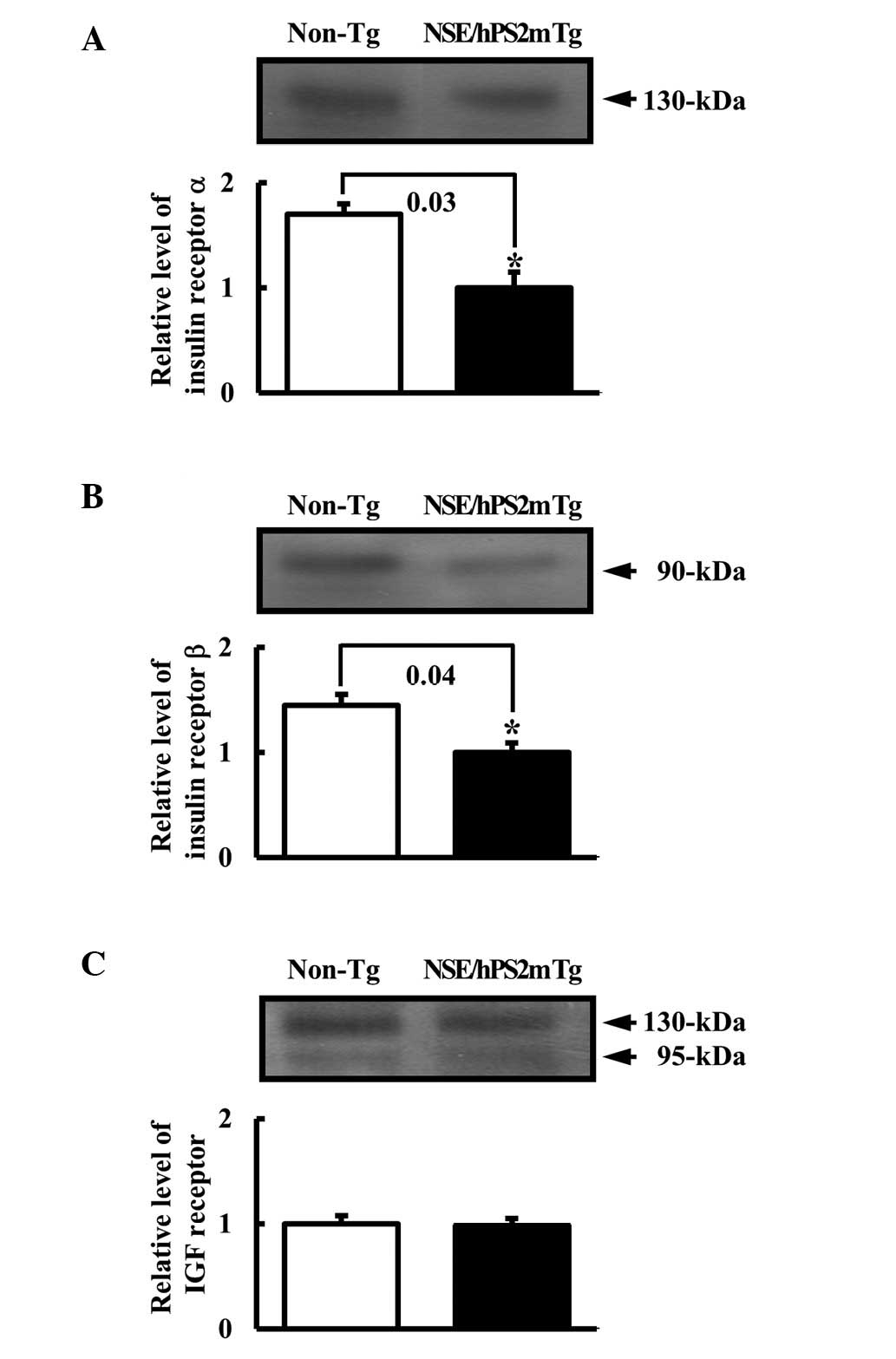
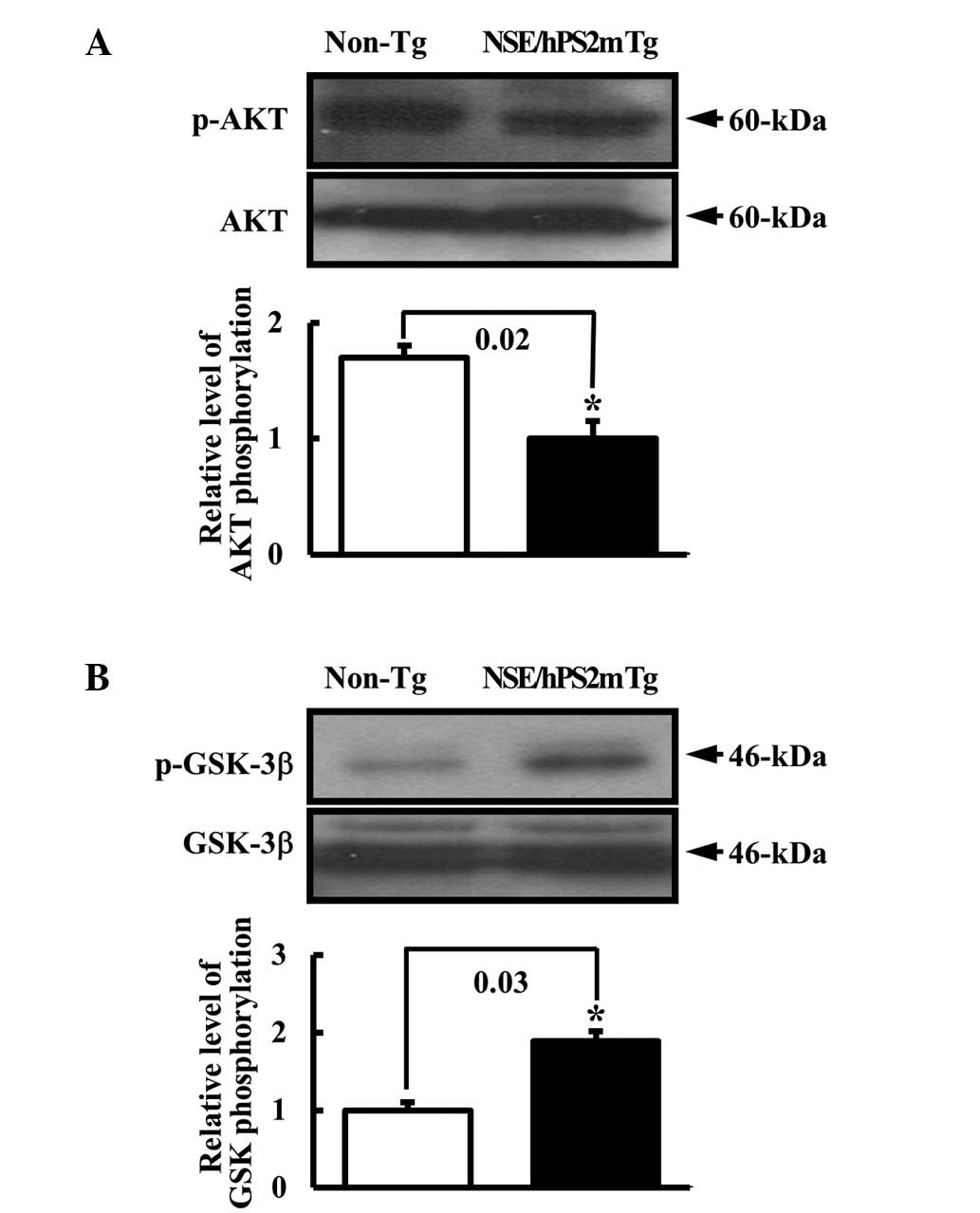
|
1
|
Gsell W, Moll G, Sofic E and Riederer P:
Cholinergic and monoaminergic neurotransmitter systems in patients
with Alzheimer’s disease and senile dementia of Alzheimer type: a
clinical evaluation. Dementias-neurochemistry, neuropathology,
neuroimaging, neuropsychology and genetics. Maurer K: Braunschweig;
Vieweg: pp. 25–51. 1993
|
|
2
|
Hoyer S: Senile dementia and Alzheimer’s
disease. Brain blood flow and metabolism. Prog Neuropsychopharmacol
Biol Psychiatry. 10:447–478. 1986.
|
|
3
|
Hoyer S, Oesterreich K and Wanger O:
Glucose metabolism as the site of the primary abnormality in
early-onset dementia of Alzheimer type? J Neurol. 235:143–148.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoyer S, Nitsch R and Oesterreich K:
Predominant abnormality in cerebral glucose utilization in
late-onset dementia of the Alzheimer type: a cross-sectional
comparison against advanced late-onset and incipient early-onset
cases. J Neural Transm Park Dis Dement Sect. 3:1–14. 1991.
View Article : Google Scholar
|
|
5
|
Bigl M, Apelt J, Eschrich K and Schliebs
R: Cortical glucose metabolism is altered in aged transgenic Tg2576
mice that demonstrate Alzheimer plaque pathology. J Neural Transm.
110:77–94. 2003.PubMed/NCBI
|
|
6
|
Mooradian AD, Chung HC and Shah GN: Glut-1
expression in the cerebral of patients with Alzheimer’s disease.
Neurobiol Aging. 18:469–474. 1997.
|
|
7
|
Simpson IA, Chundu KR, Davies-Hill T,
Honer WG and Davies P: Decreased concentrations of Glut1 and Glut3
glucose transporters in the brains of patients with Alzheimer’s
disease. Ann Neurol. 35:546–551. 1994.PubMed/NCBI
|
|
8
|
Baskin DG, Wilcox BJ, Figlewicz DP and
Dorsa DM: Insulin and insulin-like growth factors in the CNS.
Trends Neurosci. 11:107–111. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wozniak M, Rydzewski B, Baker SP and
Raizada MK: The cellular and physiological actions of insulin in
the central nervous system. Neurochem Int. 22:1–10. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harvankova J, Schmerchel D, Roth J and
Brownstein M: Identification of insulin in rat brain. Proc Natl
Acad Sci USA. 75:5737–5741. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Devasker SU, Giddings SJ, Rajakumar PA,
Carnaghi LR, Menon RK and Zahm DS: Insulin gene expression and
insulin synthesis in mammalian neuronal cells. J Biol Chem.
269:8445–8454. 1994.PubMed/NCBI
|
|
12
|
Hoyer S, Prem L, Sorbi S and Amsucci L:
Stimulation of glycolytic key enzymes in cerebral cortex by
insulin. Neuroreport. 4:991–993. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Pablo F and de la Rosa E: The
developing CNS: a scenario for the action of proinsulin, insulin
and insulin-like growth factors. Trends Neurosci. 18:143–150.
1995.PubMed/NCBI
|
|
14
|
Calissano P, Ciotti MT, Battistini L, Zona
C, Angelini A, Merlo D and Mercanti D: Recombination insulin-like
growth factor I exerts a trophic action and confers glutamate
sensitivity on glutamate-resistant cerebellar cells. Proc Natl Acad
Sci USA. 90:8752–8756. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Quirion R, Araujo DM, Lapehak PA, Seto D
and Chabot JG: Growth factors and lymphokines: modulators of
cholinergic neuronal activity. Can J Neurol Sci. 18:390–393.
1991.PubMed/NCBI
|
|
16
|
Sun XJ, Rothenberg P, Kahn CR, Backer JM,
Araki E, Wilden PA, Cahill DA, Goldstein BJ and White MF: Structure
of the insulin receptor substrate ISR-1 defines a unique signal
transduction protein. Nature. 352:73–77. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
White MF and Kahn CR: The insulin
signaling system. J Biol Chem. 269:1–4. 1994.
|
|
18
|
Frolich L, Blum-Degen D, Bernstein HG,
Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk
A, Hoyer S, Zochling R, Boissle KW, Jellinger K and Riederer P:
Brain insulin and insulin receptors in aging and sporadic
Alzheimer’s disease. J Neural Transm. 105:423–438. 1998.
|
|
19
|
Hwang DY, Chae KR, Kang TS, Hwang JH, Lim
CH, Kang HK, Goo JS, Lee MR, Lim HJ, Min SH, Cho JY, Hong JT, Song
CW, Paik SG, Cho JS and Kim YK: Alterations in behavior, amyloid
β-42, caspase-3, and Cox-2 in mutant PS2 transgenic mouse model of
Alzheimer’s disease. FASEB J. 16:805–813. 2002.
|
|
20
|
Hwang DY, Cho JS, Oh JH, Shim SB, Jee SW,
Lee SH, Seo SJ, Lee SK, Lee SH and Kim YK: Differentially expressed
genes in transgenic mice carrying human mutant presenilin-2
(N141I): correlation of selenoprotein M with Alzheimer’s disease.
Neurochem Res. 30:1009–1019. 2005.PubMed/NCBI
|
|
21
|
Garofalo RS, Orena SJ, Rafidi K, Torchia
AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks
JR, McNeish JD and Coleman KG: Severe diabetes, age-dependent loss
of adipose tissue, and mild growth deficiency in mice lacking
Akt2/PKB beta. J Clin Invest. 112:197–208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meijer L, Flajolet M and Greengard P:
Pharmacological inhibitors of glycogen synthase kinase 3. Trends
Pharmacol Sci. 25:471–480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boyt AA, Taddei TK, Hallmayer J,
Helmerhorst E, Gandy SE, Craft S and Martins RN: The effect of
insulin and glucose on the plasma concentration of Alzheimer’s
amyloid precursor protein. Neuroscience. 95:727–734. 2000.
|
|
24
|
Meier-Ruge WA and Bertoni-Freddari C:
Pathogenesis of decreased glucose turnover and oxidative
phosphorylation in ischemic and trauma-induced dementia of the
Alzheimer type. Ann N Y Acad Sci. 826:229–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoyer S: Brain glucose and energy
metabolism abnormalities in sporadic Alzheimer disease. Causes and
consequences: an update. Exp Gerontol. 35:1363–1372. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoyer S: The brain insulin signal
transduction system and sporadic (type II) Alzheimer disease: an
update. J Neural Transm. 109:341–360. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oyama F, Sawamura N, Kobayashi K,
Morishima-Kawashima M, Kuramochi T, Ito M, Tomita T, Maruyama K,
Saido TC, Iwatsubo T, Capell A, Walter J, Grunberg J, Ueyama Y,
Haass C and Ihara Y: Mutant presenilin 2 transgenic mouse: effect
on an age-dependent increase of amyloid beta-protein 42 in the
brain. J Neurochem. 71:313–322. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Polonsky KS and Rubenstein AH: C-peptide
as a measure of the secretion an hepatic extraction of insulin.
Pitfalls and limitations Diabetes. 33:486–494. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kurochkin IV and Goto S: Alzheimer’s
beta-amyloid peptide specifically interacts with and is degraded by
insulin degrading enzyme. FEBS Lett. 345:33–37. 1994.
|
|
30
|
Authier F, Posner BI and Bergeron JJ:
Insulin-degrading enzyme. Clin Invest Med. 19:149–160. 1996.
|
|
31
|
Perez A, Morelli L, Cresto JC and Castano
EM: Degradation of soluble amyloid beta-peptides 1–40, 1–42, and
the Dutch variant 1–40Q by insulin degrading enzyme from Alzheimer
disease and control brains. Neurochem Res. 25:247–255. 2000.
|
|
32
|
Vekrellis K, Ye Z, Qui WQ, Walsh D,
Hartley D, Cheseneau V, Rosner MR and Selkoe DJ: Neurons regulate
extracellular levels of amyloid-protein via proteolysis by
insulin-degrading enzyme. J Neurosci. 20:1657–1665. 2000.PubMed/NCBI
|
|
33
|
Henneberg N and Hoyer S: Short-term or
long-term intracerebroventricular (i. cv) infusion of insulin
exhibits a discrete anabolic effect on cerebral energy metabolism
in the rat. Neurosci Lett. 175:153–156. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
De Keyser J, Wilczak N and Goosens A:
Insulin-like growth factor-1 receptor densities in human frontal
cortex and white matter during aging, in Alzheimer’s disease, and
in Huntington’s disease. Neurosci Lett. 172:93–96. 1994.PubMed/NCBI
|
|
35
|
Crew FT, McElhaney R, Freund G, Ballinger
WE Jr and Raizada MK: Insulin-like growth factor I receptor binding
in brains of Alzheimer’s and alcoholic patients. J Neurochem.
58:1205–1210. 1992.
|
|
36
|
Stein TD and Johnson JA: Lack of
neurodegeneration in transgenic mice overexpressing mutant amyloid
precursor protein is associated with increased level of
transthyretin and the activation of cell survival pathways. J
Neurosci. 22:7380–7388. 2002.PubMed/NCBI
|
|
37
|
Craft S, Dagogo-Jack SE, Wiethop BV,
Murphy C, Nevins R, Fleschman S, Rice V, Newcomber JW and Cryer PE:
Effects of hyperglycemia on memory and hormone levels in dementia
of the Alzheimer type: a longitudinal study. Behav Neurosci.
107:926–940. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Manning CA, Ragozzino ME and Gold PE:
Glucose enhancement of memory in patients with probable senile
dementia of the Alzheimer’s type. Neurobiol Aging. 14:523–528.
1993.
|
|
39
|
Harr SD, Simonian NA and Hyman BT:
Functional alterations in Alzheimer’s disease: decreased glucose
transporter 3 immunoreactivity in the perforant pathway terminal
zone. J Neuropathol Exp Neurol. 54:38–41. 1995.
|
|
40
|
Sivitz WS, DeSautel PS and Pessin JE:
Regulation of the glucose transporter in developing rat brain.
Endocrinology. 124:1875–1880. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Craft S, Asthana S, Cook DG, Baker LD,
Cherrier M, Purganan K, Wait C, Petrova A, Latendresse S, Watson
GS, Newcomer JW, Schellenberg GD and Krohn AJ: Insulin
dose-response effects on memory and plasma amyloid precursor
protein in Alzheimer’s disease: interactions with apolipoprotein E
genotype. Psychoneuroendocrinology. 28:809–822. 2003.
|