology, Inc.) and rabbit
anti-mouse IgG (1:5,000; Santa Cruz Biotechnology, Inc.). Secondary
antibodies were visualized using a BeyoECL Plus kit (Beyotime
Institute of Biotechnology, Jiangsu, China) using ChemDoc XRS with
Quantity One software (Bio-Rad, Hercules, CA, USA). Band
intensities were quantified using Image-Pro Plus 6.0 software. The
blots were repeated ≥3 times for each condition.
Statistical analysis
Data were analyzed using the SPSS 13.0 software, by
a one-way analysis of variance (ANOVA) and post hoc multiple
comparisons were assessed using Tukey's test. Results are expressed
as the mean ± standard error of the mean (SEM). P<0.05 was
considered to indicate a statistically significant difference.
Results
Motor function evaluation
As shown in Fig. 1,
the difference in the SFI between the control and experimental
(crush and crush + ES) groups was statistically significant
(P<0.05) at 1 and 2 weeks after surgery, whereas the difference
between the two experimental groups was not significant
(P>0.05). The mean SFI at 3 and 4 weeks after surgery was
−25.99±3.04 and −18.55±4.10, respectively, in the crush group,
whereas that of the crush + ES group was −16.15±3.95 and
−10.81±4.00, respectively. There was a significant difference in
the SFI between the crush and crush + ES groups (P<0.05).
Electrophysiological assessment
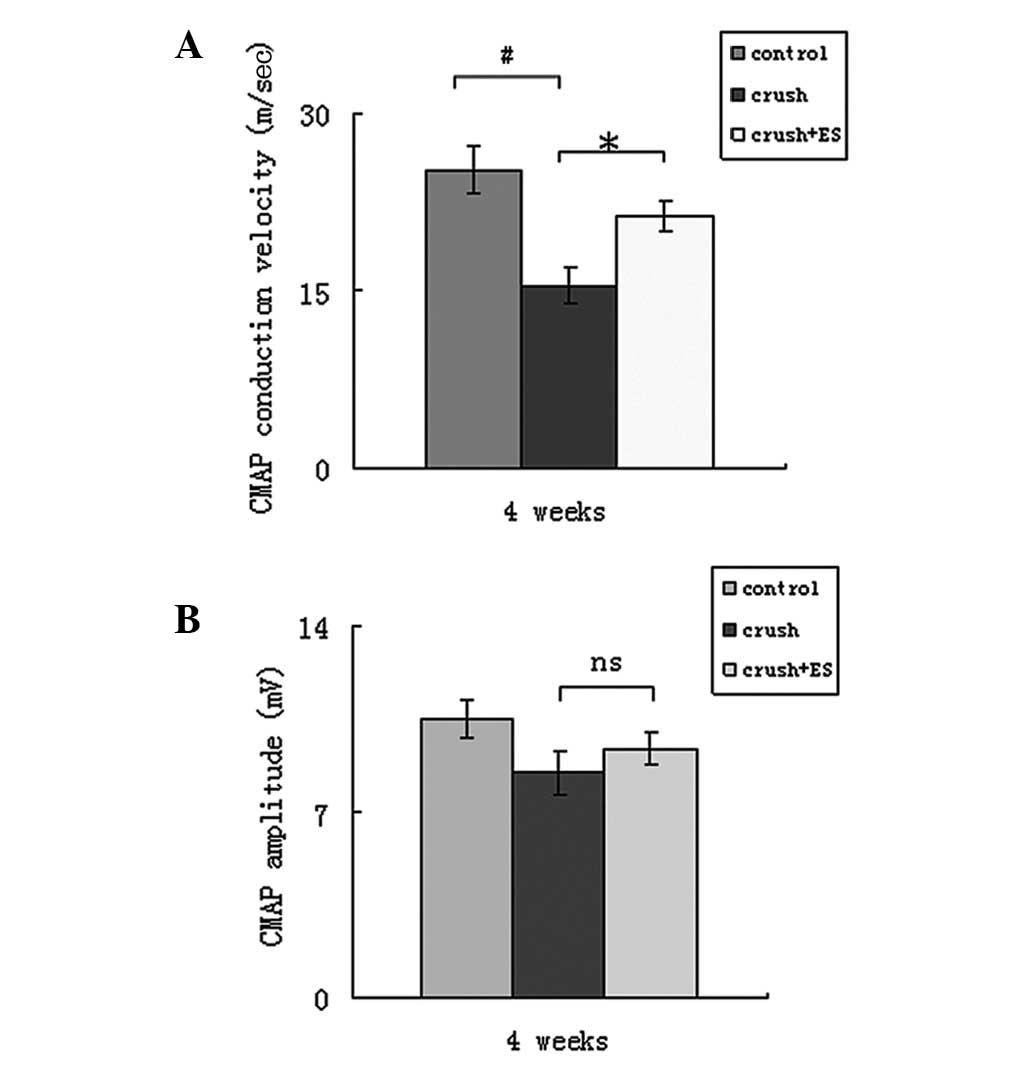
Four weeks after surgery, the mean CMAP conduction
velocity was 25.27±2.00 m/sec in the control group, 15.34±1.50
m/sec in the crush group and 21.36±1.25 m/sec in the crush + ES
group (Fig. 2A). A significant
difference was observed between the control and crush groups
(P<0.05) and the crush and crush + ES groups (P<0.05).
However, no significant difference in CMAP conduction velocity was
identified between the control and crush + ES groups (P>0.05).
As shown in Fig. 2B, the mean CMAP
amplitude was 10.5±0.7 mV in the control group, 8.5±0.8 mV in the
crush group and 9.4±0.6 mV in the crush + ES group. No significant
differences were found between the control and experimental groups
(P>0.05).
Nerve histomorphometry
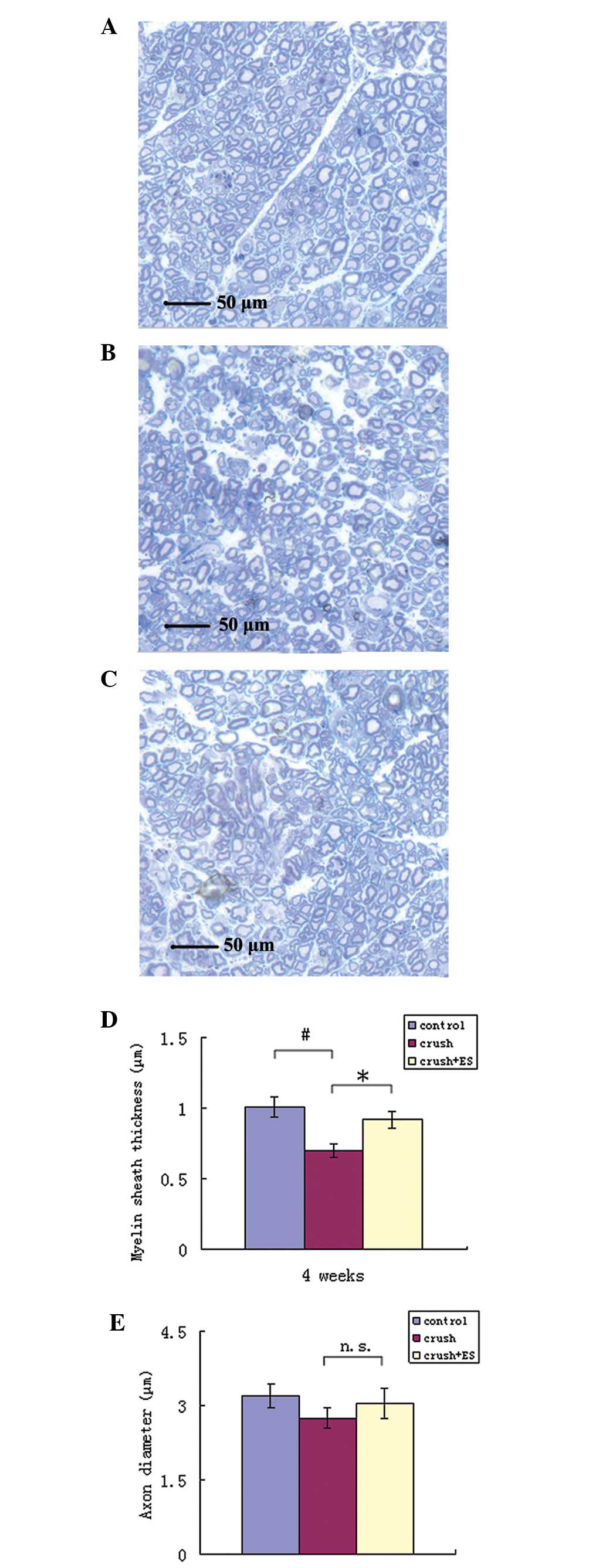
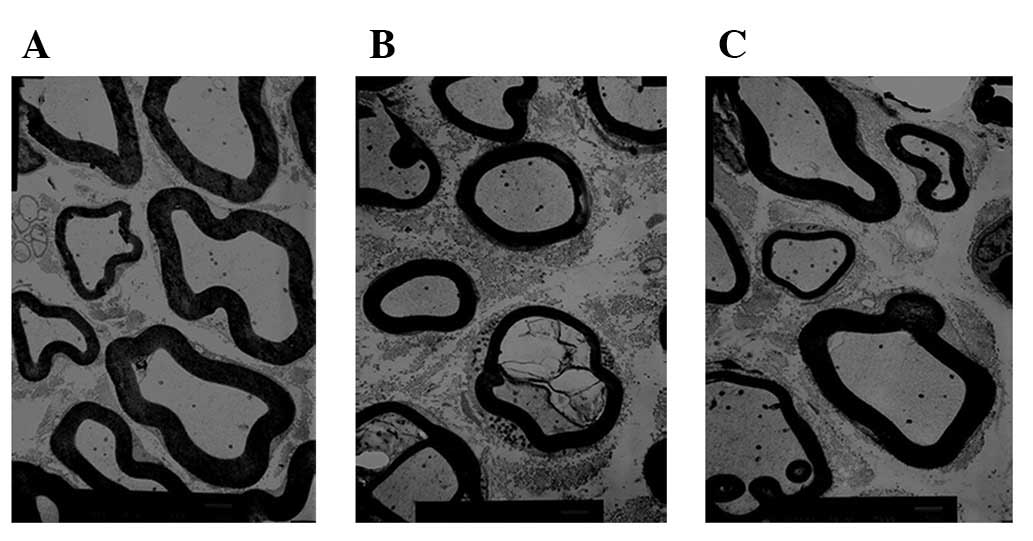
The myelin sheath thickness and axonal diameter were
evaluated in the crush (Figs. 3A
and 4A), crush + ES (Figs. 3B and 4B) and control groups (Figs. 3C and 4C) four weeks after surgery. The mean
myelin sheath thickness was 1.01±0.07 μm in the control, 0.70±0.05
μm in the crush and 0.92±0.06 μm in the crush + ES group (Fig. 3D). A significant difference was
observed between the crush and crush + ES groups (P<0.05) and
the crush and control groups (P<0.05). As shown in Fig. 3E, the mean axonal diameter was
3.20±0.23 μm in the control, 2.75±0.22 μm in the crush and
3.05±0.30 μm in the crush + ES group; no significant differences
were identified between the control and experimental groups
(P>0.05).
P0 mRNA and protein levels
Four weeks after surgery, the levels of P0
mRNA/GAPDH mRNA (Fig. 5) and P0
protein (Fig. 6) were
significantly decreased in the crush group compared with the
control group (P<0.05). The levels of P0 mRNA/GAPDH mRNA and P0
protein were increased in the crush + ES group compared with the
crush group (P<0.05).
Discussion
There have been conflicting results from previous
studies on the effect of ES on injured peripheral nerves (5). The present study showed that ES (20
Hz, 0.1 ms, 3 V, 1 h) enhances axonal regeneration when applied
immediately after nerve injury, which supports the results of a
number of previous studies (1,3,4). The
SFI has often been used to assess the recovery of motor function
following nerve injury. In the present study, walking track
analysis was used to determine the SFI every week for 4 weeks
following surgery. A significantly improved SFI was observed in the
crush + ES group at 3 and 4 weeks after surgery. Further
physiological and histological measures also demonstrated an
improvement, with the exception of the CMAP amplitude and axonal
diameter, indicating that ES is a potential early intervention
therapy for peripheral nerve injury.
During the course of peripheral nerve repair,
Schwann cells proliferate and form the myelin sheath to promote
axonal regeneration from the proximal to the distal end. P0 is the
most abundant protein within the peripheral myelin sheath (25). P0 mediates cell-to-cell
interactions via homophilic binding and stabilizes the major dense
line in the peripheral nervous system, which is essential for
normal myelin formation and maintenance (26). Mirsky et al(27) suggested that the P0 gene is
upregulated during Schwann cell myelination. In the present study,
P0 mRNA and protein levels were increased in the myelin sheath
following ES, indicating an upregulation of the mRNA and protein
levels. Combined with the enhanced CMAP conduction velocity and
thickened myelin sheath following ES, these data supported our
hypothesis that ES promotes Schwann cell proliferation and
myelination.
One potential confounding factor in the present
study was the 1 h delay for the skin to be sutured after surgery,
which may have delayed axonal regeneration. Future studies should
include a group that undergoes suturing immediately after the crush
injury. Furthermore, it is important to note that the stimulator
should be placed at an appropriate site, as described by Al-Majed
et al(1). The stimulator
may induce continuous vigorous contractions of the nearby muscles
when not insulated from surrounding tissues, which may hinder
regeneration and recovery.
The present study demonstrated that ES accelerates
axonal regeneration after nerve injury, which may provide an early
therapeutic strategy for nerve injury.
Acknowledgements
This study was supported by grants from the Shenyang
Science and Technology Development Fund (no. F10-205-1-69) and the
Liaoning Science and Technology Development Fund (no.
2010225029).
References
|
1
|
Al-Majed AA, Brushart TM and Gordon T:
Electrical stimulation accelerates and increases expression of BDNF
and trkB mRNA in regenerating rat femoral motoneurons. Eur J
Neurosci. 12:4381–4390. 2000.PubMed/NCBI
|
|
2
|
Gordon T, Brushart TM and Chan KM:
Augmenting nerve regeneration with electrical stimulation. Neurol
Res. 30:1012–1022. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lal D, Hetzler LT, Sharma N, Wurster RD,
Marzo SJ, Jones KJ and Foecking EM: Electrical stimulation
facilitates rat facial nerve recovery from a crush injury.
Otolaryngol Head Neck Surg. 139:68–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim J, Han SJ, Shin DH, Lee WS and Choi
JY: Subthreshold continuous electrical stimulation facilitates
functional recovery of facial nerve after crush injury in rabbit.
Muscle Nerve. 43:251–258. 2011. View Article : Google Scholar
|
|
5
|
Al-Majed AA, Neumann CM, Brushart TM and
Gordon T: Brief electrical stimulation promotes the speed and
accuracy of motor axonal regeneration. J Neurosci. 20:2602–2608.
2000.
|
|
6
|
Alrashdan MS, Park JC, Sung MA, et al:
Thirty minutes of low intensity electrical stimulation promotes
nerve regeneration after sciatic nerve crush injury in a rat model.
Acta Neurol Belg. 110:168–179. 2010.
|
|
7
|
Wan L, Xia R and Ding W: Short-term
low-frequency electrical stimulation enhanced remyelination of
injured peripheral nerves by inducing the promyelination effect of
brain-derived neurotrophic factor on Schwann cell polarization. J
Neurosci Res. 88:2578–2587. 2010.
|
|
8
|
Al-Majed AA, Tam SL and Gordon T:
Electrical stimulation accelerates and enhances expression of
regeneration-associated genes in regenerating rat femoral
motoneurons. Cell Mol Neurobiol. 24:397–402. 2004.PubMed/NCBI
|
|
9
|
Geremia NM, Gordon T, Brushart TM,
Al-Majed AA and Verge VM: Electrical stimulation promotes sensory
neuron regeneration and growth-associated gene expression. Exp
Neurol. 205:347–359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
English AW, Schwartz G, Meador W, Sabatier
MJ and Mulligan A: Electrical stimulation promotes peripheral axon
regeneration by enhanced neuronal neurotrophin signaling. Dev
Neurobiol. 67:158–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alrashdan MS, Sung MA, Kwon YK, Chung HJ,
Kim SJ and Lee JH: Effects of combining electrical stimulation with
BDNF gene transfer on the regeneration of crushed rat sciatic
nerve. Acta Neurochir (Wien). 153:2021–2029. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeh CC, Lin YC, Tsai FJ, Huang CY, Yao CH
and Chen YS: Timing of applying electrical stimulation is an
important factor deciding the success rate and maturity of
regenerating rat sciatic nerves. Neurorehabil Neural Repair.
24:730–735. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahlborn P, Schachner M and Irintchev A:
One hour electrical stimulation accelerates functional recovery
after femoral nerve repair. Exp Neurol. 208:137–144. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Asensio-Pinilla E, Udina E, Jaramillo J
and Navarro X: Electrical stimulation combined with exercise
increase axonal regeneration after peripheral nerve injury. Exp
Neurol. 219:258–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vivó M, Puigdemasa A, Casals L, Asensio E,
Udina E and Navarro X: Immediate electrical stimulation enhances
regeneration and reinnervation and modulates spinal plastic changes
after sciatic nerve injury and repair. Exp Neurol. 211:180–193.
2008.
|
|
16
|
Huang J, Lu L, Hu X, et al: Electrical
stimulation accelerates motor functional recovery in the rat model
of 15-mm sciatic nerve gap bridged by scaffolds with longitudinally
oriented microchannels. Neurorehabil Neural Repair. 24:736–745.
2010. View Article : Google Scholar
|
|
17
|
Yao CH, Chang RL, Chang SL, Tsai CC, Tsai
FJ and Chen YS: Electrical stimulation improves peripheral nerve
regeneration in streptozotocin-induced diabetic rats. J Trauma
Acute Care Surg. 72:199–205. 2012.PubMed/NCBI
|
|
18
|
Baptista AF, Gomes JR, Oliveira JT, Santos
SM, Vannier-Santos MA and Martinez AM: High- and low-frequency
transcutaneous electrical nerve stimulation delay sciatic nerve
regeneration after crush lesion in the mouse. J Peripher Nerv Syst.
13:71–80. 2008. View Article : Google Scholar
|
|
19
|
Gigo-Benato D, Russo TL, Geuna S,
Domingues NR, Salvini TF and Parizotto NA: Electrical stimulation
impairs early functional recovery and accentuates skeletal muscle
atrophy after sciatic nerve crush injury in rats. Muscle Nerve.
41:685–693. 2010. View Article : Google Scholar
|
|
20
|
Hamilton SK, Hinkle ML, Nicolini J, et al:
Misdirection of regenerating axons and functional recovery
following sciatic nerve injury in rats. J Comp Neurol. 519:21–33.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu MC, Ho CY, Hsu SF, Lee HC, Lin JH, Yao
CH and Chen YS: Effects of electrical stimulation at different
frequencies on regeneration of transected peripheral nerve.
Neurorehabil Neural Repair. 22:367–373. 2008.PubMed/NCBI
|
|
22
|
Li FQ, Fowler KA, Neil JE, Colton CA and
Vitek MP: An apolipoprotein E-mimetic stimulates axonal
regeneration and remyelination after peripheral nerve injury. J
Pharmacol Exp Ther. 334:106–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beer GM, Steurer J and Meyer VE:
Standardizing nerve crushes with a non-serrated clamp. J Reconstr
Microsurg. 17:531–534. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bain JR, Mackinnon SE and Hunter DA:
Functional evaluation of complete sciatic, peroneal, and posterior
tibial nerve lesions in the rat. Plast Reconstr Surg. 83:129–138.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao L and Zheng Y: Correlation and
toxicological significance between myelin protein zero and
peripheral nerve disease. Wei Sheng Yan Jiu. 39:635–638. 2010.(In
Chinese).
|
|
26
|
Shen D, Zhang Q, Gao X, Gu X and Ding F:
Age-related changes in myelin morphology, electrophysiological
property and myelin-associated protein expression of mouse sciatic
nerves. Neurosci Lett. 502:162–167. 2011. View Article : Google Scholar
|
|
27
|
Mirsky R, Jessen KR, Brennan A, et al:
Schwann cells as regulators of nerve development. J Physiol Paris.
96:17–24. 2002. View Article : Google Scholar : PubMed/NCBI
|