Introduction
Cancer develops as a result of multiple genetic and
epigenetic alterations. Functional imbalances between oncogenes and
tumor suppressor genes have been identified in most types of
cancer, including gastric cancer (1). Better knowledge of the changes in
gene expression that occur during gastric carcinogenesis may lead
to improvements in diagnosis, treatment and prevention of gastric
cancer (2). Increasing evidence
shows that there are disorders of signaling pathways and
disequilibria between oncogenes and tumor suppressor genes in the
occurrence and development of gastric cancer (3,4). The
homeostatic self-renewal of the stomach depends on a complex
interplay between processes involved in cell proliferation,
differentiation, migration, adhesion and cell death (5). This panoply of cellular responses is
coordinated by a relatively small number of highly conserved
signaling pathways, which include the bone morphogenetic protein
(BMP) (6,7), Sonic Hedgehog (8), Notch (9) and Wnt (10) signaling pathways. Deregulation of
these pathways may lead to pathological conditions, such as cancer.
This notion is particularly well-illustrated by the role of the
Notch and Wnt pathway in the gastric mucosa cells (11).
The Notch signaling pathway is a highly conserved
signaling system in mammals, which has been found to play central
roles in stem cell maintenance, cell fate decisions, as well as
cancer in humans (12,13). Notch receptors are single-span
membrane proteins (Notch1–4 in mammals). The ligands for Notch are
similar but smaller single-pass transmembrane proteins, consisting
of three δ-like proteins (DLL-1, -3 and -4) and two Jagged proteins
(JAG-1 and -2) (14,15). Binding to Notch by ligand on an
adjacent cell triggers two enzymatic cleavages of Notch,
extracellularly by an α-secretase (a disintegrin and
metalloprotease ADAM-10 and ADAM-17) and intracellularly by the
γ-secretase/presenilin complex. The liberated Notch intracellular
domain (NICD) then travels to the nucleus, displaces corepressors
from CBF1/Suppressor of hairless/LAG-1 (CSL) transcription factors,
and recruits coactivators such as histone acetyl-transferase and
Mastermind-like to activate downstream target genes such as the
Hes and Hey gene families (16–18).
A number of relevant studies have showed that the expression levels
of the Notch signaling pathway components change in gastric cancer
tissues (9,12). However, there is not a final
conclusion about whether it plays a cancer-suppressor or a
cancer-promoting role on the occurrence and development of gastric
cancer.
The classic Wnt/β-catenin signaling pathway is also
known to play a critical role on cell proliferation and
homeostasis. In the absence of Wnt signals, free cytoplasmic
β-catenin is actively targeted for degradation. This is
accomplished by glycogen synthase kinase-3β (GSK-3β) and two
scaffolding proteins, the tumor suppressors adenomatous polyposis
coli (APC) and Axin/Axin2, in the so-called destruction complex.
Thus, β-catenin is not able to accumulate excessively (19). The binding of Wnt ligands to
corresponding frizzled receptors induces the excessive accumulation
of β-catenin in the cytoplasm (20,21).
Then, the β-catenin translocates into the nucleus and binds to the
lymphoid enhancer factor (LEF)/T cell factor (TCF) families and
activates downstream target genes such as c-Myc and cyclin D1
(22,23). Previous studies have demonstrated
that aberrant Wnt/c-Myc-catenin signaling is detected in a wide
variety of human tumors, including breast cancer, melanoma and
colon cancer (24–26). Sergio and Kalaska (27) also confirmed that the expression
changes of the Wnt/β-catenin signaling pathway occur in gastric
cancer tissues, as well as the correlation with clinical stages and
the degrees of differentiation of gastric cancer.
In the present study, we co-activated the Notch and
Wnt signaling in BGC-823 human gastric cancer cells and
investigated the effects of this co-activation on the proliferation
of tumor cells and the possible underlying mechanism.
Materials and methods
Cell line and culture
Human gastric carcinoma BGC-823 cells (China Center
for Type Culture Collection, Wuhan University, Hubei, China) were
cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO,
USA), 100 U/ml penicillin and 100 μg/ml streptomycin. All the cells
were cultured in a humidified environment (37°C and 5%
CO2). BGC-823 cells were treated using Notch1 signaling
pathway activator doxycycline (Promega, Madison, WI, USA) and
Wnt/β-catenin signaling pathway activation agent LiCl (Amresco,
Solon, OH, USA) for up to 48 h.
Western blot analysis
Cells were lysed using RIPA (1% SDS, 5% sodium
deoxycholate, 1% NP-40, 0.1% PMSF) and protein was determined
according to the Lowry method. Equal amounts of proteins were
separated in 10% SDS-polyacrylamide gel electrophoresis and
transferred to polyvinylidene fluoride membranes. The membranes
were blocked by 3% bovine serum albumin (BSA) in the Tris-buffered
saline with Tween-20 (TBST) for 2 h at room temperature and then
incubated with primary antibodies (Abcam, Cambridge, MA, USA) at
4°C overnight. The corresponding horseradish peroxidase-conjugated
secondary antibodies were incubated for 2 h at room temperature.
The protein bands were determined by electrochemical luminescence
(ECL) and exposed by GeneGenius automatic gel imaging system
(Syngene, Cambridge, UK). The experiment was repeated 3 times
independently.
MTT
(3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
assay
BGC-823 cells were seeded in 96-well microplates at
a density of 0.2×104/well for 0, 12, 24, 36 and 48 h,
respectively. MTT solution (20 μl; 5 mg/ml) was added into every
well. Cells were cultured at 37°C and 5% CO2 for 4 h,
followed by the addition of 100 μl of solubilization solution into
each well. The plates were kept in a dark room overnight, and the
absorbance (A) at 570 nm was read by a Tecan Sunrise (Tecan,
Männedorf, Switzerland).
Transmission electron microscopy
The 5-mm3 cells lumps generated by
centrifugation were soaked in the 2.5% glutaraldehyde solution for
12 h, washed using 0.1 mol/l phosphate-buffered saline (PBS) three
times and kept in PBS overnight at 4°C. Then, the lumps were soaked
in 1% osmium tetroxide acid (OsO4) for 2 h and washed
again, and incubated at 4°C overnight. The cells lumps were
dehydrated with ethanol solutions step by step for 15 min.
Following dehydration, the lumps were washed by acetone twice, then
soaked in the fluid which was mixed with the resin and an equal
volume of acetone for 2 h, and then soaked in pure resin overnight.
Following embedding, cells lumps were incubated at 37°C, 45°C, 60°C
respectively for 24 h. The lumps were fixed into pyramid shape to
be cut into ultra-thin slices using the UC6 ultrathin slice machine
(Leica, Wetzlar, Germany). The slices in nets were dyed and
observed using transmission electron microscope (Carl Zeiss,
Oberkochen, Germany).
Flow cytometry
BGC-823 cells were collected after various
treatments by trypsinization, washed with PBS, fixed overnight in
70% ethanol at 4°C, incubated for 30 min with 40 μg propidium
iodide (PI, Sigma) and 100 μg RNase A (Sigma) in PBS and analyzed
on a FACSCalibur flow cytometer (Becton-Dickinson, Mountain View,
CA, USA). Collected data were analyzed by CellQuest Pro Analysis
software.
Statistical analysis
Each group of experiments was repeated at least
three times. Data were evaluated using the Statistics Software
Package (Student’s t-test or analysis of variance). P<0.05 was
considered to indicate a statistically significant difference.
Results
Activation of Notch and Wnt signaling
pathways
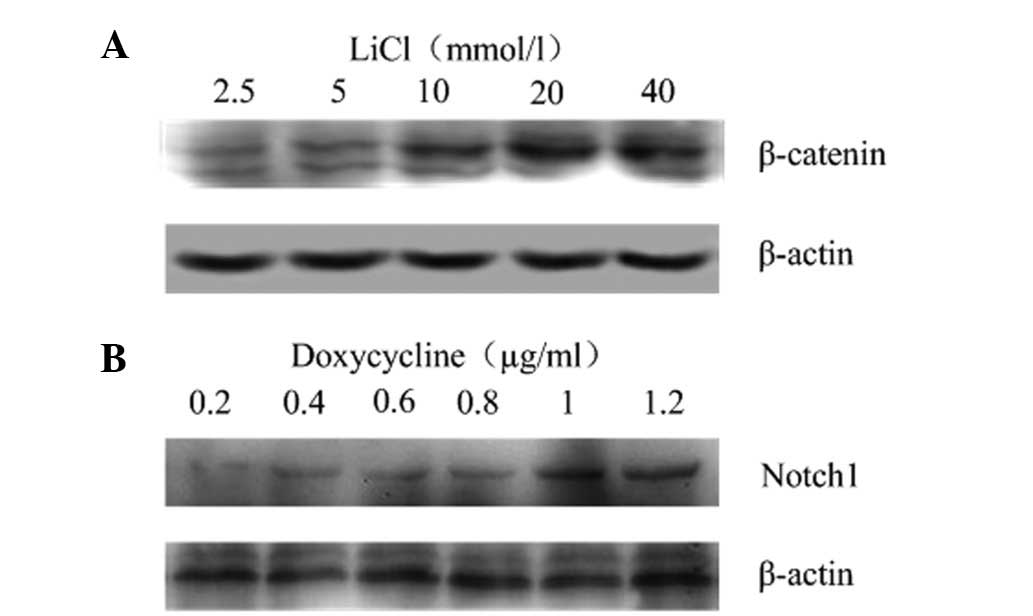
BGC-823 cells were harvested at 24 h after treatment
with different concentrations of LiCl and doxycycline. Western blot
analysis showed that β-catenin and Notch1 were expressed in an
activator concentration-dependent manner, and that β-catenin or
Notch1 proteins were significantly increased by 10 mmol/l LiCl or 1
μg/ml doxycycline, respectively (Fig.
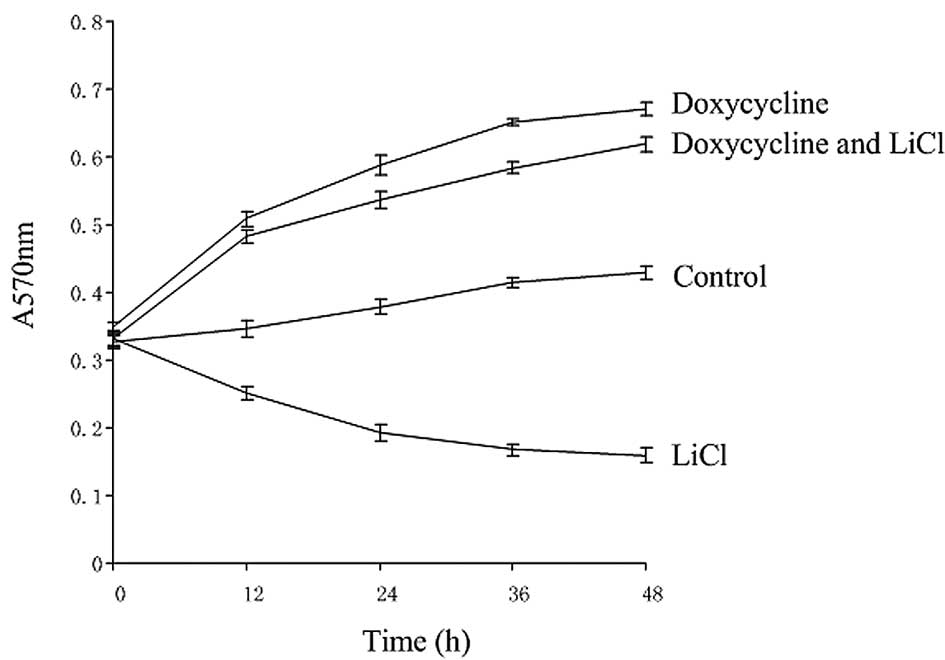
1). Compared with the control, cell proliferation was
significantly inhibited when the Notch1 signaling pathway was
activated, while cell proliferation was enhanced when the
Wnt/β-catenin signaling pathway was activated or when the Wnt and
Notch pathways were coactivated (Fig.
2).
Expression of key components of two
signaling pathways in BGC-823 cells
Firstly, the protein expression of Notch1 and the
Notch target gene Hes1 were measured by western blot analysis using
polyclonal antibodies against Notch1 (including epitopes of NICD)
and Hes1. Upon activation of the Notch1 pathway by doxycycline or
the activation of both Notch1 and Wnt pathways by doxycycline and
LiCl, the expression of Notch1 and Hes1 was significantly increased
compared with the control, while it was reduced in LiCl-treated
cells (Fig. 3A). Secondly, the
protein expression of β-catenin and cyclin D1 in single or both
activator-treated cells were detected. The expression levels of
β-catenin and cyclin D1 were significantly increased in
doxycycline-treated and co-activated cells (Fig. 3B).
Ultrastructural alterations of BGC-823
cells under Wnt and Notch co-activation
When Notch1 signaling was activated, the cell
membrane microvilli were reduced, the nucleus atrophied and some
vacuoles were observed in the cytoplasm of cells (Fig. 4B). These data suggest that cell
necrosis occurred when the Notch1 pathway was activated. When Wnt
or both Wnt and Notch pathways were activated, we found that the
membrane microvilli were developed, and that a limited number of
vacuoles was observed in the cytoplasm (Fig. 4C and D). Cell membrane microvilli
were developed and the organelles were normal. The nucleolus was
clear in the control (Fig.
4A).
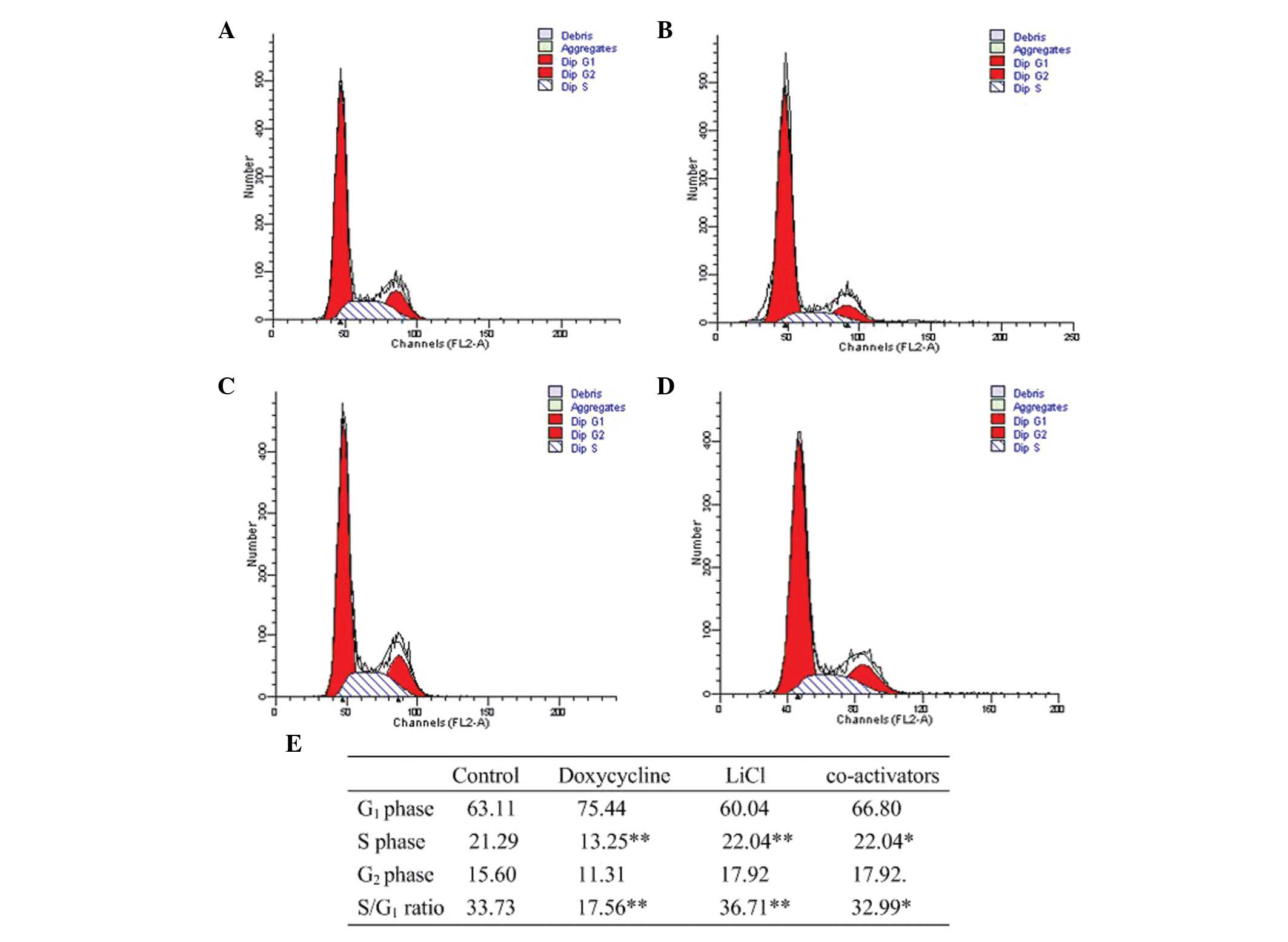
Cell cycle analysis of BGC-823 cells when
Notch and Wnt were co-activated
Compared with the control, G1/S
transition was blocked when Notch1 signaling was activated, while
G1/S transition was promoted when Wnt/β-catenin
signaling was activated. The proportion of the cells in S phase in
the coactivated cells was higher compared with the control, while
lower than the group in which only the Wnt/β-catenin signaling was
activated (Fig. 5).
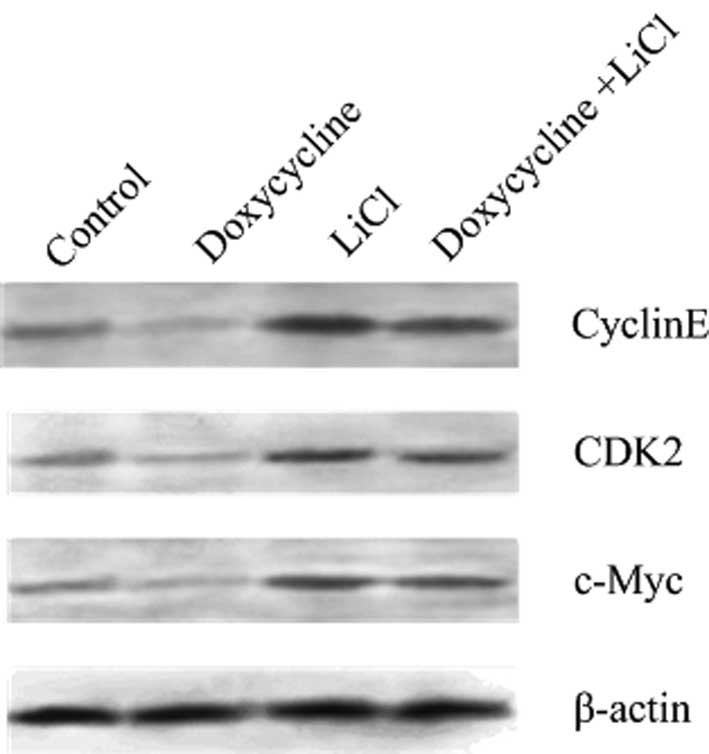
Expression of CDK2, cyclin E and c-Myc in
BGC-823 cells under Wnt and Notch co-activation
The expression of cyclin E, CDK2 and c-Myc in
activation of Notch1 signaling were significantly lower compared
with the control, suggesting that Notch1 signaling inhibits cell
proliferation by blocking the G1/S transition. When the
Wnt pathway was activated by LiCl, the expression of cyclin E, CDK2
and c-Myc was significantly increased, suggesting that
Wnt/β-catenin signaling promotes BGC-823 cell proliferation by
advancing G1/S transition and accelerating the cell
cycle process. When both Wnt and Notch1 were activated, the
expression levels of cyclin E, CDK2 and c-Myc were significantly
increased compared with the control, while they were lower compared
with the expression levels when only the Wnt pathway was activated,
suggesting that the Wnt/β-catenin signaling had a certain
antagonistic effect on Notch1 signaling (Fig. 6).
Discussion
In the present study, the effects of the activation
of Notch1 pathway by doxycycline, the activation of Wnt pathway by
LiCl and the co-activation of the two pathways were observed in
BGC-823 cells. It was found that the activation of Wnt signaling
overcomes the pro-apoptotic role of Notch in gastric cancer
cells.
Firstly, the two pathways were triggered using
doxycycline and LiCl, respectively. Treatment with doxycycline, an
activator of the Notch1 signaling pathway, resulted in higher
expression levels of the Notch1 and Hes1 proteins. Treatment with
LiCl, an activator of the Wnt/β-catenin signaling pathway, resulted
in higher expression levels of the β-catenin and cyclin D1
proteins. CDK2-cyclin E complexes, phosphorylating the
retinoblastoma protein, drive cells into the S phase of the cell
cycle. The proto-oncogene c-Myc protein promotes cell proliferation
and induces cytologic atypia, through binding to the specific DNA
sequence. c-Myc also has a close association with the
G1/S transition.
Compared with the control, when only the Notch1
signaling pathway was activated, the expression of β-catenin and
cyclin D1 were stable, while the expression of cyclin E, CDK2 and
c-Myc decreased, suggesting that the Notch1 signaling pathway had
no marked effect on the Wnt/β-catenin signaling pathway. The
inhibition of cyclin E, CDK2 and c-Myc resulted in cell cycle
retardation and inhibited the proliferation of BGC-823 cells. When
only the Wnt/β-catenin signaling pathway was activated, the
expression of Notch1 and Hes1 significantly decreased, while the
expression of cyclin E, CDK2 and c-Myc increased, suggesting that
the Notch1 signaling pathway may be regulated to some extent by the
Wnt/β-catenin signaling pathway. Previous studies have shown that
Dsh has an antagonistic effect on the Notch1 signaling pathway by
binding to the intracellular domain of Notch1 (28). The high levels of cyclin D1, cyclin
E, CDK2 and c-Myc promote G1/S transition and the cell
proliferation of BGC-823 cells. When the Notch1 and Wnt/β-catenin
signaling pathways were simultaneously activated, the expression of
Notch1 and Hes1 was higher compared with the control, while it was
lower compared with the group in which only the Notch1 signaling
pathway was activated. The expression of β-catenin and cyclin D1
was higher compared with the control, while it was not different
when only the Wnt/β-catenin signaling was significantly activated.
The expression levels of cyclin E, CDK2 and c-Myc were higher
compared with the control, suggesting that the activated
Wnt/β-catenin signaling pathway was able to overcome the inhibitory
effects of the Notch1 signaling pathway on cyclin E, CDK2 and
c-Myc.
In conclusion, it was found that the Notch1
signaling pathway plays an inhibitory role in the process of
BGC-823 cell development. The activated Notch1 signaling inhibits
the proliferation of BGC-823 cells by the downregulation of c-Myc,
cyclin E and CDK2, while the highly regulated Wnt signaling cascade
plays a key role during BGC-823 cell development. The activated
Wnt/β-catenin signaling promotes the proliferation of BGC-823 cells
by the upregulation of c-Myc, cyclin D1, cyclin E and CDK2.
Furthermore, Wnt/β-catenin signaling had an antagonistic effect on
the Notch1 signaling pathway. When the two signaling pathways were
simultaneously activated, there was a combined effect of promoting
the proliferation of BGC-823 cells by upregulating the expression
of c-Myc, cyclin D1, cyclin E and CDK2.
Strong evidence indicates that the Wnt cascade
constitutes the major driving force behind the proliferative
potential of adenomas and adenocarcinomas of the stomach. Recent
data indicate that active Notch signaling plays an important role
in the maintenance of the undifferentiated state of gastric cells,
suggesting that Notch may provide an alternative targeted-drug
strategy for the treatment of gastric neoplastic diseases. A
further study would be to explore the balance between the Wnt
signaling cascade and Notch1 signaling pathway of gastric cancer
cells.
Acknowledgements
The authors would like to thank Rongjian Su
(Liaoning Medical University, Jinzhou, China) for reviewing and
providing helpful advice for this manuscript. This project was
supported by the Natural Science Foundation of Liaoning Province
(no. 201102125).
Abbreviations:
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
References
|
1
|
Ushijima T and Sasako M: Focus on gastric
cancer. Cancer Cell. 5:121–125. 2004. View Article : Google Scholar
|
|
2
|
Gonzalez CA, Sala N and Capella G: Genetic
susceptibility and gastric cancer risk. Int J Cancer. 100:249–260.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo JL, Zheng SJ, Li YN, et al:
Toxicarioside A inhibits SGC-7901 proliferation, migration and
invasion via NF-kappaB/bFGF signaling. World J Gastroenterol.
18:1602–1609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Rifai W and Powell SM: Molecular
biology of gastric cancer. Semin Radiat Oncol. 12:128–140. 2002.
View Article : Google Scholar
|
|
5
|
Smith MG, Hold GL, Tahara E and El-Omar
EM: Cellular and molecular aspects of gastric cancer. World J
Gastroenterol. 12:2979–2990. 2006.
|
|
6
|
Kang MH, Oh SC, Lee HJ, Kang HN, Kim JL,
Kim JS and Yoo YA: Metastatic function of BMP-2 in gastric cancer
cells: the role of PI3K/AKT, MAPK, the NF-kappaB pathway, and MMP-9
expression. Exp Cell Res. 317:1746–1762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maloum F, Allaire JM, Gagne-Sansfacon J,
et al: Epithelial BMP signaling is required for proper
specification of epithelial cell lineages and gastric endocrine
cells. Am J Physiol Gastrointest Liver Physiol. 300:G1065–G1079.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin J, Donnelly JM, Houghton J and
Zavros Y: The role of sonic hedgehog reemergence during gastric
cancer. Dig Dis Sci. 55:1516–1524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsu KW, Hsieh RH, Huang KH, et al:
Activation of the Notch1/STAT3/Twist signaling axis promotes
gastric cancer progression. Carcinogenesis. 33:1459–1467. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Radulescu S, Ridgway RA, Cordero J, et al:
Acute WNT signalling activation perturbs differentiation within the
adult stomach and rapidly leads to tumour formation. Oncogene. Jun
4–2012.(Epub ahead of print). View Article : Google Scholar
|
|
11
|
Kim TH and Shivdasani RA: Notch signaling
in stomach epithelial stem cell homeostasis. J Exp Med.
208:677–688. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun Y, Gao X, Liu J, et al: Differential
Notch1 and Notch2 expression and frequent activation of Notch
signaling in gastric cancers. Arch Pathol Lab Med. 135:451–458.
2011.PubMed/NCBI
|
|
13
|
Yeh TS, Wu CW, Hsu KW, et al: The
activated Notch1 signal pathway is associated with gastric cancer
progression through cyclooxygenase-2. Cancer Res. 69:5039–5048.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katoh M: Notch ligand, JAG1, is
evolutionarily conserved target of canonical WNT signaling pathway
in progenitor cells. Int J Mol Med. 17:681–685. 2006.PubMed/NCBI
|
|
15
|
Piazzi G, Fini L, Selgrad M, et al:
Epigenetic regulation of Delta-Like1 controls Notch1 activation in
gastric cancer. Oncotarget. 2:1291–1301. 2011.PubMed/NCBI
|
|
16
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roy M, Pear WS and Aster JC: The
multifaceted role of Notch in cancer. Curr Opin Genet Dev.
17:52–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kadesch T: Notch signaling: the demise of
elegant simplicity. Curr Opin Genet Dev. 14:506–512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barth AI, Nathke IS and Nelson WJ:
Cadherins, catenins and APC protein: interplay between cytoskeletal
complexes and signaling pathways. Curr Opin Cell Biol. 9:683–690.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kikuchi A, Yamamoto H and Kishida S:
Multiplicity of the interactions of Wnt proteins and their
receptors. Cell Signal. 19:659–671. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cajanek L, Ribeiro D, Liste I, Parish CL,
Bryja V and Arenas E: Wnt/beta-catenin signaling blockade promotes
neuronal induction and dopaminergic differentiation in embryonic
stem cells. Stem Cells. 27:2917–2927. 2009.PubMed/NCBI
|
|
22
|
Wang QM, Zhang Y, Yang KM, Zhou HY and
Yang HJ: Wnt/beta-catenin signaling pathway is active in pancreatic
development of rat embryo. World J Gastroenterol. 12:2615–2619.
2006.PubMed/NCBI
|
|
23
|
Zhang Y, Li XM, Zhang FK and Wang BE:
Activation of canonical Wnt signaling pathway promotes
proliferation and self-renewal of rat hepatic oval cell line
WB-F344 in vitro. World J Gastroenterol. 14:6673–6680. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prasad CP, Rath G, Mathur S, Bhatnagar D
and Ralhan R: Potent growth suppressive activity of curcumin in
human breast cancer cells: Modulation of Wnt/beta-catenin
signaling. Chem Biol Interact. 181:263–271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Larue L and Delmas V: The WNT/Beta-catenin
pathway in melanoma. Front Biosci. 11:733–742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park JK, Song JH, He TC, Nam SW, Lee JY
and Park WS: Overexpression of Wnt-2 in colorectal cancers.
Neoplasma. 56:119–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sergio LE and Kalaska JF: Systematic
changes in motor cortex cell activity with arm posture during
directional isometric force generation. J Neurophysiol. 89:212–228.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hansson EM, Lendahl U and Chapman G: Notch
signaling in development and disease. Semin Cancer Biol.
14:320–328. 2004. View Article : Google Scholar : PubMed/NCBI
|