Introduction
M phase-promoting factor (MPF) is a universal
regulator of M phase in the eukaryotic cell cycle (1,2). Its
activation induces entry into M phase and its subsequent
inactivation is necessary to exit from M phase. The activity of MPF
in higher eukaryotic cells is primarily regulated by the formation
of a complex between the catalytic cyclin-dependent kinase 1 (CDK1,
previously known as CDC2) subunit and the regulatory CCNB1
(previously known as cyclin B) subunit, and further by
phosphorylation/dephosphorylation at Thr 14 and Tyr 15 of CDK1
associated with CCNB1 (1–4). Until the end of G2 phase,
MPF remains inactive through the inhibitory phosphorylation of
these residues by the WEE1 kinase family. WEE1 proteins are
dual-specificity kinases that uniquely function to phosphorylate
specific Tyr/Thr residues on CDKs. There are three WEE1 family
members: WEE1 homolog 1 (WEE1, also designated as somatic WEE1A);
WEE1 homolog 2 (WEE2, also known as WEE1B) and PKMYT1 (previously
known as MYT1). Various genetic and biochemical studies have
indicated that WEE1, which was originally identified in the fission
yeast Schizosaccharomyces pombe mutant with a small cell or
WEE phenotype, is a Tyr-specific protein kinase conserved in all
eukaryotes (5). In eukaryotic
cells, WEE1A is a nuclear protein that is capable of
phosphorylating Tyr 15 but not Thr 14 of CDK1 and thus inhibiting
its kinase activity, thereby preventing entry into mitosis
(6–9). PKMYT1, a membrane-associated
inhibitory kinase, is present in metazoans and phosphorylates CDK1
efficiently on Thr 14 and Tyr 15 residues (6,10–15)
and the human PKMYT1 kinase preferentially phosphorylates CDK1 on
Thr 14 in a cyclin-dependent manner (13). WEE1B, a newly identified member of
the WEE1 kinase family, is conserved from yeast to humans and has
recently been shown to suppress CDK1 activity by phosphorylating
its inhibitory sites (16–21). While WEE1A and PKMYT1 are well
documented as important regulators of mitosis in multiple cell
types in numerous species (22),
the role and regulation of WEE1B in zygotes and early embryo
development remain to be elucidated.
Protein kinase A (PKA), a cAMP-dependent Ser/Thr
kinase, is composed of two catalytic (C) subunits held in an
inactive state in association with a regulatory (R) subunit dimer.
PKA constitutes one of the most important components of several
signal transduction pathways, which are important in cell growth,
differentiation, proliferation and cell cycle control. Studies in
starfish, Xenopus and mammalian oocytes demonstrate that the
maintenance of high levels of cAMP and active PKA are important in
meiotic arrest (23–26). Previous studies have suggested that
PKA may directly phosphorylate and regulate the activity of CDC25B
phosphatase (25,27–29)
and WEE1A kinase (20,30,31)
to form a bidirectional regulatory loop for MPF activity.
Generally, cAMP levels are high in meiosis-arrested oocytes and
high levels of cAMP may result in high PKA activity, both of which
are essential for the maintenance of meiotic arrest (32–34).
In particular, when cAMP levels decrease, PKA is deactivated and
unable to phosphorylate CDC25 phosphatase, which is subsequently
activated. As a result, activated CDC25 phosphatase translocates to
the nucleus where it dephosphorylates CDK1 and thus activates MPF
(35,36). The concurrent deactivation of WEE1A
kinase allows the inhibitory phosphates to be removed from CDK1 by
CDC25 phosphatase (12,36,37).
However, the pathway between high PKA activity and the repression
of MPF activity has yet to be fully elucidated. To examine the
effects of PKA on WEE1B in mammalian cells, WEE1B has been
predicted as a potential PKA substrate in mice using the scansite
software (http://scansite.mit.edu/) and two
potential PKA phosphorylation sites, including Ser 15 and Thr 170
were predicted. The two sites lie within a consensus sequence for
PKA (KKLS), which includes a positively charged R or K residue
upstream of an S residue. Acting as a candidate target of PKA, it
has been demonstrated that Ser 15 is the major phosphorylation site
in vitro and is involved in the regulation of meiotic arrest
in mouse oocytes by modification of phosphorylation and
dephosphorylation (21). However,
the role of Ser 15 in the mitotic cell cycle of one-cell stage
mouse embryos remains unknown. In the present study, using a highly
specific antibody against phospho-Ser 15 of WEE1B in Western
blotting, we demonstrated that WEE1B-Ser 15 was phosphorylated
during G1 and S phases, whereas Ser 15 was
dephosphorylated during G2 and M phases in vivo.
Furthermore, we preliminarily examined the role of Ser 15 of WEE1B
as the major PKA phosphorylation site in vivo and the
inhibitory effects of the kinase are strengthened when this residue
is phosphorylated. Collectively, our results suggest that Ser 15 of
WEE1B is a potential PKA phosphorylation target in G2/M
transition of one-cell stage mouse embryos and WEE1B acts as a
direct downstream substrate of PKA in mammals. Thus, WEE1B inhibits
mitosis by negatively regulating MPF activity.
Materials and methods
Animals and reagents
Kunming genealogy-specific pathogen-free mice were
purchased from the Department of Laboratory Animals, China Medical
University [license: SCXK 2008-0005 (Liaoning, China)]. The female
and male mice, which were 4 weeks, 18 g and 8 weeks, 30 g,
respectively, were housed under controlled environmental conditions
(19°C and 12 h light per day) and provided with food and water
ad libitum. The study was performed in strict accordance
with the recommendations outlined in the Guide for the Care and Use
of Laboratory Animals released by the National Institutes of
Health. The protocol for animal handling and the treatment
procedures were approved by the Committee on the Ethics of Animal
Experiments of the China Medical University. Rabbit anti-WEE1B
(mWEE1B-specific antibodies raised in New Zealand white rabbits
against the keyhole limpet hemocyanin-conjugated peptide
MADTETDQGLNKK), rabbit anti-phospho-WEE1B-pSer 15
(phospho-WEE1B-pSer 15 antibodies raised in New Zealand white
rabbits against the keyhole limpet hemocyanin-conjugated
phosphor-peptide TDQGLNKKLpSFSF) and rabbit
anti-non-phospho-WEE1B-pSer 15 (non-phospho-WEE1B-pSer 15
antibodies raised in New Zealand white rabbits against the keyhole
limpet hemocyanin-conjugated non-phospho-peptide TDQGLNKKLSFSF)
were purchased from Signalway Antibody Co., Ltd. (Baltimore, MD,
USA). Goat anti-pTyr 15 of CDK1, rabbit anti-β-actin and H-89 were
provided by Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
mMESSAGE mMACHINE T7 Ultra kit was purchased from Ambion (Carlsbad,
CA, USA) and [γ-32P] ATP from Peking Yahui Biotechnology
Co., Ltd. (Beijing, China). Unless otherwise specified, all other
reagents were purchased from Sigma (St. Louis, MO, USA).
Collection and culture of one-cell stage
mouse embryos
Mouse embryos at the one-cell stage were collected
and cultured as previously described (38). Female mice were injected with 10 IU
of pregnant mare serum gonadotropin (PMSG) and then with 10 IU of
human chorionic gonadotropin (hCG) 46–48 h post-PMSG, and caged
with Kunming males. One-cell stage embryos were obtained from
females 18–20 h post-hCG injection and incubated for 2 min in M2
medium suspended in 300 μg/ml hyaluronidase to remove cumulus
cells, washed extensively using M2 medium and then the embryos were
microinjected or fixed. Following microinjection, embryos were
cultured to the different cell cycle stages in M16 medium at 37°C
in a humidified atmosphere of 5% CO2. Embryos pretreated
with dibutyryl cyclic AMP (dbcAMP) or H-89 were incubated and
collected at different time points. Mitotic stages (G1,
S, G2 and M phases) were defined as previously described
(38,39): G1 phase, 12–21 h
post-hCG injection; S phase, 21–27 h; G2 phase, 27–30 h
and M phase, 30–33 h. The rate of cleavage, i.e., the number of
two-cell embryos resulting from the division of a one-cell embryo,
was counted in three independent experiments under a phase contrast
microscope at 31, 35 or 30 h post-hCG injection in the absence or
presence of dbcAMP and/or H-89.
Plasmid construction and site-directed
mutagenesis
The plasmids of wild-type Wee1B
(pcDNA3.1/V5-His-TOPO- Wee1B-WT) and kinase-dead
Wee1B (pcDNA3.1/V5-His-TOPO-Wee1B-KD) were kindly
provided by Professor Marco Conti (University of California, San
Francisco, USA). The pcDNA3.1/V5-His-TOPO-Wee1B-S15A/D
construct was prepared by mutating Ser 15 of Wee1B to
alanine (A) and aspartic acid (D), which acts to mimick
phosphorylation. Wee1B-WT was used as a template and the
site-directed mutagenesis kit (Stratagene, Santa Clara, CA, USA).
The primers for Wee1B-S15A were
5′-CTGAACAAGAAATTAGCCTTCTCCTTT-3′ and
5′-GCTAATTTCTTGTTCAGTCCCTGGTCA-3′. The primers used to construct
Wee1B-S15D were 5′-CTGAACAAGAAATTAGACTTCTCCTTT-3′ and
5′-TCTAATTTCTTGTTCAGTCCCTGGTCAG-3′. Both recombinant plasmids were
sequenced to verify the correct gene insertion and successful
mutation.
In vitro transcription
According to our previous study (40), all the pcDNA3.1/V5-His-TOPO
constructs were linearized with XhoI as templates for RNA
transcription. 5′-capped mRNAs were generated using the mMESSAGE
mMACHINE T7 Ultra kit according to the manufacturer's instructions
and DNA templates were removed using TURBO DNase. Synthesized mRNAs
were dissolved in nuclease-free TE buffer (5 mM Tris, 0.5 mM EDTA,
pH 7.4) and the concentration of mRNAs was determined using a
Hitachi U-2900 spectrophotometer (Hitachi High-Technologies
Corporation, Tokyo, Japan) or agarose gel electrophoresis.
Synchronizing the embryos and
microinjection
To synchronize the embryos, embryos were placed in a
drop of M2 medium under mineral oil in the lid of a 3-cm Falcon
culture dish and synchronized for 7 h following microinjection and
release from a thymidine block. Alternatively, asynchronous cells
were recorded 20 h following microinjection. The typical injection
volumes were 5% (10 pl, cytoplasmic) of the total cell volume of
embryos. Non-microinjection or microinjection with TE buffer were
selected as controls. Following microinjection, embryos were
transferred to M16 medium and incubated at 37°C in 5%
CO2, and the percentage of cell cleavage was scored
under a dissecting microscope. Morphological analysis was performed
at the indicated times.
Western blotting
Protein was extracted from ~160 mouse embryos using
20 μl protein extraction buffer (150 mM NaCl, 1 mM
Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium
pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4, 1 μg/ml leupeptin and 1 mM PMSF). The
protein extracts were separated by 10% polyacrylamide gel
electrophoresis and the protein was transferred onto polyvinylidene
fluoride membranes. The membranes were blocked using 3% (w/v) BSA
in Tris-buffered saline containing 0.05% Tween-20 for 1 h at room
temperature and incubated with primary antibody overnight at 4°C.
The blots were then incubated with HRP-linked secondary antibody
and visualized using the enhanced chemiluminescence detection
system (Pierce Biotechnology, Inc., Rockford, IL, USA).
Assay of MPF activity
MPF kinase activity was assayed as previously
described (41,42). Ten embryos were collected in 5 μl
collection buffer (PBS containing 1 mg/ml polyvinyl alcohol, 5 mM
EDTA, 10 mM Na3VO4 and 10 mM NaF) and
immediately stored at −70°C until the kinase assay was performed.
Embryos were lysed by three rounds of freezing and thawing in 20 μl
of extraction buffer [50 mM Tris-HCl (pH 7.4), 250 mM NaCl, 1 mM
EDTA, 50 mM NaF, 1 mM DTT, 0.1% Triton X-100, 10 μM leupeptin, 100
μg/ml aprotinin and 0.5 μl PMSF]. Embryo lysate (5 μl) was added to
the 20 μl MPF buffer [250 mM Tris-HCl (pH 7.5), 50 mM
MgCl2, 5 mM EGTA, 10 mM DTT, 200 mM β-glycerophosphate,
100 mM p-nitrophenylphosphate, 0.5 mM sodium vanadate, 0.05% Brij
35, 2 mg/ml histone (type III-S), 2.4 μM PKA inhibitor (PKI), 0.5
mM ATP and 10 mCi/ml [γ-32P] ATP], and incubated at 30°C
for 10 min. Then, 25 μl of reaction mixture was spotted on Whatman
p81 paper and the reaction was stopped using 5%
H3PO4 solution. Following thorough washing,
the radioactivity on the filter paper was counted using a Beckman
scintillation counter.
A parallel incubation was performed to confirm the
phosphorylation of histone H1. Protein extracted from 20 embryos
was incubated with 50 μl of MPF buffer containing 20 mCi/ml
[γ-32P] ATP at 37°C for 30 min and the reaction was
stopped by adding an equal amount of 2X SDS buffer. The mixtures
were then separated by a 12% SDS-PAGE and the incorporation of
32P into histone H1 was visualized by
autoradiography.
Statistical analysis
Experiments were performed independently at least
three times. Values were analyzed by one-way analysis of variance
or Student's t-test with GraphPad Prism 5 software. P<0.05 was
considered to indicate a statistically significant result.
Results
Phosphorylation status of WEE1B-Ser 15 in
one-cell stage mouse embryos
To identify whether WEE1B-Ser 15 was phosphorylated
in vivo, we collected one-cell stage mouse embryos at
different phases in the absence or presence of dbcAMP and/or H-89,
then measured the phosphorylation status of WEE1B-Ser 15 using the
phosphor-specific antibody and non-phosphorylation status using the
non-phosphor-specific antibody. On the basis of previous
experimental studies (27,40), 2 mmol/l of dbcAMP, a PKA activator,
led to maximal G2 arrest, suggesting inhibition of the
G2/M transition in one-cell stage mouse embryos. It has
been well documented that H-89 inhibits the PKA C subunit and
readily diffuses through the cell membrane (43,44).
Previously, we (27) demonstrated
that 40 μmol/l H-89 induced all of the mouse embryos to enter the M
phase of mitosis, suggesting activation of the G2/M
transition in one-cell stage mouse embryos. The results
demonstrated that a phosphorylated WEE1B-Ser 15 band was observed
at G1 and S phases, whereas no phosphorylation of
WEE1B-Ser 15 was observed at G2 and M phases in one-cell
stage embryos with or without H-89 (Fig. 1A and C). The strong phosphorylated
WEE1B-Ser 15 band was detected in the G1 and S phases,
and also in the G2 and M phases in the presence of
dbcAMP (Fig. 1B). Conversely, a
non-phosphorylated WEE1B-Ser 15 band was mainly detected during the
G2 and M phases, and is strongest in the presence of
H-89 (Fig. 1C). Therefore, these
results demonstrated that WEE1B-Ser 15 is phosphorylated at
G1 and S phases and dephosphorylated at G2
and M phases in vivo.
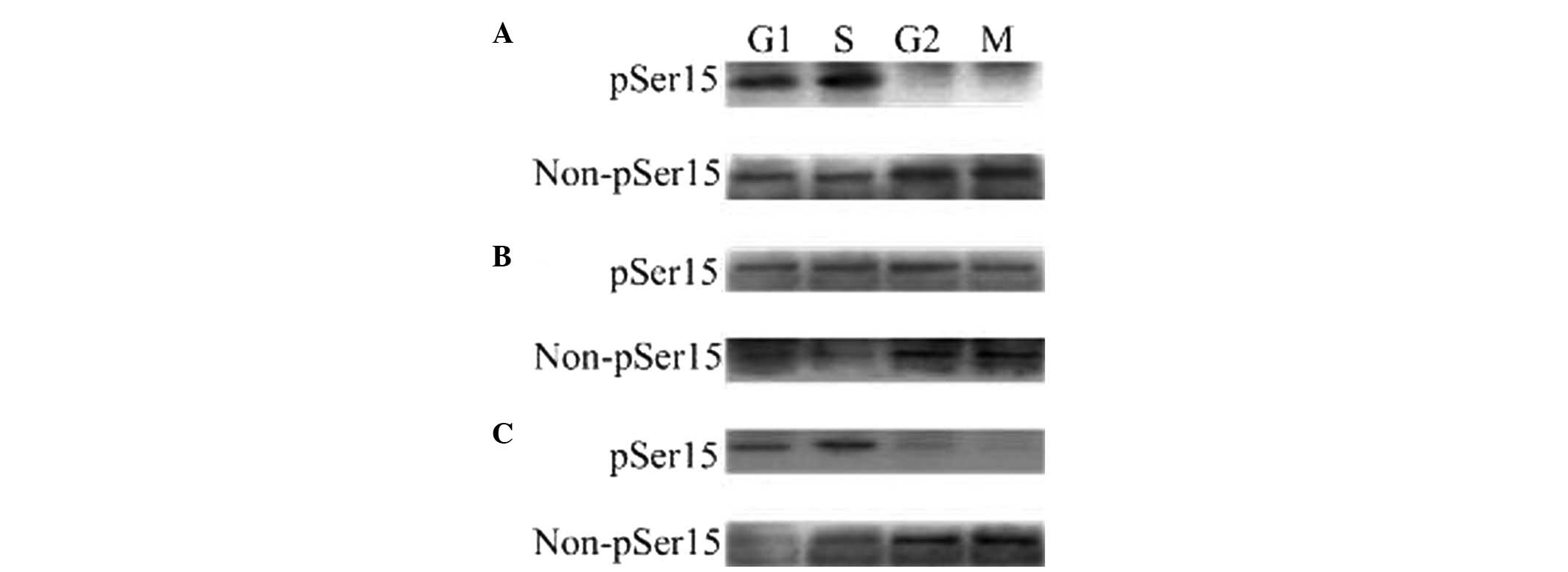 | Figure 1The phosphorylated and
unphosphorylated status of WEE1B-Ser 15 in one-cell stage mouse
embryos at different phases of the cell cycle in the
absence/presence of 2 mmol/l dbcAMP and 40 μmol/l H-89. A total of
160 embryos were loaded onto each lane in Western blotting. Gels
were then transferred to the PVDF membrane and probed with
phosphor-WEE1B-Ser 15 and non-phosphor-WEE1B-Ser 15 antibodies,
respectively. (A) Western blotting analysis of the phosphorylated
and non-phosphorylated status of WEE1B-Ser 15 in mouse embryos
during G1, S, G2 and M phases with a
phosphor-specific and a non-phosphor-specific WEE1B-Ser 15
antibody, respectively, in the absence of dbcAMP and H-89. (B) The
phosphorylated and unphosphorylated status of WEE1B-Ser 15 during
G1, S, G2 and M phases were measured in the
presence of 2 mmol/l dbcAMP. (C) The phosphorylated and
unphosphorylated form of WEE1B-Ser 15 at G1, S,
G2 and M phases were detected in the presence of 40
μmol/l H-89. dbcAMP, dibutyryl cyclic AMP. |
Wee1B-WT and Wee1B-S15D inhibit MPF
activity
To investigate the roles of Ser 15 phosphorylation
of WEE1B, we mutated Ser 15 of WEE1B and generated Wee1B-15A
and Wee1B-15D mutants. Various WEE1B mutants were
overexpressed in one-cell stage mouse embryos. Immunoblotting with
anti-WEE1B antibody confirmed that the WEE1B protein accumulated at
higher levels in Wee1B-WT/KD and Wee1B-15A/D
mRNA-microinjected embryos compared with the control, 5 h following
microinjection (Fig. 2A),
demonstrating that the exogenous mRNA of four types of Wee1B
were able to be translated efficiently in one-cell stage mouse
embryos. Furthermore, there was no difference among the
microinjection groups.
 | Figure 2Phosphorylation on Ser 15 is
essential for WEE1B. (A) Western blot analysis of WEE1B expression
5 h following microinjection of Wee1B mRNA (upper panel). A
total of 0.03 ng mRNA encoding Wee1B-WT/KD and
Wee1B-S15A/S15D were microinjected into each embryo. Control
embryos were microinjected with or without TE buffer. Western
blotting was performed using anti-WEE1B antibody in different
microinjected groups. β-actin was used as an internal control. Band
intensities for WEE1B were quantified and normalized to β-actin
level (bottom panel). Each value was expressed as mean ± SEM from
three independent experiments. ΔP<0.01 and
*P<0.05, compared with no injection group. (B)
Western blot analysis of the phosphorylation status of CDK1-Tyr 15
in the control and various Wee1B mRNA-injected embryos. The
embryos were collected at 26.5, 27, 27.5, 28, 28.5, 29 and 29.5 h
post-hCG injection. A total of 160 embryos were lysed and then
subjected to western blot analysis. A representative of three
independent experiments is shown. (C) MPF activity in embryos
injected with various Wee1B mRNAs and controls. Embryos in
controls were microinjected with or without TE buffer. The embryos
were collected at 26.5, 27, 27.5, 28, 28.5, 29 and 29.5 h post-hCG
injection. For each point, 10 embryos were collected and MPF
activity was examined by scintillation counting (upper panel) and
autoradiography (bottom panel). Each value is expressed as the mean
± SEM and shown is a representative of three independent
experiments. (D) The cleavage rate in cultured mouse embryos
injecting various Wee1B mRNAs and controls at 31 h post-hCG
injection. The cleavage rate was calculated and each value was
expressed as the mean ± SEM from three independent experiments.
ΔP<0.01 and *P<0.05, compared with the
no injection group. Wee1B-WT, Wee1B-wild type;
Wee1B-KD, Wee1B-kinase dead; MPF, M phase-promoting
factor; hCG, human chorionic gonadotropin; CDK1, cyclin-dependent
kinase 1. |
To investigate the effects of Wee1B on the
phosphorylation status of CDK1-Tyr 15 in mouse embryos, western
blotting was carried out at 26.5, 27, 27.5, 28, 28.5, 29 and 29.5 h
post-hCG injection in the control and Wee1B
mRNA-microinjected embryos (Fig.
2B). In the control group, there were strong signals of
CDK1-Tyr 15 phosphorylation at 26.5–28 h, a reduced phosphorylation
level at 28.5 h and no signal at 29 h post-hCG injection, which
demonstrated that MPF was fully activated. Moreover, there was a
weak CDK1-Tyr 15 phosphorylation signal at 29 h and no signal at
29.5 h post-hCG injection in the Wee1B-WT and S15A-injected
embryos. When Wee1B-KD was overexpressed, strong signals of
CDK1-Tyr 15 phosphorylation were detected at 26.5 h; however, no
signal was detected at 29 h post-hCG injection, as in the control.
By contrast, the inhibitory phosphorylation signals of CDK1-Tyr 15
were still observed at 29.5 h post-hCG injection in the
Wee1B-S15D-overexpressed embryos. These results were
consistent with the changes of MPF activity, suggesting that Ser 15
phosphorylation of WEE1B is critical for the regulation of MPF.
Furthermore, to identify the correlation between MPF activity and
phosphorylation status of CDK1-Tyr 15, we measured the MPF activity
at 26.5 h post-hCG injection every 30 min. In the control group,
MPF activity was gradually increased at 28.5 h post-hCG injection,
reaching its maximal level at 29 h before gradually decreasing. In
the Wee1B-WT and S15A mRNA-microinjected embryos, MPF
activity reached its peak value at 29.5 h post-hCG injection, ~30
min later than the control group. In the Wee1B-KD
mRNA-microinjected embryos, MPF activity reached its peak value at
29 h post-hCG injection, which was similar to that observed in the
control group. However, MPF activity remained consistently low
until 29.5 h post-hCG injection in the Wee1B-S15D
mRNA-injected embryos (Fig. 2C).
These results were consistent with the changes in the
phosphorylation status of CDK1-Tyr 15.
Wee1B-WT and Wee1B-S15D decrease the
cleavage of one-cell stage embryos
To examine the role of Ser 15 phosphorylation of
WEE1B during the cleavage of one-cell stage embryos, the division
of mouse embryos was observed at 26.5–29.5 h post-hCG injection,
and the cleavage rate was calculated at 31 h. In the control group,
cleavage of the embryos started at 28.5–29 h post-hCG injection and
58.97 (no injection) and 59.87% (TE injection) of embryos had
reached the two-cell stage at 31 h. Embryos microinjected with
Wee1B-WT mRNAs entered M phase until 29–29.5 h post-hCG
injection, and the cleavage rates were ~35.1% at 31 h. Embryos
microinjected with mRNA of Wee1B-KD entered M phase at
28.5–29 h post-hCG injection, and almost 60.33% of embryos had
reached the two-cell stage at 31 h, similar to the control group
(P>0.05). By contrast, embryos injected with Wee1B-S15A
mRNA had reached the two-cell stage at 29–29.5 h post-hCG
injection, and the cleavage rate was 32.78% at 31 h, markedly lower
than that of the control (P<0.01); however, similar to the
Wee1B-WT mRNA-injected embryos (P>0.05). However, embryos
microinjected with Wee1B-S 15D mRNAs rarely entered M phase
at 29.5 h post-hCG injection, and the cleavage rate was ~24.77% at
31 h, markedly lower than that of the control group (P<0.01;
Fig. 2D). These results
demonstrated that the phosphorylation of the Ser 15 residue of
WEE1B is essential for its activity during the cleavage of one-cell
stage embryos.
Microinjection of Wee1B mRNA suppresses
MPF activity induced by dbcAMP
To further examine whether PKA is capable of
phosphorylating Ser 15 of WEE1B, various Wee1B mRNAs were
microinjected into embryos during S phase in the presence of 2
mmol/l dbcAMP. The WEE1B protein was highly expressed at 5 h in
each mRNA-microinjected embryo compared with the control (Fig. 3A) and there was no significant
difference among the microinjection groups, indicating that various
exogenous Wee1B mRNAs were able to be translated efficiently
in mouse embryos.
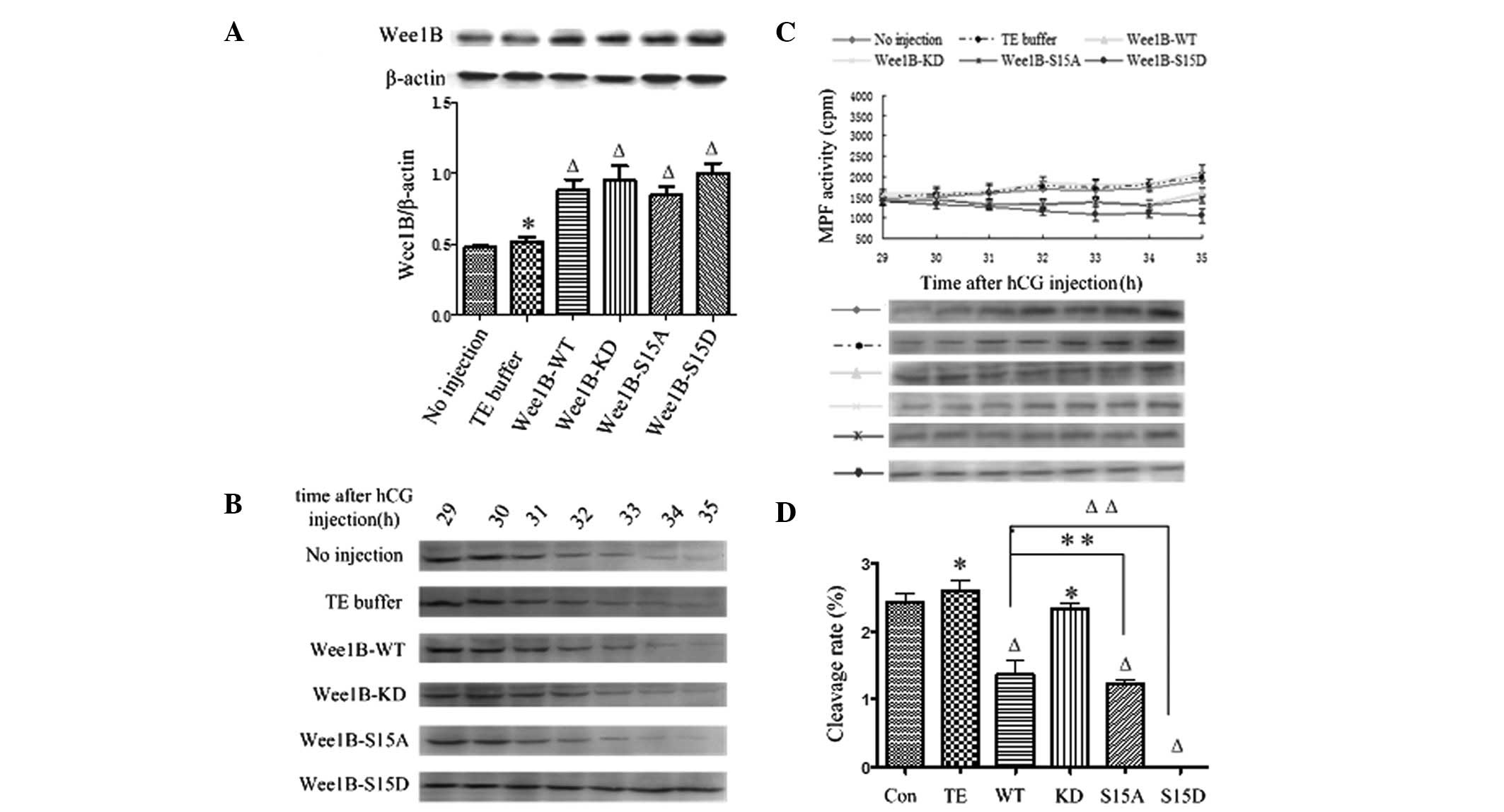 | Figure 3WEE1B functions downstream of PKA.
(A) Western blotting analysis of WEE1B expression at 5 h following
microinjection of Wee1B mRNA in the presence of 2 mmol/l
dbcAMP. In total, 0.03 ng of mRNA encoding Wee1B-WT/KD and
Wee1B-S15A/S15D were microinjected into each embryo. Embryos
in the control group were microinjected with or without TE buffer.
Western blotting was performed using anti-WEE1B antibody in
different microinjected groups. β-actin was used as an internal
control. Band intensities for WEE1B were quantified and normalized
to β-actin level (bottom panel). Each value is expressed as the
mean ± SEM from three independent experiments.
ΔP<0.01 and *P<0.05, compared with the
no injection group. (B) Western blotting analysis of the
phosphorylation status of CDK1-Tyr 15 in the control and various
Wee1B mRNA-injected embryos in the presence of 2 mmol/l
dbcAMP. The embryos were collected at 29, 30, 31, 32, 33, 34 and 35
h post-hCG injection. A total of 160 embryos were loaded onto each
lane and fractionated on 12% SDS-PAGE. Gels were then transferred
to the PVDF membrane and probed with CDK1-Tyr 15 antibody. Shown is
a representative of three independent experiments. (C) MPF activity
in embryos injected with various Wee1B mRNAs and controls.
Embryos in controls were microinjected with or without TE buffer.
Embryos in the presence of 2 mmol/l dbcAMP were collected at 29,
30, 31, 32, 33, 34 and 35 h post-hCG injection. For each point, 10
embryos were collected and MPF activity was examined by
scintillation counting (upper panel) and autoradiography (bottom
panel). Each value is expressed as the mean ± SEM, shown is a
representative of three independent experiments. (D) The cleavage
rate in cultured mouse embryos injected with various Wee1B
mRNAs and controls at 35 h post-hCG injection in the presence of 2
mmol/l dbcAMP. The cleavage rate was calculated and each value was
expressed as mean ± SEM from three independent experiments.
ΔP<0.01 and *P<0.05, compared with the
no injection group. Wee1B-WT, Wee1B-wild type;
Wee1B-KD, Wee1B-kinase dead; MPF, M phase-promoting
factor; hCG, human chorionic gonadotropin; CDK1, cyclin-dependent
kinase 1. |
The phosphorylation status of CDK1-Tyr 15 was
detected by western blotting at 29, 30, 31, 32, 33, 34 and 35 h
post-hCG injection in various exogenous Wee1B mRNAs in the
presence of 2 mmol/l dbcAMP. In the control and Wee1B-KD
mRNA-injected embryos, strong bands of phosphorylated CDK1-Tyr 15
were identified at 29–35 h post-hCG injection, indicating that MPF
activity was inhibited. In the Wee1B-WT/S15A-injected
embryos, weak CDK1-Tyr 15 phosphorylation was detected at 35 h
post-hCG injection, which was coincident with MPF activity.
However, when Wee1B-S15D was overexpressed, a strong signal
was present at 35 h post-hCG injection, suggesting that MPF was
completely inactivated (Fig. 3B).
The results suggested that Ser 15 phosphorylation of WEE1B is
required for PKA-induced CDK1-Tyr 15 phosphorylation and therefore
for PKA-induced MPF inhibition.
To investigate the effects of Ser 15 phosporylation
on MPF inhibition induced by PKA in mouse embryos, MPF activity was
measured beginning at 29 h post-hCG injection at 30-min intervals.
As shown in Fig. 3C, the MPF
activity was stably low at 29–35 h in the control and
Wee1B-KD mRNA-injected embryos, which indicated that MPF
activity was inhibited by PKA activation. Additionally, MPF
activity in Wee1B-WT/S15A mRNA-injected embryos increased
weakly at 35 h post-hCG injection. By contrast, MPF activity in
embryos injected with the Wee1B-S15D mutants remained at a
low level at 29–35 h post-hCG injection, indicating G2/M
arrest. These results demonstrated that WEE1B functions downstream
of PKA.
Overexpression of Wee1B prohibits the
mitotic entry of mouse embryos treated by dbcAMP
In the presence of 2 mmol/l dbcAMP, the cleavage of
mouse embryos was observed at 29–35 h post-hCG injection, and the
cleavage rate was calculated at 35 h. As shown in Fig. 3D, the cleavage rate of embryos
treated with dbcAMP in each group evidently decreased, and
extremely few embryos reached the two-cell stage at 35 h post-hCG
injection in the control, Wee1B-WT/KD and
Wee1B-S15A-microinjected embryos. No embryos microinjected
with Wee1B-S15D mRNA entered M phase at 35 h.
Wee1B-S15A/D inhibits MPF activity
induced by H-89
Previously, we and other authors have demonstrated
that H-89 activates MPF through the inhibition of PKA (27,43,44).
To investigate the role of Ser 15 phosphorylation of WEE1B during
PKA-induced MPF inhibition, various Wee1B mRNAs were
microinjected into mouse embryos during S phase in the presence of
40 μmol/l H-89. The WEE1B protein was more highly expressed at 5 h
in the mRNA-microinjected embryos than in the control group
(Fig. 4A). No significant
differences among the microinjection groups were observed,
indicating that the various exogenous Wee1B mRNAs were able
to be translated efficiently in mouse embryos.
 | Figure 4Wee1B-S15D blocks H-89
inhibition of PKA. (A) Western blotting analysis of WEE1B
expression 5 h following microinjection of Wee1B mRNA in the
presence of 40 μmol/l H-89. A total of 0.03 ng mRNA encoding
Wee1B-WT/KD and Wee1B-S15A/S15D were microinjected
into each embryo. Embryos in the control group were microinjected
with or without TE buffer. Western blotting was performed using
anti-WEE1B antibody in different microinjected groups. β-actin
served as an internal control. Band intensities for WEE1B were
quantified and normalized to β-actin level (bottom panel). Each
value was expressed as mean ± SEM from three independent
experiments. ΔP<0.01 and *P<0.05,
compared with the no injection group. (B) Western blotting analysis
of the phosphorylation status of CDK1-Tyr 15 in the control and
various Wee1B mRNA-injected embryos in the presence of 40
μmol/l H-89. The embryos were collected at 26, 26.5, 27, 27.5, 28,
28.5 and 29 h post-hCG injection. A total of 160 embryos were
loaded onto each lane and fractionated on 12% SDS-PAGE. Gels were
then transferred to the PVDF membrane and probed with CDK1-Tyr 15
antibody. Shown is a representative of three independent
experiments. (C) MPF activity in embryos injected with various
Wee1B mRNAs and controls. Embryos in the control group were
microinjected with or without TE buffer. The embryos in the
presence of 40 μmol/l H-89 were collected at 26, 26.5, 27, 27.5,
28, 28.5 and 29 h post-hCG injection. For each point, 10 embryos
were collected and MPF activity was examined by scintillation
counting (upper panel) and autoradiography (bottom panel). Each
value is expressed as the mean ± SEM and shown is a representative
of three independent experiments. (D) The cleavage rate in cultured
mouse embryos injected with various Wee1B mRNAs and controls
at 30 h post-hCG in the presence of 40 μmol/l H-89. The cleavage
rate was calculated and each value was expressed as mean ± SEM from
three independent experiments. ΔP<0.01 and
*P<0.05, compared with the no injection group.
Wee1B-WT, Wee1B-wild type; Wee1B-KD,
Wee1B-kinase dead; MPF, M phase-promoting factor; hCG, human
chorionic gonadotropin; CDK1, cyclin-dependent kinase 1. |
To identify the phosphorylation of CDK1-Tyr 15 in
the mouse embryos treated with H-89, the embryos were collected at
26, 26.5, 27, 27.5, 28, 28.5 and 29 h post-hCG injection and
subjected to western blotting. The inhibitory phosphorylation
signals of CDK1-Tyr 15 were detected at 26–27 h, and no
phosphorylation band was detected at 27.5 h post-hCG injection in
the control and Wee1B-KD mRNA-injected embryos (Fig. 4B). However, in the Wee1B-WT
and Wee1B-S15A mutant-injected embryos, there were strong bands of
CDK1-Tyr 15 phosphorylation at 26 h, and weak signals were detected
at 28 h post-hCG injection. Conversely, the inhibitory
phosphorylation bands of CDK1-Tyr 15 were observed at 26–28.5 h
when Wee1B-S15D was overexpressed, and there was still a
strong CDK1-Tyr 15 phosphorylation signal detected at 29 h post-hCG
injection.
In the presence of H-89, MPF was initially detected
at 26 h post-hCG injection at 30-min intervals. The MPF activity
oscillated in different groups. In the control, there was a marked
increase in MPF activity at 27.5 h post-hCG injection, then MPF
activity peaked at 28 h, indicating that inhibition of PKA
activates MPF early. Furthermore, MPF activity in Wee1B-KD
mRNA-injected embryos reached its maximal level at 28 h post-hCG
injection, then decreased gradually, as in the control. However,
MPF activity in embryos injected with Wee1B-WT and
Wee1B-S15A mRNAs peaked at 28.5 h and the MPF peak lagged
behind the control. However, MPF activity in embryos injected with
Wee1B-S15D-phosphor-mimic mutants remained low and stable at
26–29 h post-hCG injection (Fig.
4C).
Microinjection of Wee1B-WT and
Wee1B-S15A/D mutants overcomes G2/M transition in mouse
embryos induced by H-89
In the presence of 40 μmol/l H-89, the cleavage of
mouse embryos was observed at 26–29 h post-hCG injection, and the
cleavage rate was calculated at 30 h (Fig. 4D). The results demonstrated that
the cleavage rate of embryos in controls induced by H-89 increased
markedly and embryos entered M phase at 27.5–28 h post-hCG
injection, almost 79.2 (no injection) and 81.3% (TE injection) of
embryos had developed into the two-cell stage at 30 h post-hCG
injection. However, embryos injected with Wee1B-WT and
Wee1B-S15A mRNAs resumed mitosis at 28.5 h post-hCG
injection, and ~40% of embryos had developed to the 2-cell stage at
30 h, markedly lower than that in the control (P<0.01). The
embryos injected with Wee1B-KD mutants entered M phase at
27.5–28 h, and 82.13% of embryos had reached the two-cell stage at
30 h post-hCG injection, which was similar to the control; however,
embryos injected with Wee1B-S15D mRNA rarely entered into
the two-cell stage at 30 h post-hCG injection. Taken together,
these results demonstrate that Ser 15 phosphorylation of WEE1B is
important in G2/M transition of mouse embryos.
Discussion
In the present study, we analyzed the regulation of
mouse WEE1B and examined the role of Ser 15 of WEE1B in regulating
the development of one-cell stage mouse embryos.
PKA is important in regulating the cell cycle
progression of eukaryotic organisms. A study by Shimaoka et
al suggested that continuous high PKA activity is a primary
cause of the meiotic incompetence of pig-growing oocytes, and that
this PKA activity is, not only caused by an insufficient expression
level of PKA subunits, but may be attributed to more complex
spatial-temporal regulation mechanisms (45). Our studies have demonstrated that
PKA negatively regulates cell cycle progression in one-cell stage
mouse embryos by inhibiting MPF (46) and CDC25B acts as a direct substrate
of PKA (27). PKA activity
oscillates with cell cycle progression. The activity of the free C
subunit of PKA was high during interphase, decreased to a minimum
level at the onset of mitosis and increased again at the
metaphase-anaphase transition (46). PKA directly phosphorylates mouse
WEE1B in a cAMP concentration-dependent manner, at least in
vitro(21). Ser 15 in the N
terminus of mouse WEE1B is a major PKA phosphorylation target, and
phosphorylation at this site enhances the autophosphorylation
activity of WEE1B and its ability to inhibit CDK1 and oocyte
maturation (21). In the present
study, to further examine the role of the Ser 15 site of WEE1B in
regulating the development of one-cell stage mouse embryos, we
demonstrated that overexpressed Wee1B-WT and
Wee1B-S15A, not only inhibits the mitotic G2/M
transition, but also decreases the cleavage rate of mouse embryos.
However, inhibition by the S15A mutant did not differ from that of
Wee1B-WT. Since Wee1B-KD is a kinase-dead mutant, Lys
237 of Wee1B-WT was altered to Met, no significant
difference with the control was observed. Overexpression of
phosphor-mimic Wee1B-S15D mutants inhibited mitosis more
efficiently than Wee1B-WT and S15A mutant in the absence of
dbcAMP and H-89, indicating that overexpression of WEE1B prevents
the activation of MPF in the nucleus, which is a prerequisite for
the induction of mitotic resumption. Residue Ser 15 of WEE1B is
likely a direct target of PKA in mouse embryos.
To identify the PKA phosphorylation target of WEE1B,
affinity-purified phosphor-specific and non-phosphor-specific
antibodies were used to detect the phosphorylation status of
WEE1B-Ser 15 in embryos at G1, S, G2 and M
phases by western blotting in the absence or presence of dbcAMP
and/or H-89. The results suggest that WEE1B-Ser 15 is
phosphorylated at the G1 and S phases, whereas WEE1B-Ser
15 is dephosphorylated at the G2 and M phases in
vivo. Taken together, our results indicate that PKA regulates
the early development of mouse embryos by phosphorylation of Ser 15
whether dbcAMP is present or not. If PKA phosphorylated Ser 15 of
WEE1B during the G1 and S phases, WEE1B kinase was
activated to some extent, which is capable of inactivating MPF,
resulting in the retardation of mitosis progression. By contrast,
PKA levels are low in G2/M transition, dephosphorylation
of Ser 15 in the G2 phase by an unknown protein induced
the inactivation of WEE1B, and MPF activity was activated by CDC25B
phosphatase, thus resuming mitosis by the direct dephosphorylation
of CDK1-Tyr 15 (27).
To directly test whether the inhibition of mouse
embryos by Ser 15 of WEE1B was due to levels of endogenous PKA,
specifically endogenous PKA activity was activated or inhibited and
the role of WEE1B in one-cell stage mouse embryos was examined. The
inhibitory effects of S15D mutant delays the re-entry of embryos
into mitosis, which was strengthened compared with Wee1B-WT
and S15A mutant in the presence of dbcAMP and H-89. MPF activity
remained at a relatively low level and the phosphorylation status
of CDK1-Tyr 15 at indicated times in each group was coincident with
the MPF activity. Therefore, the 15D mutant-enhanced inhibition of
division rate of mouse embryos is due to direct phosphorylation of
CDK1-Tyr 15 and the inhibition of MPF activity. Conversely, the
cleavage rate of embryos induced by dbcAMP decreased markedly, and
a low number of embryos reached the two-cell stage at 35 h post-hCG
injection in the control group and Wee1B-WT/KD and
Wee1B-S15A mRNA-microinjected embryos, suggesting that PKA
activation is responsible for the inhibition of MPF activity and
enhanced CDK1-Tyr 15 phosphorylation status. By contrast, in the
presence of 40 μmol/l H-89, mouse embryos in the control group and
those injected with Wee1B-KD mRNA initiated mitosis rapidly,
which suggests that PKA inhibition is responsible for the
activation of MPF activity. However, embryos microinjected with
Wee1B-WT and Wee1B-S15A/D mutants overcome
G2/M transition induced by H-89. Our results indicate
that sustained activation or inhibition of PKA by treatment with
dbcAMP or H-89 into mouse embryos may prolong or promote interphase
in control and Wee1B-KD mRNA-injected embryos; however,
overexpressed Wee1B-WT and Wee1B-S15A/D mutants lead
to G2 arrest. The Wee1B-S15D mutant inhibited CDK1-Tyr
15 phosphorylation more potently than Wee1B-WT and
Wee1B-S15A. These results strongly suggest that PKA
phosphorylates Ser 15 of WEE1B and this phosphorylation causes an
inactivation of MPF activity by the direct phosphorylation of
CDK1-Tyr 15 with a consequently enhanced inhibitory effect in
embryo maturation.
Notably, Wee1B-S15A did not exhibit a
dominant-negative effect compared with the Wee1B-WT in
detecting MPF activity and cleavage rate in the absence or presence
of dbcAMP and/or H-89 in one-cell stage embryos, suggesting that
endogenous WEE1B may be important in cell cycle progression.
Therefore, the knockdown and overexpression of Wee1B into
mouse embryos concurrently may be a way to examine whether
inhibition of mouse embryos occurs by phosphorylation of Ser 15 of
WEE1B or due to the endogenous WEE1B. Further experiments are
required to verify this hypothesis.
Taken together, the present study combined with our
previous studies (27), provides
new insights into the molecular mechanisms of G2/M
transition involved in the early development of mammalian embryos.
A decrease of cAMP in G2/M transition of one-cell stage
embryos and inactivation of PKA (46) causes a rapid and progressive CDC25B
translocation to the nucleus. Thus, the accumulation of CDC25B in
the nucleus causes dephosphorylation and activation of the fraction
of MPF that is shuttling into the nucleus (27). Following this, the activated MPF
promotes the export of WEE1B to the cytoplasm by an unknown
mechanism. The translocation of WEE1B functions as an amplification
step to further promote the activation of the nuclear MPF until a
threshold of activity sufficient for G2/M transition for
WEE1B has no access to its MPF substrate and that this is
sufficient to cause MPF activation. However, it is also possible
that WEE1B becomes inactivated during the export process or during
its accumulation in the cytosol (47), further suppressing its cytostatic
activity for low PKA activity leading to the inactivation of WEE1B.
CDC25B may then be transferred from the nucleus to the cytosol
(27), WEE1B is also exported from
the cytoplasm to the nucleus. The reciprocal translocation between
CDC25B and WEE1B is the prerequisite for the first embryonic
divisions.
Acknowledgements
The authors would like to thank Professor Marco
Conti (University of California, San Francisco, USA) for providing
mouse wild-type Wee1B and kinase-dead Wee1B and Dr Rongjian Su
(Liaoning Medical University, Liaoning, China) for revising the
manuscript and providing valuable advice and encouragement. This
study was supported by the National Nature Science Foundation of
China (81070489 and 81270654) and the Educational Commission of
Liaoning Province of China (L2012303).
Abbreviations:
|
CDK1
|
cyclin-dependent kinase 1
|
|
Wee1B-WT
|
Wee1B-wild type
|
|
Wee1B-KD
|
Wee1B-kinase dead
|
|
PKA
|
protein kinase A
|
|
MPF
|
M phase-promoting factor
|
|
PMSG
|
pregnant mare serum gonadotropin
|
|
hCG
|
human chorionic gonadotropin
|
References
|
1
|
Nigg EA: Mitotic kinases as regulators of
cell division and its checkpoints. Nat Rev Mol Cell Biol. 2:21–32.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nurse P: Universal control mechanism
regulating onset of M-phase. Nature. 344:503–508. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coleman TR and Dunphy WG: Cdc2 regulatory
factors. Curr Opin Cell Biol. 6:877–882. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lew DJ and Kornbluth S: Regulatory roles
of cyclin dependent kinase phosphorylation in cell cycle control.
Curr Opin Cell Biol. 8:795–804. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakajima H, Yonemura S, Murata M, Nakamura
N, Piwnica-Worms H and Nishida E: Myt1 protein kinase is essential
for Golgi and ER assembly during mitotic exit. J Cell Biol.
181:89–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mueller PR, Coleman TR, Kumagai A and
Dunphy WG: Myt1: a membrane-associated inhibitory kinase that
phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science.
270:86–90. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Booher RN, Deshaies RJ and Kirschner MW:
Properties of Saccharomyces cerevisiae wee1 and its
differential regulation of p34CDC28 in response to G1 and G2
cyclins. EMBO J. 12:3417–3426. 1993.
|
|
8
|
McGowan CH and Russell P: Human Wee1
kinase inhibits cell division by phosphorylating p34cdc2
exclusively on Tyr15. EMBO J. 12:75–85. 1993.PubMed/NCBI
|
|
9
|
McGowan CH and Russell P: Cell cycle
regulation of human WEE1. EMBO J. 14:2166–2175. 1995.PubMed/NCBI
|
|
10
|
Mueller PR, Coleman TR and Dunphy WG: Cell
cycle regulation of a Xenopus Wee1-like kinase. Mol Biol
Cell. 6:119–134. 1995. View Article : Google Scholar
|
|
11
|
Kornbluth S, Sebastian B, Hunter T and
Newport J: Membrane localization of the kinase which phosphorylates
p34cdc2 on threonine 14. Mol Biol Cell. 5:273–282. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Booher RN, Holman PS and Fattaey A: Human
Myt1 is a cell cycle-regulated kinase that inhibits Cdc2 but not
Cdk2 activity. J Biol Chem. 272:22300–22306. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu F, Stanton JJ, Wu Z and Piwnica-Worms
H: The human Myt1 kinase preferentially phosphorylates Cdc2 on
threonine 14 and localizes to the endoplasmic reticulum and Golgi
complex. Mol Cell Biol. 17:571–583. 1997.PubMed/NCBI
|
|
14
|
Liu F, Rothblum-Oviatt C, Ryan CE and
Piwnica-Worms H: Overproduction of human Myt1 kinase induces a G2
cell cycle delay by interfering with the intracellular trafficking
of Cdc2-cyclin B1 complexes. Mol Cell Biol. 19:5113–5123.
1999.PubMed/NCBI
|
|
15
|
Wells NJ, Watanabe N, Tokusumi T, Jiang W,
Verdecia MA and Hunter T: The C-terminal domain of the Cdc2
inhibitory kinase Myt1 interacts with Cdc2 complexes and is
required for inhibition of G(2)/M progression. J Cell Sci. 112(Pt
19): 3361–3371. 1999.PubMed/NCBI
|
|
16
|
Hanna CB, Yao S, Patta MC, Jensen JT and
Wu X: WEE2 is an oocyte-specific meiosis inhibitor in rhesus
macaque monkeys. Biol Reprod. 82:1190–1197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shimaoka T, Nishimura T, Kano K and Naito
K: Critical effect of pigWee1B on the regulation of meiotic
resumption in porcine immature oocytes. Cell Cycle. 8:2375–2384.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakanishi M, Ando H, Watanabe N, et al:
Identification and characterization of human Wee1B, a new member of
the Wee1 family of Cdk-inhibitory kinases. Genes Cells. 5:839–847.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishimura T, Shimaoka T, Kano K and Naito
K: Insufficient amount of Cdc2 and continuous activation of Wee1 B
are the cause of meiotic failure in porcine growing oocytes. J
Reprod Dev. 55:553–557. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han SJ and Conti M: New pathways from PKA
to the Cdc2/cyclin B complex in oocytes: Wee1B as a potential PKA
substrate. Cell Cycle. 5:227–231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han SJ, Chen R, Paronetto MP and Conti M:
Wee1B is an oocyte-specific kinase involved in the control of
meiotic arrest in the mouse. Curr Biol. 15:1670–1676. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mueller PR and Leise WF 3rd: Measurement
of Wee kinase activity. Methods Mol Biol. 296:299–328.
2005.PubMed/NCBI
|
|
23
|
Islam MS, Kawase O, Hase S, Hoshi M and
Matsumoto M: PKA activation in concert with ARIS and asterosap
induces the acrosome reaction in starfish. Zygote. 14:329–340.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J and Liu XJ: Progesterone inhibits
protein kinase A (PKA) in Xenopus oocytes: demonstration of
endogenous PKA activities using an expressed substrate. J Cell Sci.
117:5107–5116. 2004.PubMed/NCBI
|
|
25
|
Pirino G, Wescott MP and Donovan PJ:
Protein kinase A regulates resumption of meiosis by phosphorylation
of Cdc25B in mammalian oocytes. Cell Cycle. 8:665–670. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kovo M, Kandli-Cohen M, Ben-Haim M,
Galiani D, Carr DW and Dekel N: An active protein kinase A (PKA) is
involved in meiotic arrest of rat growing oocytes. Reproduction.
132:33–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao J, Liu C, Hou J, et al: Ser149 is
another potential PKA phosphorylation target of Cdc25B in G2/M
transition of fertilized mouse eggs. J Biol Chem. 286:10356–10366.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schultz R: PKA and CDC25B: at last
connected. Cell Cycle. 8:516–517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Zhang Z, Xu XY, et al: Protein
kinase A modulates Cdc25B activity during meiotic resumption of
mouse oocytes. Dev Dyn. 237:3777–3786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okamoto K and Sagata N: Mechanism for
inactivation of the mitotic inhibitory kinase Wee1 at M phase. Proc
Natl Acad Sci USA. 104:3753–3758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burrows AE, Sceurman BK, Kosinski ME, et
al: The C. elegans Myt1 ortholog is required for the proper
timing of oocyte maturation. Development. 133:697–709. 2006.
|
|
32
|
Maller JL, Butcher FR and Krebs EG: Early
effect of progesterone on levels of cyclic adenosine
3′:5′-monophosphate in Xenopus oocytes. J Biol Chem.
254:579–582. 1979.
|
|
33
|
Conti M, Andersen CB, Richard FJ,
Shitsukawa K and Tsafriri A: Role of cyclic nucleotide
phosphodiesterases in resumption of meiosis. Mol Cell Endocrinol.
145:9–14. 1998.PubMed/NCBI
|
|
34
|
Conti M, Andersen CB, Richard F, et al:
Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell
Endocrinol. 187:153–159. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dekel N: Protein
phosphorylation/dephosphorylation in the meiotic cell cycle of
mammalian oocytes. Rev Reprod. 1:82–88. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Okamoto K, Nakajo N and Sagata N: The
existence of two distinct Wee1 isoforms in Xenopus:
implications for the developmental regulation of the cell cycle.
EMBO J. 21:2472–2484. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Parker LL and Piwnica-Worms H:
Inactivation of the p34cdc2-cyclin B complex by the human WEE1
tyrosine kinase. Science. 257:1955–1957. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hogan B, Beddington R, Constantini F and
Lacy E: Manipulating the mouse embryo: a laboratory manual. Cold
Harbor Laboratory Press; New York: 1986
|
|
39
|
Zhang Z, Su WH, Feng C, et al: Polo-like
kinase 1 may regulate G2/M transition of mouse fertilized eggs by
means of inhibiting the phosphorylation of Tyr 15 of Cdc2. Mol
Reprod Dev. 74:1247–1254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cui C, Zhao H, Zhang Z, et al: CDC25B acts
as a potential target of PRKACA in fertilized mouse eggs. Biol
Reprod. 79:991–998. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gallicano GI, McGaughey RW and Capco DG:
Activation of protein kinase C after fertilization is required for
remodeling the mouse egg into the zygote. Mol Reprod Dev.
46:587–601. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu XY, Zhang Z, Su WH, et al: Involvement
of the p110 alpha isoform of PI3K in early development of mouse
embryos. Mol Reprod Dev. 76:389–398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vitolo OV, Sant'Angelo A, Costanzo V,
Battaglia F, Arancio O and Shelanski M: Amyloid β-peptide
inhibition of the PKA/CREB pathway and long-term potentiation:
reversibility by drugs that enhance cAMP signaling. Proc Natl Acad
Sci USA. 99:13217–13221. 2002.
|
|
44
|
Yamada T, Matsuda K and Uchiyama M: Atrial
natriuretic peptide and cGMP activate sodium transport through
PKA-dependent pathway in the urinary bladder of the Japanese tree
frog. J Comp Physiol B. 176:203–212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shimaoka T, Nishimura T, Kano K and Naito
K: Analyses of the regulatory mechanism of porcine WEE1B: the
phosphorylation sites of porcine WEE1B and mouse WEE1B are
different. J Reprod Dev. 57:223–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu B, Wang Y, Liu Y, et al: Protein kinase
A regulates cell cycle progression of mouse fertilized eggs by
means of MPF. Dev Dyn. 232:98–105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Oh JS, Han SJ and Conti M: Wee1B, Myt1,
and Cdc25 function in distinct compartments of the mouse oocyte to
control meiotic resumption. J Cell Biol. 188:199–207. 2010.
View Article : Google Scholar : PubMed/NCBI
|


















