Introduction
Lipopolysaccharide (LPS), a component of the
Gram-negative bacterial cell wall, is a potent inducer of
inflammation (1). The inflammatory
response to Gram-negative bacterial infections in the central
nervous system remains a major cause of neurological disease
(2). Several studies have
suggested that neuroinflammation is implicated in the pathology of
several neurodegenerative diseases, including Alzheimer’s disease,
Parkinson’s disease and multiple sclerosis (3–5). It
has been demonstrated that elevated levels of pro-inflammatory
cytokines are capable of inducing the onset of a number of
neurodegenerative diseases (6).
Although the pathologies of different neurodegenerative diseases
are distinct, neuroinflammation is commonly involved (7). It has been suggested that the
inhibition of neuroinflammation prevents the onset and reduces the
symptoms of neurodegenerative diseases (6,7). A
significant body of evidence has observed LPS-induced
neuroinflammation, characterized by the elevated expression levels
of interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α, in
the central nervous system of animal models with neurological
diseases (8–10).
Ketamine, a commonly used anesthetic agent, is
recommended for use in patients with sepsis (11). Clinically, the majority of
anesthesiologists have observed that ketamine is capable of
increasing blood pressure, particularly in patients with sepsis.
Collectively, several studies have demonstrated that ketamine is
able to maintain circulatory stability, mainly as a result of
inhibiting the secretion of pro-inflammatory cytokines (12–14).
Koga et al(15) have
demonstrated that ketamine is capable of decreasing the mortality
rate in rats with endotoxin-induced shock, as well as the activity
of TNF-α. Another study by Taniguchi et al(16) demonstrated that ketamine is able to
inhibit the release of IL-1β and IL-6. Furthermore, it has been
shown that ketamine inhibits the LPS-induced inflammatory response
in a dose-dependent manner (17).
However, a paucity of data exists with regard to the
anti-inflammatory effects of ketamine in the central nervous
system. Therefore, in the present study, we aimed to investigate
the effect of ketamine on the LPS-induced inflammatory response in
cultured Neuro2a (N2a) cells and to elucidate its potential
mechanism of action.
Materials and methods
Reagents
Ketamine was purchased from Fujian Gutian
Pharmaceutical Company (Fujian, China). IL-1β, IL-6 and TNF-α ELISA
kits were purchased from Nanjing Jiancheng Bioengineering Company
(Nanjing, China). Primary antibodies against nuclear factor (NF)-κB
and inducible nitric oxide synthase (iNOS) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). The study was
approved by the ethics committee of Soochow University, Suzhou,
China.
Cell culture
N2a cells were obtained from Medical College,
Soochow University, Suzhou, China. The mouse N2a neuroblastoma cell
culture was performed as described previously (18,19).
N2a cells were maintained in DMEM (Gibco Co., New York, USA)
culture solution supplemented with 10% FBS (Gibco), 0.3 mM
L-glutamine and 50 U/ml penicillin/streptomycin. N2a cells with 70%
confluence were randomly divided into 3 groups (6 duplicates), and
each culture well contained 2×105 N2a cells. DMEM
solution, 0.5 μmol/l LPS or 1 μmol/l ketamine plus 0.5 μmol/l LPS
(Sigma, Oakville, ON, Canada) were randomly administered into 18
culture wells (n=6/group). Forty-eight hours after incubation, N2a
cells were prepared for analysis.
ELISA
The concentration of cytokines was determined using
ELISA kits. Forty-eight-well microtiter plates were coated with a
monoclonal antibody to mouse cytokines and were incubated in
hydrogen bicarbonate buffer (pH 9.6) overnight at 4°C. In order to
avoid non-specific binding, the wells were blocked with 0.5% bovine
serum albumin (BSA, Equitech-Bio Inc., Kerrville, TX, USA) in
phosphate-buffered saline (PBS, Sigma; 60 mmol/l, pH 7.4) for 1 h
at room temperature. Undiluted culture supernatants were added in
duplicates and incubated for 2 h at 37°C. Antibodies to human
cytokines coupled with biotin (Pharmingen, San Diego, CA, USA) were
used as secondary antibodies (1.25 μg/ml) and were incubated with
the plates at 37°C for 2 h. Following incubation, avidin-peroxidase
(2.5 μg/ml) was added for 1 h and then 0.5 mg/ml
orthophenylenediamine dissolved in citrate buffer (pH 5.0) with
0.01% H2O2 was used as a substrate solution.
After stopping the enzyme reaction with 25% sulphuric acid, optical
density was measured in a microtiter plate reader at 450 nm.
Recombinant cytokines were used to establish a standard curve to
which the results obtained with the culture supernatants were
correlated.
Western blotting
The N2a cells were washed with PBS and lysed in 1.3X
SDS-containing sample buffer without 1,4-dithio-dl-threitol (DTT)
or bromophenol blue containing 100 μM orthovanadate. Protein levels
were determined using the bicinchoninic acid (BCA) method,
according to the manufacturer’s instructions. BSA was used as a
standard. Prior to electrophoresis, a mixture of bromophenol blue
and DTT (final concentration, 10 mM) was added to the samples. For
western blotting, 50 μg of the total protein from each sample was
separated by SDS-PAGE under reducing conditions. The proteins were
then transferred onto polyvinylidene fluoride (PVDF) membranes
(Nanjing Jiancheng Bioengineering Company, Nanjing, China). The
membranes were blocked for 2 h at room temperature using non-fat
dried milk blotting grade blocker and incubated overnight with
primary antibodies. The primary antibodies used were goat
anti-NF-κB (1:2,000) and rabbit anti-iNOS (1:1,000). Primary
antibodies were diluted in Tris-+6buffered saline (TBS, Thermo
Fisher Scientific Inc., Rockford, IL, USA) containing 0.1% Tween-20
(TBS-T) and 1% BSA. Following extensive washing (three times for 15
min each in TBS-T), the NF-κB and iNOS protein levels were measured
with horseradish peroxidase-conjugated rabbit anti-goat IgG
(1:100,000 dilution) using chemiluminescence (ECL) reagents
(Beyotime Company, Nantong, China). Equal protein loading and
transfer were assessed by the subjection of each sample to western
blotting for GAPDH (rabbit anti-GAPDH IgG, 1:5,000 dilution).
Statistical analysis
Data are expressed as the mean ± SD. Statistical
analyses were performed using one-way ANOVA and post hoc analyses
were performed using the least significant difference test. These
statistical analyses were conducted using SPSS version 17.0 (IBM,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant result.
Results
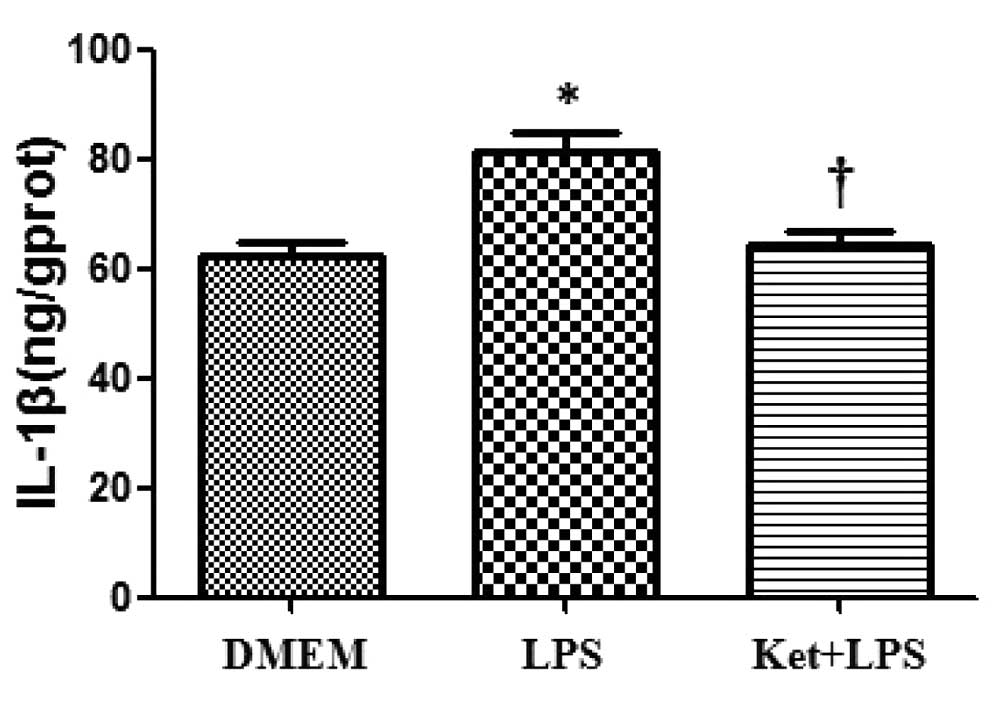
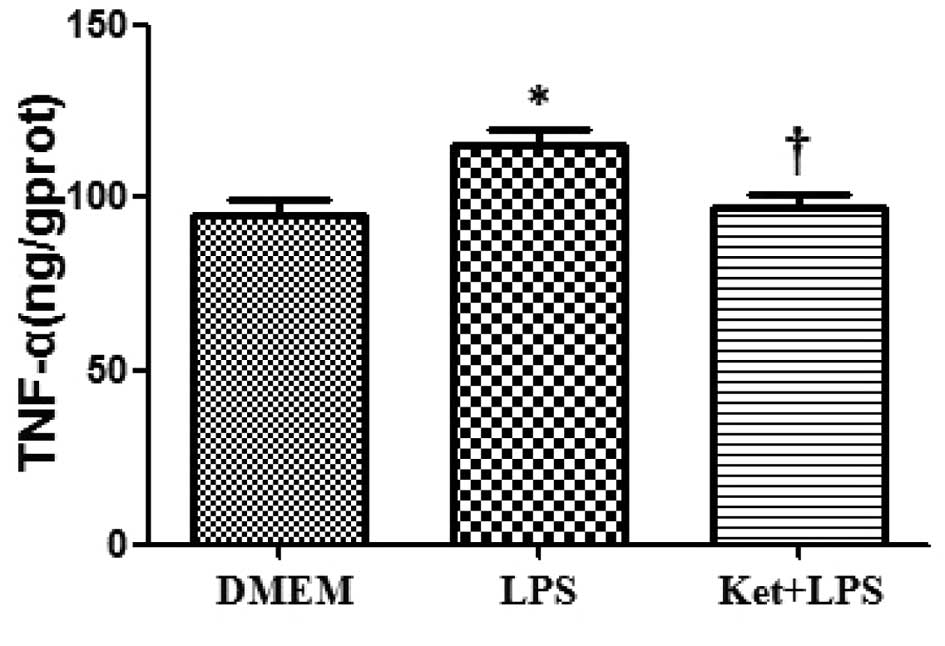
LPS administration increases the
expression levels of IL-1β, IL-6 and TNF-α in cultured N2a
cells
The administration of LPS alone significantly
increased the expression levels of IL-1β, IL-6 and TNF-α compared
with DMEM administration alone in cultured N2a cells (P<0.05;
Figs. 1–3).
Ketamine attenuates the LPS-induced
upregulation of IL-1β, IL-6 and TNF-α in cultured N2a cells
Compared with the LPS administration alone group,
the administration of LPS plus ketamine group demonstrated a
significant decrease in the levels of IL-1β, IL-6 and TNF-α in
cultured N2a cells (P<0.05; Figs.
1–3).
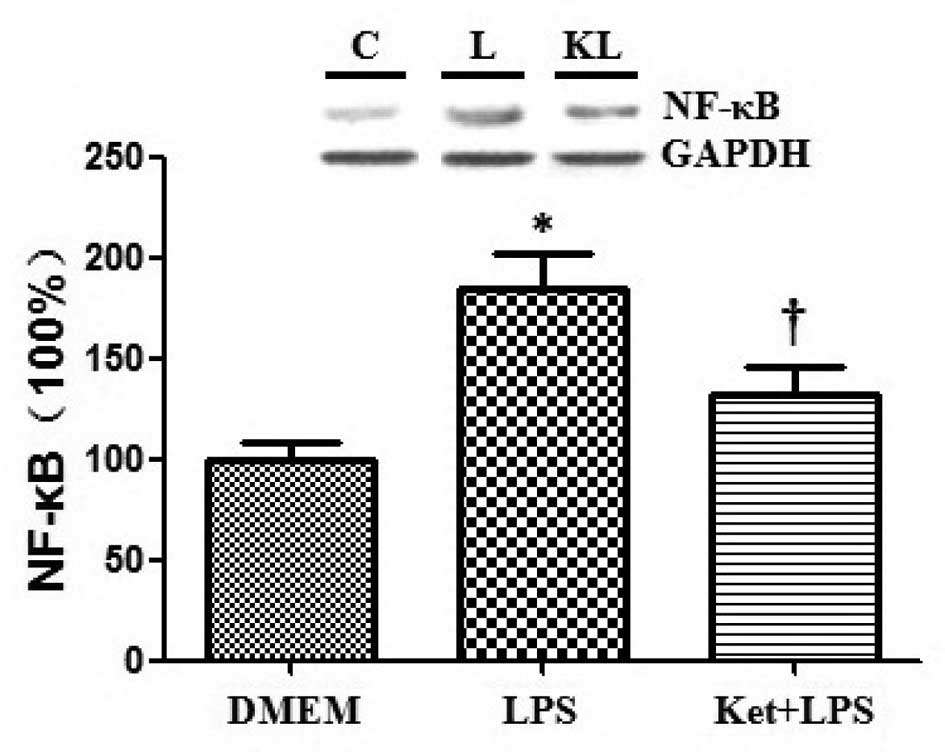
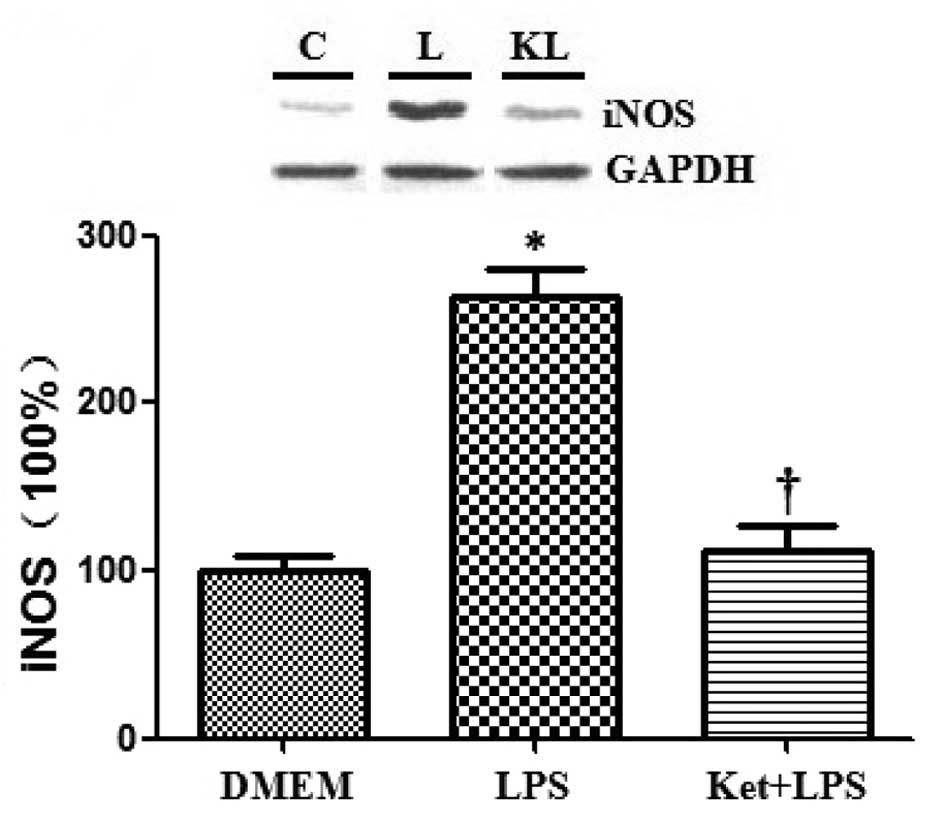
Effects of ketamine and/or LPS on the
expression levels of NF-κB and iNOS in cultured N2a cells
Western blotting was used to assess the expression
levels of NF-κB and iNOS, and the results demonstrated that the
administration of LPS alone significantly increased the expression
levels of NF-κB and iNOS compared with DMEM administration alone in
cultured N2a cells. By contrast, the administration of ketamine
attenuated the LPS-induced upregulation of NF-κB and iNOS
(P<0.05; Figs. 4 and 5).
Discussion
In the present study, several inflammatory
cytokines, including IL-1β, IL-6 and TNF-α, were upregulated in
LPS-treated N2a cells, while the administration of ketamine
attenuated the LPS-induced inflammatory response. In addition, we
demonstrated that the expression levels of NF-κB and iNOS were
increased following LPS treatment, while they were downregulated
following ketamine treatment.
Several lines of evidence have indicated that
ketamine, an N-methyl-D-aspartic acid (NMDA) receptor antagonist,
has a significant positive effect on the regulation of the
inflammatory response (13,14).
Several studies have reported that ketamine exhibits an inhibitory
effect on the inflammatory response in vitro(20–22).
In addition, Shibakawa et al(23) demonstrated that ketamine
significantly inhibits the LPS-induced inflammatory response in
microglial cells. However, in the present study, we used N2a cells
to investigate the anti-inflammatory effect of ketamine. Our
results demonstrated that ketamine exerted an inhibitory effect on
the LPS-stimulated inflammatory response in N2a cells.
NF-κB has been shown to be important in regulating
the expression of a number of genes involved in cell survival,
inflammatory processes and neuronal degeneration (24). Dysregulation of the NF-κB signaling
pathway may result in chronic neuroinflammation and neuronal death,
thus the NF-κB signaling pathway is important in the regulation of
neuronal function (25,26). A recent study by Wu et
al(27) demonstrated that the
inhibitory effect of ketamine on the LPS-induced inflammatory
response may be mediated by the suppression of NF-κB activation. In
addition, Yu et al(28)
revealed that ketamine is able to inhibit pulmonary NF-κB activity
during endotoxemia in rats. The results of the present study were
consistent with previous findings demonstrating that LPS
significantly activates the expression of NF-κB, while ketamine is
capable of inhibiting the LPS-induced activation of NF-κB.
In the present study, the upregulation of NF-κB was
observed in LPS-treated N2a cells. It has been reported that NF-κB
activation upregulates the production of iNOS, which leads to the
upregulation of nitric oxide production during the inflammatory
response (29). NF-κB-dependent
activation of iNOS promotes the inflammatory response (30). Therefore, we hypothesized that
NF-κB and iNOS are important in mediating the inhibitory effect of
ketamine on the inflammatory response in the central nervous
system. Our results demonstrated that ketamine was capable of
inhibiting the LPS-induced activation of iNOS, which confirmed our
hypothesis.
Several studies have reported that the pathogenesis
of numerous types of neurodegenerative diseases, including
Alzheimer’s disease, Parkinson’s disease and multiple sclerosis,
are likely to be associated with increased levels of
pro-inflammatory cytokines in the central nervous system (3–5).
Notably, as a widely used anesthetic agent, ketamine has attracted
increasing attention for the treatment of refractory depression. To
the best of our knowledge, no other study has reported the
therapeutic effects of ketamine in other inflammation-associated
neurological and/or psychiatric diseases to date. Large-scale
studies are required to fully elucidate the therapeutic effects of
ketamine, thereby expanding its clinical application.
In conclusion, ketamine is capable of exerting an
inhibitory effect on the LPS-induced inflammatory response in
cultured N2a cells, and its mechanism is likely to be associated
with the inhibition of NF-κB and iNOS.
References
|
1
|
Tuo W, Ott TL, Liu S and Bazer FW:
Intrauterine infusion of bacterial lipopolysaccharide (LPS) prior
to mating has no adverse effect on fertility, fetal survival and
fetal development. J Reprod Immunol. 42:31–39. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glass CK, Saijo K, Winner B, Marchetto MC
and Gage FH: Mechanisms underlying inflammation in
neurodegeneration. Cell. 140:918–934. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dhib-Jalbut S, Arnold DL, Cleveland DW, et
al: Neurodegeneration and neuroprotection in multiple sclerosis and
other neurodegenerative diseases. J Neuroimmunol. 176:198–215.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rozemuller AJ, Jansen C, Carrano A, et al:
Neuroinflammation and common mechanism in Alzheimer’s disease and
prion amyloidosis: amyloid-associated proteins, neuroinflammation
and neurofibrillary degeneration. Neurodegener Dis. 10:301–304.
2012.
|
|
5
|
Cooper-Knock J, Kirby J, Ferraiuolo L,
Heath PR, Rattray M and Shaw PJ: Gene expression profiling in human
neurodegenerative disease. Nat Rev Neurol. 8:518–530. 2012.
View Article : Google Scholar
|
|
6
|
Weiner HL and Selkoe DJ: Inflammation and
therapeutic vaccination in CNS diseases. Nature. 420:879–884. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Minghetti L: Role of inflammation in
neurodegenerative diseases. Curr Opin Neurol. 18:315–321. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ambrosini A, Louin G, Croci N, Plotkine M
and Jafarian-Tehrani M: Characterization of a rat model to study
acute neuroinflammation on histopathological, biochemical and
functional outcomes. J Neurosci Methods. 144:183–191. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burd I, Bentz AI, Chai J, et al:
Inflammation-induced preterm birth alters neuronal morphology in
the mouse fetal brain. J Neurosci Res. 88:1872–1881. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Belarbi K, Jopson T, Tweedie D, et al:
TNF-α protein synthesis inhibitor restores neuronal function and
reverses cognitive deficits induced by chronic neuroinflammation. J
Neuroinflammation. 9:232012.
|
|
11
|
Park GR, Manara AR, Mendel L and Bateman
PE: Ketamine infusion. Its use as a sedative, inotrope and
bronchodilator in a critically ill patient. Anaesthesia.
42:980–983. 1987.PubMed/NCBI
|
|
12
|
Cho JE, Shim JK, Choi YS, Kim DH, Hong SW
and Kwak YL: Effect of low-dose ketamine on inflammatory response
in off-pump coronary artery bypass graft surgery. Br J Anaesth.
102:23–28. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsao CM, Wu CC, Wang JJ, Wong CS, Tsai SK
and Ho ST: Intravenous anesthetics in sepsis. Acta Anaesthesiol
Taiwan. 43:153–163. 2005.PubMed/NCBI
|
|
14
|
Taniguchi T and Yamamoto K:
Anti-inflammatory effects of intravenous anesthetics on
endotoxemia. Mini Rev Med Chem. 5:241–245. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koga K, Ogata M, Takenaka I, Matsumoto T
and Shigematsu A: Ketamine suppresses tumor necrosis factor-alpha
activity and mortality in carrageenan-sensitized endotoxin shock
model. Circ Shock. 44:160–168. 1994.PubMed/NCBI
|
|
16
|
Taniguchi T, Kanakura H, Takemoto Y,
Kidani Y and Yamamoto K: Effects of ketamine and propofol on the
ratio of interleukin-6 to interleukin-10 during endotoxemia in
rats. Tohoku J Exp Med. 200:85–92. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taniguchi T, Takemoto Y, Kanakura H,
Kidani Y and Yamamoto K: The dose-related effects of ketamine on
mortality and cytokine responses to endotoxin-induced shock in
rats. Anesth Analg. 97:1769–1772. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strenge K, Schauer R and Kelm S: Binding
partners for the myelin-associated glycoprotein of N2A
neuroblastoma cells. FEBS Lett. 444:59–64. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Björkdahl C, Sjögren MJ, Winblad B and Pei
JJ: Zinc induces neurofilament phosphorylation independent of p70
S6 kinase in N2a cells. Neuroreport. 16:591–595. 2005.PubMed/NCBI
|
|
20
|
Kawasaki T, Ogata M, Kawasaki C, Ogata J,
Inoue Y and Shigematsu A: Ketamine suppresses proinflammatory
cytokine production in human whole blood in vitro. Anesth Analg.
89:665–669. 1999.PubMed/NCBI
|
|
21
|
Takenaka I, Ogata M, Koga K, Matsumoto T
and Shigematsu A: Ketamine suppresses endotoxin-induced tumor
necrosis factor alpha production in mice. Anesthesiology.
80:402–408. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimaoka M, Iida T, Ohara A, et al:
Ketamine inhibits nitric oxide production in mouse-activated
macrophage-like cells. Br J Anaesth. 77:238–242. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shibakawa YS, Sasaki Y, Goshima Y, et al:
Effects of ketamine and propofol on inflammatory responses of
primary glial cell cultures stimulated with lipopolysaccharide. Br
J Anaesth. 95:803–810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jones E, Adcock IM, Ahmed BY and Punchard
NA: Modulation of LPS stimulated NF-kappaB mediated Nitric Oxide
production by PKCepsilon and JAK2 in RAW macrophages. J Inflamm
(Lond). 4:232007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bonini SA, Ferrari-Toninelli G, Uberti D,
et al: Nuclear factor κB-dependent neurite remodeling is mediated
by Notch pathway. J Neurosci. 31:11697–11705. 2011.
|
|
26
|
Makarov SS: NF-kappaB as a therapeutic
target in chronic inflammation: recent advances. Mol Med Today.
6:441–448. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu Y, Li W, Zhou C, et al: Ketamine
inhibits lipopolysaccharide-induced astrocytes activation by
suppressing TLR4/NF-κB pathway. Cell Physiol Biochem. 30:609–617.
2012.PubMed/NCBI
|
|
28
|
Yu M, Shao D, Feng X, Duan M and Xu J:
Effects of ketamine on pulmonary TLR4 expression and NF-kappa-B
activation during endotoxemia in rats. Methods Find Exp Clin
Pharmacol. 29:395–399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: new discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han S, Lee JH, Kim C, et al: Capillarisin
inhibits iNOS, COX-2 expression, and proinflammatory cytokines in
LPS-induced RAW 264.7 macrophages via the suppression of ERK, JNK,
and NF-κB activation. Immunopharmacol Immunotoxicol. 35:34–42.
2013.PubMed/NCBI
|