Introduction
Diabetes mellitus (DM) is a metabolic disease
associated with hyperglycemia, which is caused by absolute or
relative insulin deficiency and resistance (1). In addition to the condition, numerous
secondary complications are associated with DM. Of these, diabetic
retinopathy is one of the most serious complications caused by
DM.
Apoptosis, also known as programmed cell death, is a
form of cell death that occurs during several pathological
processes in multicellular organisms and contributes to cell
replacement, tissue remodeling and the removal of damaged cells
under normal conditions. However, inappropriate apoptosis is also
implicated in a number of neurodegenerative diseases (2–4).
Apoptosis is a well-known hallmark associated with the underlying
mechanisms of diabetic retinopathy (5–7).
Hyperglycemia has been demonstrated to induce neuronal cell death
through an apoptotic pathway in diabetic retinas (8,9).
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end
labeling (TUNEL) staining detects DNA fragmentation which is
characteristic of apoptotic cell death (2,3).
Caspases are cysteine proteases which regulate apoptotic cell death
in a variety of cells, including neurons (4). In particular, caspase-3, an
executioner caspase, functions as a downstream death signal and
activates other caspases. Previous studies have reported that
DM-induced apoptosis occurs through caspase-dependent pathways,
including caspase-3 (6,10). In addition, hyperglycemia-induced
apoptotic cell death in the retina is triggered by caspase-3
activation (8,9,11).
The Bcl-2 family of proteins function as critical
regulators in the pathways of apoptosis, inhibiting or promoting
cell death. Bax forms a high molecular weight oligomer in the
mitochondrial membrane enabling the release of cytochrome c, which
initiates apoptosis (12,13). Podestà et al(14) demonstrated that Bax expression was
enhanced in diabetic retinas and its increased expression
contributed to pericyte loss and the development of vascular
complications in diabetic retinopathy. By contrast, Bcl-2 inhibits
apoptosis by suppressing Bax (15).
Protein kinase B (Akt) is a main effector in the
phosphoinositide 3-kinase (PI3K) signaling pathway. Increased Akt
activity blocks the mitochondrial apoptotic pathway by
phosphorylating members of the Bcl-2 family via the inactivation of
pro-apoptotic members, including Bad or by directly inhibiting the
activation of caspase-9 (16,17).
Akt is important for a number of cellular processes, including cell
survival, metabolism, growth, proliferation and mobility (18). In addition, Akt is known to protect
against apoptotic neuronal cell death by targeting the activity of
several transcription factors implicated in the regulation of cell
survival (19,20).
Exercise has been recommended for the alleviation of
symptoms in patients with diabetes (3,21–23).
Regular physical exercise is known to be effective in the
prevention and delay of non-insulin-dependent diabetes onset,
increasing insulin sensitivity and ameliorating glucose metabolism
(24). Exercise is known to
relieve a number of symptoms of DM; however, the effect of exercise
on diabetes-induced apoptotic retinal cell death in association
with Akt expression has not yet been clarified. In the present
study, the effects of treadmill exercise on apoptosis and Akt
expression were investigated in the retinas of diabetic rats.
Materials and methods
Animals
Thirty-two male Sprague-Dawley rats weighing 200±10
g (7 weeks old) were used in this experiment. The rats were housed
under controlled temperature (20±2°C) and lighting conditions
(07:00–19:00 h), with food and water made available ad
libitum throughout the experiment. Experimental procedures were
performed in accordance with the animal care guidelines of the
National Institutes of Health and the Korean Academy of Medical
Sciences. The animals were randomly divided into four groups (each
group, n=4): Control, control and exercise, streptozotocin
(STZ)-induced diabetes and STZ-induced diabetes and exercise. Blood
glucose levels were measured following fasting at 0, 2, 4 and 6
weeks.
Induction of diabetes
To induce diabetes in the experimental animals, a
single intraperitoneal (i.p.) injection of STZ (50 mg/kg, dissolved
in 0.01 M citrate buffer at pH 4.5; Sigma-Aldrich, St. Louis, MO,
USA) was administered to each animal as described previously
(3). Changes in body weight and
blood glucose levels were determined 2 days following STZ injection
using a blood glucose tester (Arkray Inc., Kyoto, Japan). Animals
with blood glucose levels of ≥300 mg/dl were used as subjects in
the diabetic groups.
Treadmill exercise
Rats in the exercise groups ran on the treadmill for
30 min/day, five times a week, over 6 weeks. Exercise consisted of
running at a speed of 3 m/min for the first 5 min, 5 m/min for the
next 5 min and then 8 m/min for the last 20 min, with a 0°
incline.
Tissue preparation
Animals were anesthetized using Zoletil 50 (10
mg/kg, i.p.; Vibac Laboratories, Carros, France), transcardially
perfused with 50 mM phosphate-buffered saline and fixed with a
freshly prepared solution consisting of 4% paraformaldehyde in a
100 mM phosphate buffer (pH 7.4). The retinas were dissected,
postfixed in the same fixative overnight and then transferred into
a 30% sucrose solution for cryoprotection. Coronal sections of
20-μm thickness were generated using a freezing microtome (Leica
Biosystems GmbH, Nussloch, Germany).
TUNEL staining
DNA fragmentation was visualized by TUNEL staining
performed using an In Situ Cell Death Detection kit (Roche
Diagnostics GmbH, Mannheim, Germany) according to the
manufacturer’s instructions (2).
The retinas were suspended in 10 mM Tris-HCl buffer (pH 8.0)
containing 1 mM EDTA and incubated at 55°C for 30 min. Next, the
sections were incubated with proteinase K (100 μg/ml), rinsed,
incubated in 3% H2O2, permeabilized with 0.5%
Triton X-100, rinsed again and incubated in a TUNEL reaction
mixture. The sections were rinsed and visualized using
Converter-POD with 0.03% 3,3′-diaminobenzidine (DAB) and then
finally mounted onto gelatin-coated slides. The slides were
air-dried overnight at room temperature and coverslips were mounted
using Permount (Fisher Scientific, Fair Lawn, NJ, USA).
Caspase-3 immunohistochemistry
Caspase-3 immunohistochemistry was performed as
described previously (2). Sections
from each retina were incubated overnight with a mouse
anti-caspase-3 antibody (1:500; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) and then for a further 1 h with a biotinylated
mouse secondary antibody (1:200; Vector Laboratories, Burlingame,
CA, USA). A bounded secondary antibody was then amplified with a
Vector Elite ABC kit (1:100; Vector Laboratories).
Antibody-biotin-avidin-peroxidase complexes were visualized using
0.03% DAB and the sections were mounted onto gelatin-coated slides.
The slides were air-dried overnight at room temperature and the
coverslips were mounted using Permount.
Western blot analysis
Western blot analysis was performed as described
previously (2). For the western
blot analysis, retinal tissues were lysed in a lysis buffer
containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% deoxycholic
acid, 1% Nonidet P-40, 0.1% SDS, 1 mM PMSF and 100 mg/ml leupeptin.
Protein content was measured using a Bio-Rad colorimetric protein
assay kit (Bio-Rad, Hercules, CA, USA). The protein was separated
on SDS-polyacrylamide gels and transferred onto a nitrocellulose
membrane. Mouse anti-Bax and anti-Bcl-2 (both 1:1,000; Santa Cruz
Biotechnology, Inc.) and rabbit anti-Akt and anti-phospho-Akt
antibodies (both 1:1,000; Cell Signaling Technology Inc., Beverly,
MA, USA) were used as primary antibodies. Horseradish
peroxidase-conjugated anti-mouse antibody against Bax and Bcl-2
(1:3,000) and horseradish peroxidase-conjugated anti-rabbit
antibody against Akt and phospho-Akt (1:5,000; both Vector
Laboratories) were used as the secondary antibodies. Band detection
was performed using the enhanced chemiluminescence detection kit
(Santa Cruz Biotechnology, Inc.).
Statistical analysis
Following staining, immunoreactive cells were
counted in each of the retinal sections using Image-Pro Plus
software (Media Cybernetics, Silver Spring, MD, USA). To compare
the relative expression of proteins, detected bands were calculated
densitometrically using Image-pro Plus software. The results are
expressed as the mean ± SEM. Data were analyzed by one-way analysis
of variance followed by Duncan’s post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of treadmill exercise on body
weight and blood glucose level
At 0, 2, 4 and 6 weeks of the experiment, the
average body weight was 265.33±2.06, 338.33±12.37, 387.33±16.37 and
394.33±16.72 g in the control group, 263.33±2.82, 322.50±10.53,
361.50±12.19 and 374.00±13.70 g in the control and exercise group,
264.00±1.51, 212.00±5.98, 213.00±7.26 and 209.22±8.77 g in the
STZ-induced diabetes group and 265.20±1.63, 218.22±3.88,
223.25±5.46 and 213.20± 13.77 g in the STZ-induced diabetes and
exercise group, respectively. Loss of body weight was observed in
diabetic rats (P<0.05) and reduced body weight was not restored
following treadmill exercise in this group.
At 0, 2, 4 and 6 weeks of the experiment, the
average blood glucose level was 91.83±1.74, 108.00±2.91,
115.33±2.69 and 113.33±2.11 mg/dl in the control group, 92.00±1.21,
103.00±2.84, 101.50±2.54 and 110.66±2.18 mg/dl in the control and
exercise group, 332.50±8.96, 358.33±14.69, 498.89±18.69 and
435.44±19.65 mg/dl in the STZ-induced diabetes group and
337.90±6.67, 396.22±20.44, 459.87±45.99 and 405.40±11.56 mg/dl in
the STZ-induced diabetes and exercise group, respectively. Blood
glucose levels were observed to be significantly increased in the
STZ-induced diabetic rats (P<0.05) and treadmill exercise did
not exert any effect on the blood glucose levels in diabetic
rats.
Effect of treadmill exercise on the
numbers of TUNEL- and caspase-3-positive cells in the retinas of
diabetic rats
Photomicrographs of TUNEL-positive cells in the
retina are presented in Fig. 1A.
The number of TUNEL-positive cells was 5.17±0.98, 4.50±0.67,
78.51±4.74 and 31.50±4.95/section in the control, control and
exercise, STZ-induced diabetes and STZ-induced diabetes and
exercise groups, respectively (Fig.
1B). These results indicate that STZ-induced diabetes enhanced
DNA fragmentation (P<0.05) and treadmill exercise significantly
suppressed DNA fragmentation in diabetic retinas (P<0.05).
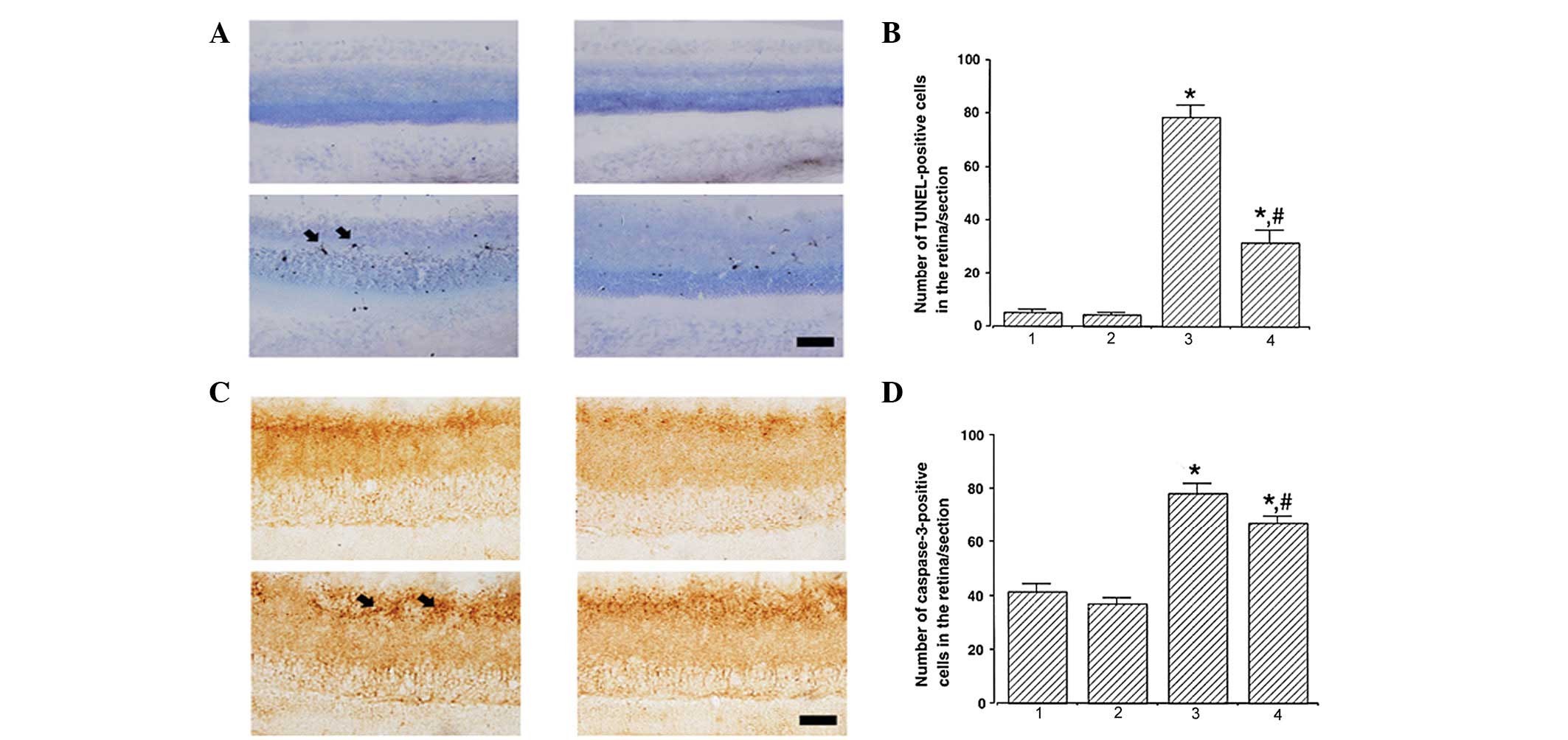 | Figure 1Effect of treadmill exercise on DNA
fragmentation and caspase-3 expression in the retina. (A and B)
Effect of treadmill exercise on DNA fragmentation in the retina.
(A) Photomicrographs of TUNEL-positive cells in the retina (scale
bar, 25 μm) and (B) quantification of TUNEL-positive cells. (C and
D) Effect of treadmill exercise on caspase-3 expression in the
retina. (C) Photomicrographs of caspase-3-positive cells in the
retina (scale bar, 25 μm) and (D) quantification of
caspase-3-positive cells. *P<0.05, vs. control;
#P<0.05, vs. STZ-induced diabetes. 1, control; 2,
control and exercise; 3, STZ-induced diabetes and 4, STZ-induced
diabetes and exercise. Data are presented as the mean ± SEM. TUNEL,
terminal deoxynucleotidyl transferase-mediated dUTP nick-end
labeling; STZ, streptozotocin. |
Photomicrographs of caspase-3-positive cells in the
retina are presented in Fig. 1C.
The number of caspase-3-positive cells was 41.17±3.11, 37.01±2.29,
78.33±3.74 and 67.17±2.69/section in the control, control and
exercise, STZ-induced diabetes and STZ-induced diabetes and
exercise groups, respectively (Fig.
1D). These results indicate that STZ-induced diabetes enhanced
caspase-3 expression (P<0.05) and treadmill exercise
significantly suppressed caspase-3 expression in diabetic retinas
(P<0.05).
Effect of treadmill exercise on the
expression of Bax and Bcl-2 in the retinas of diabetic rats
When the level of Bax protein (24 kDa) in the
control group was set at 1.00, the level of Bax was 1.18±0.06 in
the exercise group, 1.98±0.16 in the STZ-induced diabetes group and
1.40±0.12 in the STZ-induced diabetes and exercise group (Fig. 2A). These results demonstrate that
STZ-induced diabetes enhanced Bax expression (P<0.05) and
treadmill exercise significantly suppressed Bax expression in
diabetic retinas (P<0.05).
When the level of Bcl-2 protein (26–29 kDa) was set
at 1.00 in the control group, the level of Bcl-2 was 11.25±2.92 in
the control and exercise group, 8.35±1.83 in the STZ-induced
diabetes group and 19.04±3.48 in the STZ-induced diabetes and
exercise group (Fig. 2B). These
results indicate that Bcl-2 expression was increased by STZ-induced
diabetes and treadmill exercise markedly enhanced Bcl-2 expression
in diabetic retinas (P<0.05).
Effect of treadmill exercise on the
expression of p-Akt and Akt in the retinas of diabetic rats
Analysis of p-Akt and Akt expression was performed
to estimate the relative levels of these proteins (Fig. 3A). When the expression of p-Akt (60
kDa) in the control group was set as 1.00, p-Akt level was
0.92±0.01 in the control and exercise group, 0.56±0.02 in the
STZ-induced diabetes group and 0.69±0.02 in the STZ-induced
diabetes and exercise group (Fig.
3B). These results indicated that STZ-induced diabetes
suppressed p-Akt expression in the retina (P<0.05) and treadmill
exercise significantly enhanced p-Akt expression in diabetic
retinas (P<0.05).
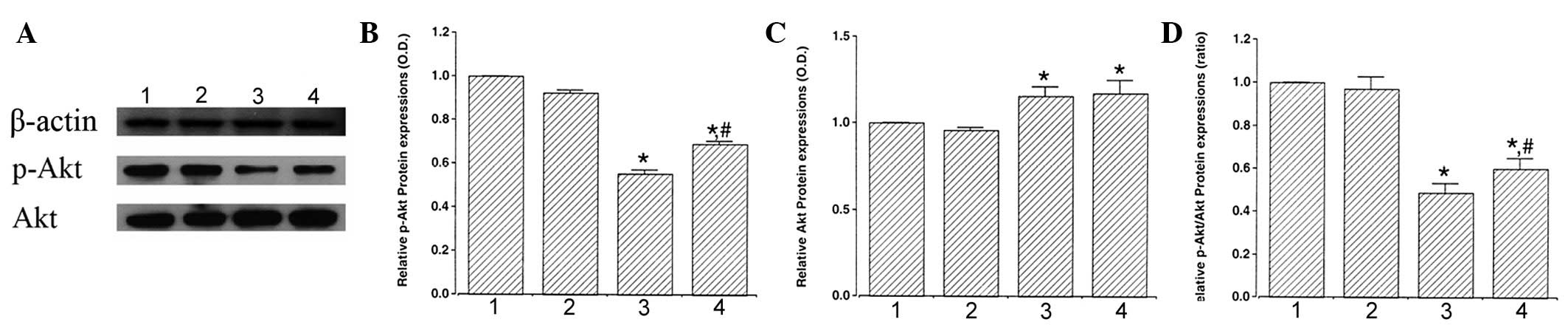 | Figure 3Effect of exercise on p-Akt and Akt
protein expression in the retina. (A) Representative western
blotting. Effect of treadmill exercise on (B) p-Akt, (C) Akt and
(D) ratio of p-Akt/Akt in the retina. 1, control; 2, control and
exercise; 3, STZ-induced diabetes and 4, STZ-induced diabetes and
exercise. Data are presented as the mean ± SEM.
*P<0.05, vs. control; #P<0.05, vs.
STZ-induced diabetes. Akt, protein kinase B; p-Akt, phosphorylated
Akt; STZ, streptozotocin. |
When the expression of Akt (60 kDa) in the control
group was set as 1.00, the level of Akt level was 0.96±0.01 in the
control and exercise group, 1.16±0.02 in the STZ-induced diabetes
group and 1.17±0.03 in the STZ-induced diabetes and exercise group
(Fig. 3C). These results revealed
that STZ-induced diabetes enhanced Akt expression (P<0.05) and
exercise was not found to significantly affect Akt expression in
diabetic retinas.
When the ratio of p-Akt/Akt in the control group was
set as 1.00, the ratio of p-Akt/Akt was 0.97±0.03 in the control
and exercise group, 0.49±0.02 in the STZ-induced diabetes group and
0.60±0.02 in the STZ-induced diabetes and exercise group (Fig. 3D). These results demonstrated that
the ratio of p-Akt to Akt decreased in diabetic retinas
(P<0.05). However, treadmill exercise increased the ratio of
p-Akt to Akt by enhancing the expression of the cell survival
factor, p-Akt (P<0.05).
Discussion
In the present study, STZ-induced DM led to reduced
body weight and significantly increased blood glucose levels.
Treadmill exercise for 6 weeks was not observed to restore lost
body weight and did not exert an effect on blood glucose levels in
the diabetic rats. These observations are consistent with previous
studies reporting that exercise exerts no significant effect on
body weight and blood glucose levels in STZ-induced diabetic rats
(3,25).
Neuronal cell apoptosis in the retina is an
important mechanism involved in the induction of ocular
complications in diabetic retinopathy, glaucoma and ischemic injury
(10,26,27).
In the present study, the number of TUNEL- and caspase-3-positive
cells in the retinas of STZ-induced diabetic rats were higher than
those in the control. Increased neuronal apoptosis in the retina
has been reported in experimental diabetic rats and in patients
with diabetes (6,10). Elevated glucose concentration
decreases cell viability (4) and
Gao et al(7) demonstrated
that the number of TUNEL-positive cells in the retina was increased
in the diabetic rats. Treadmill exercise was found to significantly
suppress the number of TUNEL- and caspase-3-positive cells in the
retinas of STZ-induced diabetic rats. Abu El-Asrar et
al(11) identified that
inhibition of caspase-3 activity reduced DM-induced apoptotic cell
death in retinas and apoptosis induced by hyperglycemia was
effectively inhibited by pre-treatment with caspase-3 inhibitors
(28). Treadmill exercise
suppressed DNA fragmentation and caspase-3 expression (2,29).
Zhang et al(29) reported
that aerobic exercise training for 8 weeks significantly decreased
the number of TUNEL-positive cells and attenuated caspase-3
activity in the heart following ischemia/reperfusion injury. The
present observations reveal that treadmill exercise may ameliorate
diabetes-induced apoptotic cell death in the retina.
In this study, a significant increase in the
expression of the pro-apoptotic molecule, Bax, was observed in the
retinas of diabetic rats. Bax is one of the main regulators of
mitochondrial permeability during apoptosis (12,13).
A significant increase in Bax expression in the retina led to
retinal neuronal cell apoptosis in the diabetic rats (30). In the present study, anti-apoptotic
Bcl-2 expression in the retinas of STZ-induced diabetic rats was
increased compared with the control. Bcl-2 is known to inhibit
apoptosis by blocking the release of cytochrome c from the
mitochondria and binding to pro-apoptotic molecules (31). Bcl-2 expression in the retinas was
increased in diabetic rats with high glucose levels (7). Increased Bcl-2 expression in the
retinas of diabetic rats is considered to represent a compensatory
mechanism against hyperglycemia. Treadmill exercise was
demonstrated to suppress Bax and increase Bcl-2 expression in
diabetic retinas. The anti-apoptotic effects of exercise via the
inhibition of Bax and increased Bcl-2 are well documented (2,25).
In rats with spinal cord injuries, cycling exercise was found to
significantly increase the mRNA expression of the anti-apoptotic
marker Bcl-2 in the spinal cord and high levels of Bcl-2 mRNA were
consistent with reduced expression of caspase-7 and -9 mRNA
(25). Treadmill exercise
suppressed the expression of Bax and increased Bcl-2 expression in
the hippocampus following traumatic brain injury (2). In the present study, decreased Bax
and increased Bcl-2 levels in diabetic retinas were observed
following treadmill exercise and may prevent retinal cells from
undergoing apoptosis.
The levels of p-Akt in retinas were decreased in the
diabetic rats and treadmill exercise markedly enhanced p-Akt
expression. The ratio of p-Akt to Akt was also increased following
treadmill exercise through increased p-Akt expression. Activation
of Akt by PI3K has been demonstrated to result in inhibition of
apoptotic signals and promotion of cell survival signals (32). Phosphorylation of Akt inactivated
the pro-apoptotic factors, Bad and procaspase-9, and increased
resistance to apoptosis (33).
Exercise increased Akt phosphorylation and reduced age-related
insulin resistance of muscle protein metabolism (34). In diabetic rats, treadmill running
activated Akt signaling and improved cognition and synaptic
plasticity in aging rats (35).
Increased phosphorylation levels of Akt by treadmill exercise also
suppressed neuronal cell death in the transgenic mouse model of
Alzheimer’s disease (36). Wang
et al(37) reported that
diabetic retinopathy was the result of increased oxidative stress
induced by chronic hyperglycemia and found that stimulation of Akt
reduced oxidative stress. The present study indicates that the
enhanced expression of p-Akt in retinas may contribute to the
anti-apoptotic effect of treadmill exercise in diabetic rats.
The anti-apoptotic and ameliorating effects of
treadmill exercise on neuropsychiatric disorders are well
documented (2,36,38–40).
In the present study, markers of apoptosis, including TUNEL- and
caspase-3-positive cells, and Bax protein, were increased with
decreased levels of p-Akt in the retinas of diabetic rats.
Treadmill exercise inhibited these apoptotic markers with increased
levels of Bcl-2 and p-Akt in the retinas of diabetic rats. The
anti-apoptotic effect of treadmill exercise on diabetic retinas is
hypothesized to be a result of the enhancing effect of treadmill
exercise on the levels of p-Akt in the retina. Under normal
conditions, treadmill exercise exerted no significant effect on
these apoptotic markers in retinas. The present study demonstrated
that treadmill exercise represents an effective strategy to delay
or prevent the onset of ocular complications in patients with
diabetes.
Acknowledgements
The present study was supported by a grant from the
National Research Foundation of Korea funded by the Korean
Government (NRF-2011-332-G00091).
References
|
1
|
Tang J, Mohr S, Du YD and Kern TS:
Non-uniform distribution of lesions and biochemical abnormalities
within the retina of diabetic humans. Curr Eye Res. 27:7–13. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim DH, Ko IG, Kim BK, Kim TW, Kim SE,
Shin MS, Kim CJ, Kim H, Kim KM and Baek SS: Treadmill exercise
inhibits traumatic brain injury-induced hippocampal apoptosis.
Physiol Behav. 101:660–665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee HH, Shin MS, Kim YS, Yang HY, Chang
HK, Lee TH, Kim CJ, Cho S and Hong SP: Early treadmill exercise
decreases intrastriatal hemorrhage-induced neuronal cell death and
increases cell proliferation in the dentate gyrus of
streptozotocin-induced hyperglycemic rats. J Diabetes
Complications. 19:356–360. 2005.
|
|
4
|
Santiago AR, Cristóvão AJ, Santos PF,
Carvalho CM and Ambrósio AF: High glucose induces
caspase-independent cell death in retinal neural cells. Neurobiol
Dis. 25:464–472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abu El-Asrar AM, Dralands L, Missotten L
and Geboes K: Expression of antiapoptotic and proapoptotic
molecules in diabetic retinas. Eye (Lond). 21:238–245.
2007.PubMed/NCBI
|
|
6
|
Busik JV, Mohr S and Grant MB:
Hyperglycemia-induced reactive oxygen species toxicity to
endothelial cells is dependent on paracrine mediators. Diabetes.
57:1952–1965. 2008. View Article : Google Scholar
|
|
7
|
Gao XY, Kuang HY, Zou W, Liu XM, Lin HB
and Yang Y: The timing of re-institution of good blood glucose
control affects apoptosis and expression of Bax and Bcl-2 in the
retina of diabetic rats. Mol Biol Rep. 36:1977–1982. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barber AJ, Antonetti DA, Kern TS, Reiter
CE, Soans RS, Krady JK, Levison SW, Gardner TW and Bronson SK: The
Ins2Akita mouse as a model of early retinal complications in
diabetes. Invest Ophthalmol Vis Sci. 46:2210–2218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martin PM, Roon P, Van TK, Ganapathy V and
Smith SB: Death of retinal neurons in streptozotocin-induced
diabetic mice. Invest Ophthalmol Vis Sci. 45:3330–3336. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Behl Y, Krothapalli P, Desta T, DiPiazza
A, Roy S and Graves DT: Diabetes enhanced tumor necrosis factor-α
production promotes apoptosis and the loss of retinal microvascular
cells in type 1 and type 2 models of diabetic retinopathy. Am J
Pathol. 172:1411–1418. 2008.
|
|
11
|
Abu El-Asrar AM, Dralands L, Missotten L,
Jadaan IA and Geboes K: Expression of apoptosis markers in the
retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci.
45:2760–2766. 2004.PubMed/NCBI
|
|
12
|
Antonsson B, Montessuit S, Sanchez B and
Martinou JC: Bax is present as a high molecular weight
oligomer/complex in the mitochondrial membrane of apoptotic cells.
J Biol Chem. 276:11615–11623. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dejean LM, Martinez-Caballero S, Guo L,
Hughes C, Teijido O, Ducret T, Ichas F, Korsmeyer SJ, Antonsson B,
Jonas EA and Kinnally KW: Oligomeric Bax is a component of the
putative cytochrome c release channel MAC, mitochondrial
apoptosis-induced channel. Mol Biol Cell. 16:2424–2432. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Podestà F, Romeo G, Liu WH, Krajewski S,
Reed JC, Gerhardinger C and Lorenzi M: Bax is increased in the
retina of diabetic subjects and is associated with pericyte
apoptosis in vivo and in vitro. Am J Pathol.
156:1025–1032. 2000.PubMed/NCBI
|
|
15
|
Mikhailov V, Mikhailova M, Pulkrabek DJ,
Dong Z, Venkatachalam MA and Saikumar P: Bcl-2 prevents Bax
oligomerization in the mitochondrial outer membrane. J Biol Chem.
276:18361–18374. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cardone MH, Roy N, Stennicke HR, Salvesen
GS, Franke TF, Stanbridge E, Frisch S and Reed JC: Regulation of
cell death protease caspase-9 by phosphorylation. Science.
282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brazil DP and Hemmings BA: Ten years of
protein kinase B signalling: a hard Akt to follow. Trends Biochem
Sci. 26:657–664. 2001.PubMed/NCBI
|
|
19
|
Nakazawa T, Shimura M, Tomita H, Akiyama
H, Yoshioka Y, Kudou H and Tamai M: Intrinsic activation of
PI3K/Akt signaling pathway and its neuroprotective effect against
retinal injury. Curr Eye Res. 26:55–63. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weishaupt JH, Rodhe G, Pölking E, Siren
AL, Ehrenreich H and Bähr M: Effect of erythropoietin
axotomy-induced apoptosis in rat retinal ganglion cells. Invest
Ophthalmol Vis Sci. 45:1514–1522. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asa C, Maria S, Katharina SS and Bert A:
Aquatic exercise is effective in improving exercise performance in
patients with heart failure and type 2 diabetes mellitus. Evid
Based Complement Alternat Med. 2012:3492092012.PubMed/NCBI
|
|
22
|
Kadoglou NP, Vrabas IS, Kapelouzou A,
Lampropoulos S, Sailer N, Kostakis A, Liapis CD and Angelopoulou N:
The impact of aerobic exercise training on novel adipokines, apelin
and ghrelin, in patients with type 2 diabetes. Med Sci Monit.
18:CR290–CR295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Selaqzi H, Buyukakilli B, Cimen B, Yilmaz
N and Erdogan S: Protective and therapeutic effects of swimming
exercise training on diabetic peripheral neuropathy of
streptozotocin-induced diabetic rats. J Endocrinol Invest.
31:971–978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Derouich M and Boutayeb A: The effect of
physical exercise on the dynamics of glucose and insulin. J
Biomech. 35:911–917. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu G, Keeler BE, Zhukareva V and Houlé
JD: Cycling exercise affects the expression of apoptosis-associated
microRNAs after spinal cord injury in rats. Exp Neurol.
226:200–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen J, Miao Y, Wang XH and Wang Z:
Elevation of p-NR2AS1232 by Cdk5/p35 contributes to retinal
ganglion cell apoptosis in a rat experimental glaucoma model.
Neurobiol Dis. 43:455–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kielczewski JL, Hu P, Shaw LC, Li Calzi S,
Mames RN, Gardiner TA, McFarland E, Chan-Ling T and Grant MB: Novel
protective properties of IGFBP-3 result in enhanced pericyte
ensheathment, reduced microglial activation, increased microglial
apoptosis and neuronal protection after ischemic retinal injury. Am
J Pathol. 178:1517–1528. 2011. View Article : Google Scholar
|
|
28
|
Mohr S, Xi X, Tang J and Kern TS: Caspase
activation in retinas of diabetic and galactosemic mice and
diabetic patients. Diabetes. 51:1172–1179. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang KR, Liu HT, Zhang HF, Zhang QJ, Li
QX, Yu QJ, Guo WY, Wang HC and Gao F: Long-term aerobic exercise
protects the heart against ischemia/reperfusion injury via PI3
kinase-dependent and Akt-mediated mechanism. Apoptosis.
12:1579–1588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oshitari T and Roy S: Diabetes: a
potential enhancer of retinal injury in rat retinas. Neurosci Lett.
390:25–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nuñez G and del Peso L: Linking
extracellular survival signals and the apoptotic machinery. Curr
Opin Neurobiol. 8:613–618. 1998.PubMed/NCBI
|
|
33
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004.PubMed/NCBI
|
|
34
|
Fujita S, Rasmussen BB, Cadenas JG,
Drummond MJ, Glynn EL, Sattler FR and Volpi E: Aerobic exercise
overcomes the age-related insulin resistance of muscle protein
metabolism by improving endothelial function and Akt/mammalian
target of rapamycin signaling. Diabetes. 56:1615–1622. 2007.
View Article : Google Scholar
|
|
35
|
Aguiar AS Jr, Castro AA, Moreira EL,
Glaser V, Santos AR, Tasca CI, Latini A and Prediger RD: Short
bouts of mild-intensity physical exercise improve spatial learning
and memory in aging rats: involvement of hippocampal plasticity via
AKT, CREB and BDNF signaling. Mech Ageing Dev. 132:560–567. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Um HS, Kang EB, Koo JH, Kim HT, Jin-Lee,
Kim EJ, Yang CH, An GY, Cho IH and Cho JY: Treadmill exercise
represses neuronal cell death in an aged transgenic mouse model of
Alzheimer’s disease. Neurosci Res. 69:161–173. 2011.PubMed/NCBI
|
|
37
|
Wang Q, Pfister F, Dorn-Beineke A, vom
Hagen F, Lin J, Feng Y and Hammes HP: Low-dose erythropoietin
inhibits oxidative stress and early vascular changes in the
experimental diabetic retina. Diabetologia. 53:1227–1238. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim H, Heo HI, Kim DH, Ko IG, Lee SS, Kim
SE, Kim BK, Kim TW, Ji ES, Kim JD, Shin MS, Choi YW and Kim CJ:
Treadmill exercise and methylphenidate ameliorate symptoms of
attention deficit/hyperactivity disorder through enhancing dopamine
synthesis and brain-derived neurotrophic factor expression in
spontaneous hypertensive rats. Neurosci Lett. 504:35–39. 2011.
View Article : Google Scholar
|
|
39
|
Sung YH, Kim SC, Hong HP, Park CY, Shin
MS, Kim CJ, Seo JH, Kim DY, Kim DJ and Cho HJ: Treadmill exercise
ameliorates dopaminergic neuronal loss through suppressing
microglial activation in Parkinson’s disease mice. Life Sci.
91:1309–1316. 2012.PubMed/NCBI
|
|
40
|
Seo JH, Kim TW, Kim CJ, Sung YH and Lee
SJ: Treadmill exercise during pregnancy ameliorates post-traumatic
stress disorder-induced anxiety-like responses in maternal rats.
Mol Med Rep. 7:389–395. 2013.PubMed/NCBI
|

















