Introduction
Endometriosis is a gynecological disease defined as
the presence of endometrial tissue outside the uterine cavity. This
tissue is located in the peritoneum, ovary or fallopian tube and
more rarely in the pleura, lung or brain. Endometriosis occurs in
5–20% of females with pelvic pain, 20–50% of infertile females and
6–10% of females of reproductive age (1). The causes of this disease include
retrograde menstruation, endometrium abnormalities, peritoneal
environment changes, increased angiogenesis, inadequate
immunological reactions and genetic and environmental factors
(1,2).
Several hypotheses linking stem cells and
endometriosis have emerged and previous studies have indicated that
endometrium-derived cells, particularly stem and progenitor cells,
contribute to the initiation of endometriosis (3,4).
Furthermore, this group observed stem cell and progenitor cell
activity in the basal layers of the endometrium and ectopic
endometrium. This finding supports the ‘retrograde menstruation
theory’, which suggests that endometriosis arises from the
implantation of endometrial tissues due to retrograde flow of
menstruation blood containing these tissues (5). It was reported that bone
marrow-derived cells may differentiate into endometrial tissues and
contribute to endometriosis (6,7).
Endometrial tissues also contain stem cells with strong
proliferative characteristics, given that cells in these tissues
are rapidly shed and regenerated during the menstruation cycles.
The abnormal existence or hyperplasia of cells is thought to be
associated with the pathogenesis of a number of diseases (8). Numerous putative stem cell markers
have been previously described (9,10).
Differentiated cells are produced by the ectopic expression of
specific transcription factors, including SRY-box containing gene 2
(SOX2), octamer-binding transcription factor 4 (Oct-4),
Krüeppel-like factor 4 (KLF-4), homeobox protein NANOG (NANOG),
C-X-C chemokine receptor type 4 (CXCR4) and cellular homolog of the
oncogenic retrovirus v-myc oncogene (c-Myc), and subsequently form
pluripotent stem cells (11–13).
Recent studies have demonstrated that stem cell markers, including
SOX2, Oct-4 and CD117 antigen (c-kit) are expressed in the
endometrium (14,15).
Proteomics is the study of protein expression in
cells, tissues and whole organisms. Proteomic methods have been
used as a therapeutic tool for the diagnosis of ovarian, lung,
colon and endometrial cancers (16,17).
This approach is a non-surgical assessment and is crucial in
diagnosis. Molecular screening methods generate an index for
investigating new biological markers by comparing protein
expression levels between patients and healthy controls (18). Several groups have used proteomics
to study endometriosis by analyzing serum, peritoneal fluid,
eutopic and ectopic endometrial tissues and endometrial fluid
(19–21). The aim of our study was to
determine whether eutopic endometrial cells recovered from
menstrual blood express undifferentiated stem cell markers. In
addition, we aimed to identify novel potential biomarkers for
endometriosis through a comparative proteomic analysis of
endometrial cells from patients with and without endometriosis.
Materials and methods
Subjects
This study was approved by the human Ethics
Committee of the Pusan National University Hospital (Institutional
Review Board: 2008072) and all of the females recruited for the
study signed a consent form prior to participation. Two independent
study populations were recruited: the first for identification of
biomarkers (n=12) and the second for biomarker validation (n=6).
The patients included individuals with a laparoscopic diagnosis of
endometriosis aged between 25 and 40 years. Half the females had
advanced endometriosis and the other half did not have
endometriosis, adenomyosis or leiomyoma.
Eutopic endometrial cells derived from
menstrual blood
The eutopic endometrial cells were isolated from ~1
ml menstrual blood collected with a suction catheter on days 2–4 of
the menstrual cycle. Menstrual blood samples were cultured in
Dulbecco's modified Eagle's medium (DMEM)/F-12 with 5% fetal bovine
serum (FBS; HyClone Laboratories, Inc., Logan, UT, USA) and 0.2%
collagenase (Invitrogen Life Technologies, Carlsbad, CA, USA) at
37°C with 5% CO2 for 1 h. To isolate eutopic endometrial
cells, the media were centrifuged at 300 × g for 10 min. The cells
were cultured in DMEM supplemented with 10% FBS, 1%
penicillin/streptomycin (v/v) and 1% amphotericin B (v/v)
(Sigma-Aldrich, St. Louis, MO, USA) for 2 days at 37°C under 5%
CO2. The cells were then washed three times with
phosphate-buffered saline (PBS) and resuspended in DMEM containing
10% FBS. After 2 weeks, the eutopic endometrial cells were
collected following detachment with trypsin (Lonza Verviers,
Belgium) and then washed twice with PBS.
RNA extraction and quantitative real-time
polymerase chain reaction (PCR)
Total RNA was extracted from the eutopic endometrial
cells using TRIzol reagent (Invitrogen Life Technologies) according
to the manufacturer's instructions. An aliquot of total RNA (3 μg)
was used as a template for single-stranded cDNA synthesis by
incubating the RNA with reverse transcriptase (Invitrogen Life
Technologies) at 37°C for 1 h. PCR amplification of the selected
genes was performed using the appropriate forward and reverse
primers (Table I). Real-time PCR
was performed in 20 μl reactions in 96-well plates using a MyiQ™
Single-Color Real-Time PCR Detection System (Bio-Rad, Hercules, CA,
USA). The reaction conditions were optimized using an iQ™
SYBR®-Green Supermix Kit (Bio-Rad). Gene expression was
analyzed using the 2−ΔΔCT method (22).
 | Table IPrimers used for quantitative
real-time polymerase chain reaction (PCR). |
Table I
Primers used for quantitative
real-time polymerase chain reaction (PCR).
| Name | Accession no. | Primers (5′-3′) |
|---|
| α-tubulin | NM_006082.2 | F:
GTACCGTGGTGACGTGGTTC
R: CTTGGCATACATCAGGTCAA |
| Oct-4 | NM_203289.3 | F:
GGAAGGTATTCAGCCAAACG
R: TAGCCTGGGGTACCAAAATG |
| CXCR4 | NM_003467.2 | F:
AATCTTCCTGCCCACCATCT
R: GACGCCAACATAGACCACCT |
| SOX2 | NM_003106.2 | F:
GCACATGAACGGCTGGAGCAACG
R: TGCTGCGAGTAGGACATGCTGTAGG |
| MET | NM_000245.2 | F:
GGGTCGCTTCATGCAGGTTGTGGT
R: ATGGTCAGCCTTGTCCCTCCTTCA |
Protein extraction
Total proteins were extracted from endometrial cells
with 8 M urea, 4% (w/v)
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS),
1% (w/v) ethylenediaminetetraacetic acid (EDTA), 10 mM Tris/HCl (pH
8.3) and protease inhibitor cocktail (GE Healthcare, Piscataway,
NJ, USA). Briefly, cells were incubated with the above cell lysis
buffer for 30 min at room temperature and stirred every 5 min. The
protein samples were then centrifuged at 18,000 × g for 15 min at
20°C and the supernatant was collected. Protein concentrations of
all the samples were determined using a PlusOne 2-D Quant Kit (GE
Healthcare). Aliquots of the protein samples were stored at −80°C
until analysis.
Two-dimensional electrophoresis (2-DE)
and protein identification by electrospray
ionization-quadrupole-time of flight/mass spectrometry
(ESI-Q-TOF/MS)
Two separate 2-DE analyses were performed in order
to analyse the difference of these two independent study
populations, separately. Electrophoretic separation of total cell
proteins was performed using a previously described method
(23). Briefly, the protein
samples of eutopic endometrial cells were diluted in isoelectric
focusing (IEF) buffer containing 9 M urea, 2 M thiourea, 4% CHAPS,
500 mM EDTA, 0.002% (w/v) bromophenol blue, 75 mM dithiothreitol
(DTT) and 1% (v/v) pharmalyte (pH 3–10 NL). A total volume of 350
μl containing 100 μg pooled proteins was then loaded onto a pH 3–10
NL immobilized pH gradient strip (GE Healthcare; 18 cm). After 12 h
rehydration at 20°C, IEF was performed with an IEF electrophoresis
unit (GE Healthcare) under the following conditions: a linear ramp
from 500 to 1,000 V for 1 h and a constant voltage of 8,000 V for 6
h to deliver a total of 56,000 Vh. Following IEF, the strips were
equilibrated twice for 15 min each in equilibration buffer
containing 50 mM Tris-HCl (pH 8.8), 6 M urea, 2% sodium dodecyl
sulfate (SDS), 30% glycerol and 0.002% (w/v) bromophenol blue. For
the first equilibration, the buffer contained 1% DTT and for the
second equilibration the buffer contained 135 mM iodoacetamide. An
Ettan DALT 2-D gel system (GE Healthcare) was used for
electrophoresis in the second dimension. The equilibrated strips
were inserted into the top of the 12% SDS-polyacrylamide gel
electrophoresis (PAGE) gel. The gels were then stained using a
PlusOne Silver Staining Kit (GE Healthcare). Spot detection, pair
matching and normalization were performed using ProteomWeaver
software (Definiens, Munich, Germany). Ratios of spot intensities
for the control and endometriosis patients were calculated. Spots
with intensities showing a change of >2-fold were selected for
ESI-Q-TOF/MS analysis. The details of ESI-Q-TOF/MS analysis have
been described previously (24).
Western blot analysis
Eutopic endometrial proteins (25 μg) were loaded
onto a 10% (w/v) polyacrylamide gel and separated with
electrophoresis. Proteins in the gels were transferred onto
nitrocellulose membranes using a Trans-Blot® SD Semi-Dry
Transfer Cell (Bio-Rad). The membranes were blocked overnight at
4°C with 5% non-fat dried milk (BD Biosciences, Franklin Lakes, NJ,
USA) in Tris-buffered saline with Tween-20 [TBST; 20 mM Tris-HCl
(pH 7.6), 137 mM NaCl and 0.01% Tween-20]. The membranes were then
incubated with an anti-collapsin response mediator protein 2
(CRMP2) antibody (ab62661, 1:10000 dilution; Abcam, Cambridge, MA,
USA) for 3 h at 4°C. The membranes were washed three times with
TBST and incubated with polyclonal goat anti rabbit IgG-horseradish
peroxidase (HRP; ab6721, 1:3000 dilution; Abcam). Immunoreactive
proteins on the membrane were visualized by enhanced
chemiluminescence using an ECL-Plus Detection Kit (GE Healthcare)
and exposure to X-ray film (Fujifilm Corporation, Tokyo, Japan) for
1–5 min. The film was then scanned and the bands were quantified
using ImageJ 1.43 software (http://rsb.info.nih.gov/ij/download.html). Protein
expression levels were normalized to those of β-actin on the same
membrane.
Statistical analysis
Values are expressed as mean ± standard error of the
mean (SEM). Student's t-test was used to compare results from the
two groups of study subjects. P<0.05 was considered to indicate
a statistically significant difference.
Results
Real-time PCR verification of
undifferentiated stem cell markers overexpressed in primary eutopic
endometrial cells collected from menstrual blood
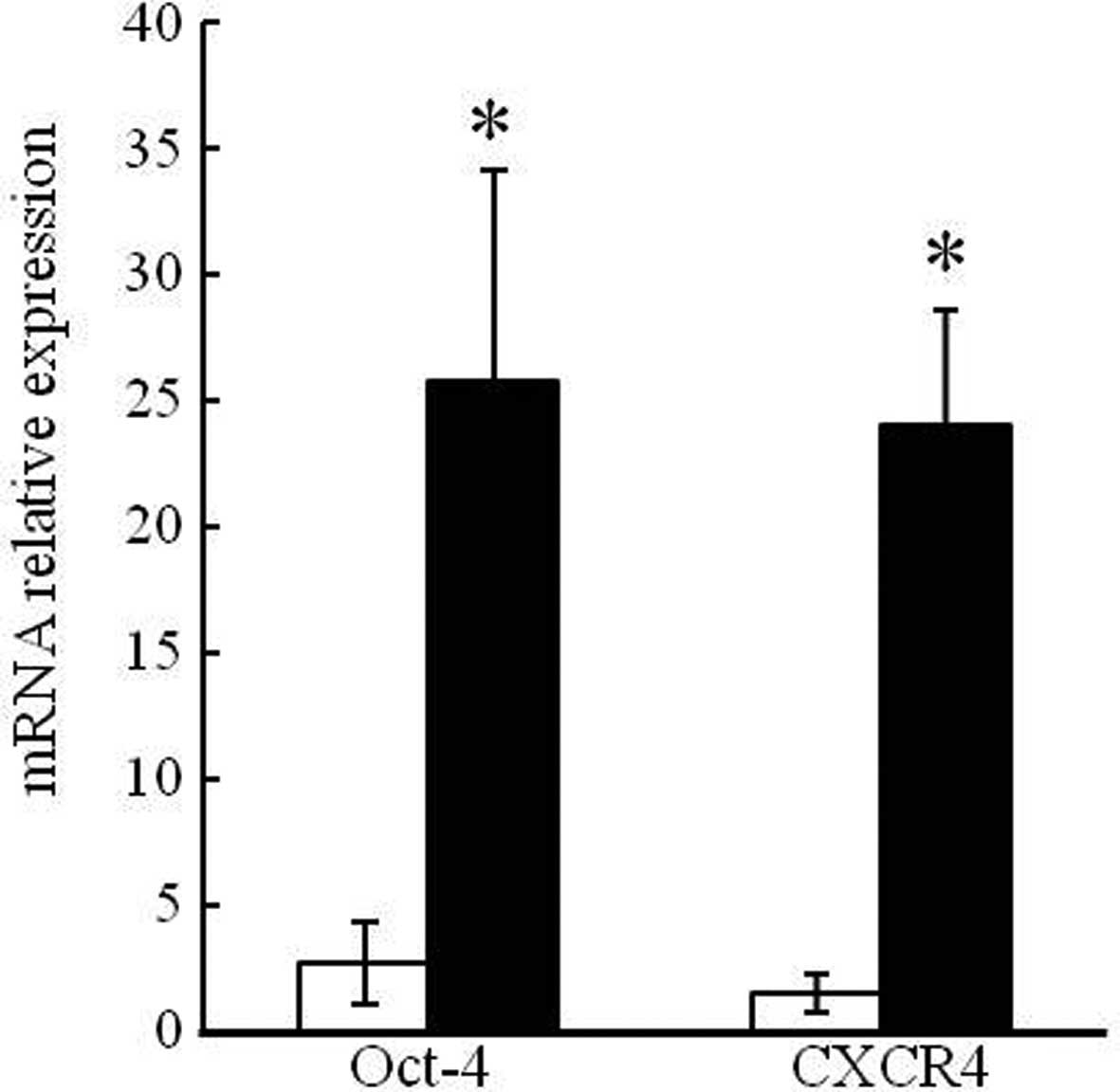
The mRNA expression levels of undifferentiated stem
cell markers (Oct-4 and CXCR4) in eutopic endometrial cells from
endometriosis patients and healthy controls were compared using
real-time PCR. Compared to the controls, mRNA expression of Oct-4
and CXCR4 was 21.36- and 23.5-fold higher, respectively, in the
endometriosis patients (Fig. 1).
These results confirm that the mRNA expression of undifferentiated
stem cell markers was higher in the endometriosis patients.
2-DE protein profiles of eutopic
endometrial cells collected from the menstrual blood of females
with or without endometriosis
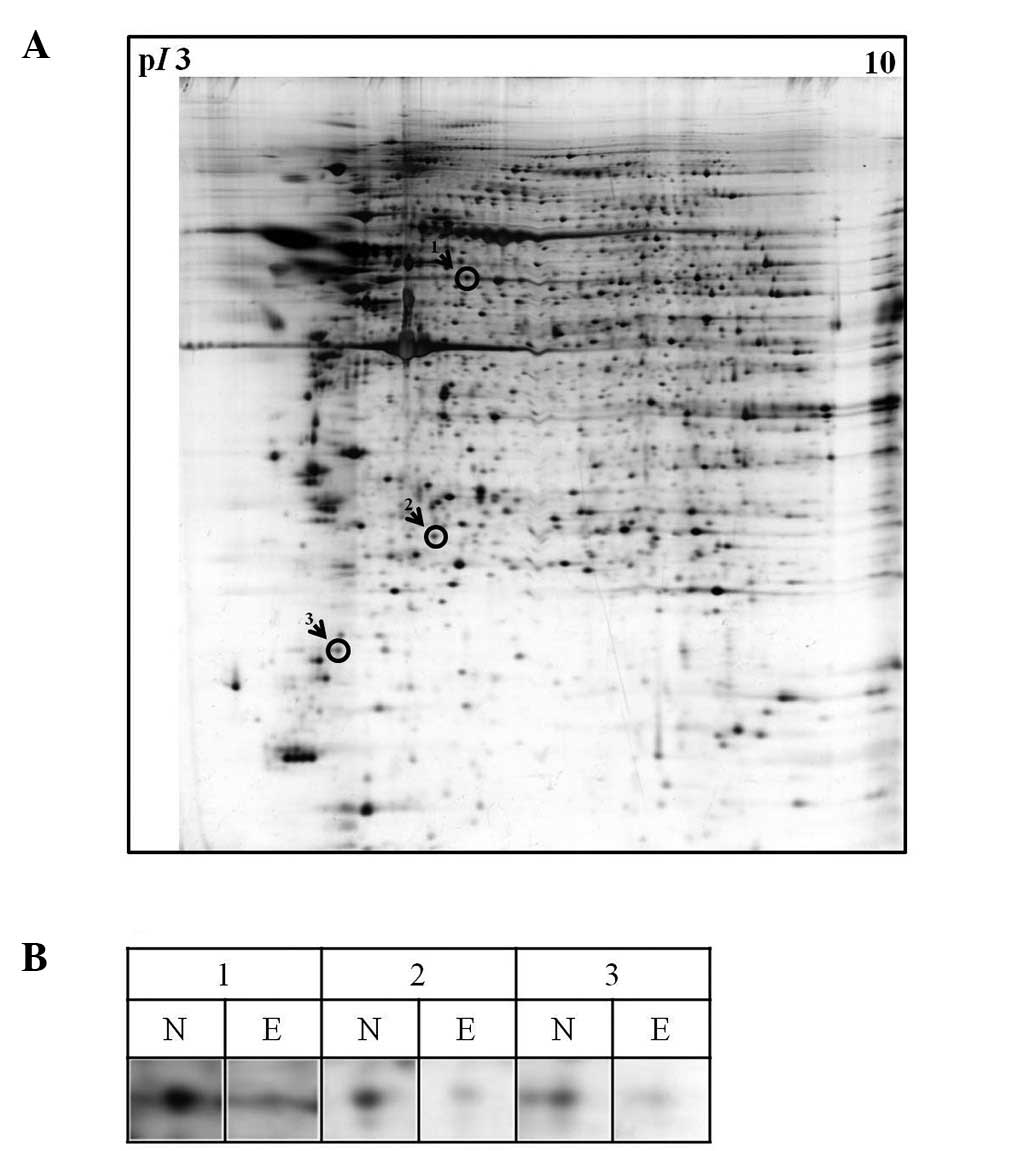
2-DE analysis was performed to characterize
differences of protein expression in eutopic endometrial cells from
endometriosis patients and normal controls. Proteins corresponding
to three selected spots on the 2-DE gel were identified using
ESI-Q-TOF/MS. These proteins had a lower (≥3-fold) expression in
endometriosis patients compared to that of the controls. Using this
technique, we identified three differentially expressed proteins as
CRMP2, UCH-L1 and MYL9 (Fig. 2;
Table II).
 | Table IIIdentification of proteins
corresponding to spots on 2-DE gels of eutopic endometrial-derived
cells showing differences in protein expression (control vs.
endometriosis) during days 2–4 of the menstrual cycle in females
with and without endometriosis. |
Table II
Identification of proteins
corresponding to spots on 2-DE gels of eutopic endometrial-derived
cells showing differences in protein expression (control vs.
endometriosis) during days 2–4 of the menstrual cycle in females
with and without endometriosis.
| Spot no. | Protein name | MW
(kDa)/pI | MOWSE score |
UniprotKB/Swiss-Prot entry | Expression |
|---|
| 1 | CRMP2 | 62.7/5.95 | 590 | Q16555 | 4.09 |
| 2 | UCH-L1 | 25.1/5.33 | 132 | P09936 | 3.10 |
| 3 | MYL9 | 19.9/4.80 | 88 | P24844 | 4.68 |
Real-time PCR analysis of
undifferentiated stem cell marker overexpression in the second set
of eutopic endometrial cells
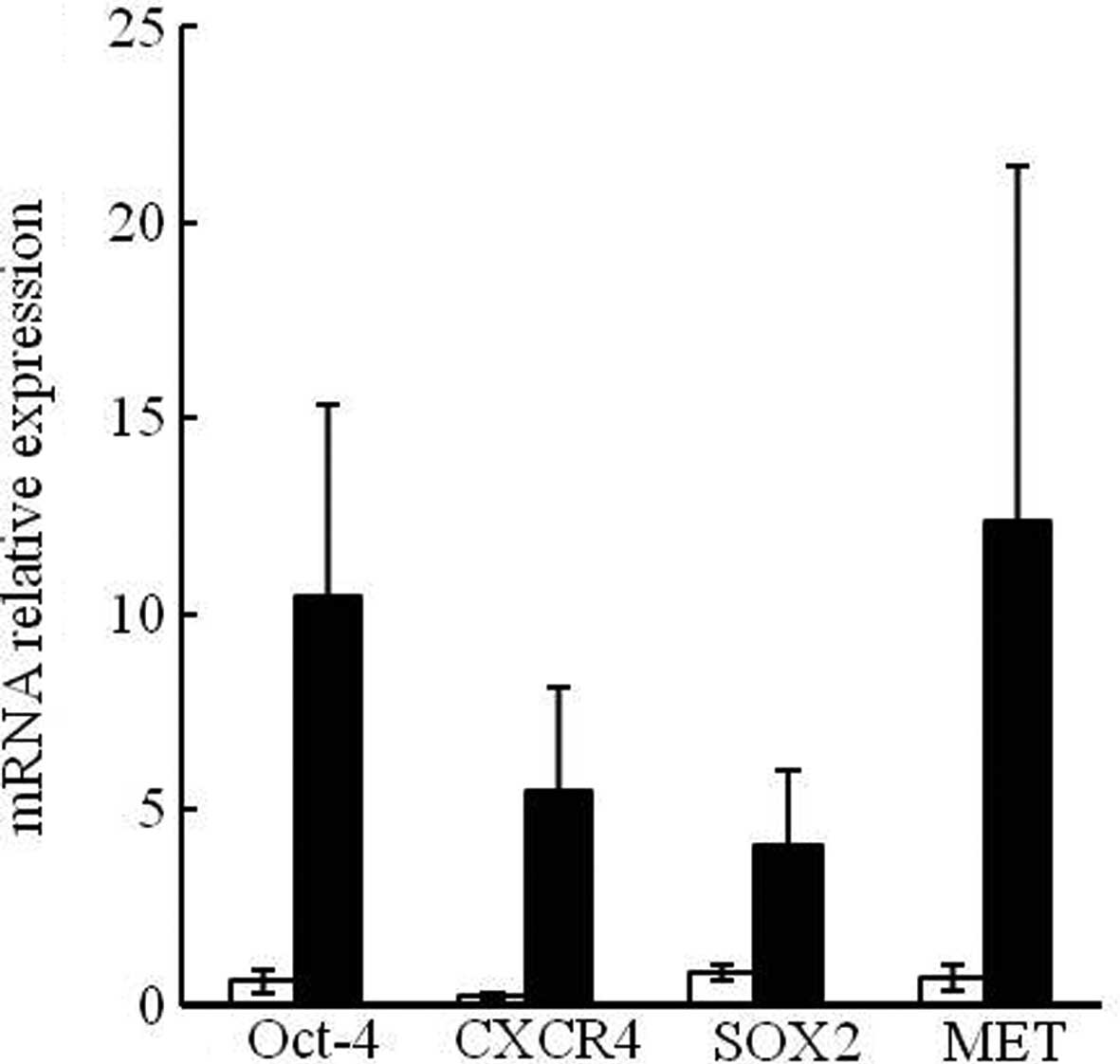
The mRNA expression of four undifferentiated stem
cell markers, including Oct-4, CXCR4, SOX2 and
mesenchymal-epithelial transition factor (MET) in the second set of
collected samples were analyzed using real-time PCR. Partially
consistent with the results from the first set of samples, mRNA
expression levels of Oct-4, CXCR4, SOX2 and MET were 16.9-, 22.36-,
4.88- and 16.95-fold higher, respectively, in the endometriosis
patients compared to those in the controls (Fig. 3). However, these differences were
not statistically significant. Increased mRNA expression of
undifferentiated stem cell markers in the first and second sets of
samples nevertheless suggests that there is a relevant connection
between undifferentiated stem cells and endometriosis.
Confirmation of CRMP2 as a candidate
endometriosis marker by 2-DE analysis of the second set of
samples
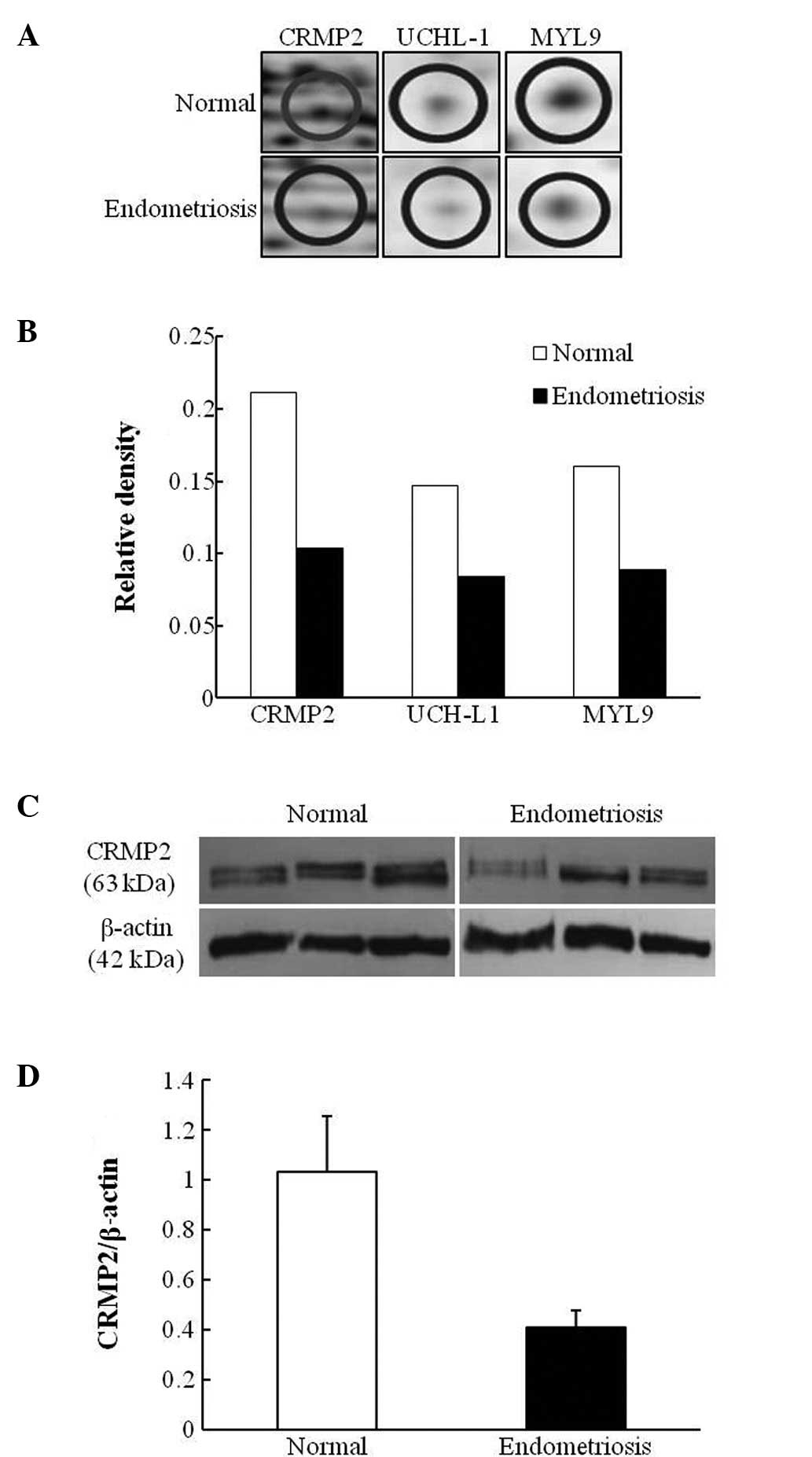
A second 2-DE analysis was performed to confirm the
candidate biomarker proteins identified by the first 2-DE analysis.
Among the three candidate proteins (CRMP2, UCH-L1 and MYL9)
identified by 2-DE analysis of the first set of samples, only CRMP2
expression demonstrated a change of >2-fold (Fig. 4B). Therefore, CRMP2 was selected
for further western blot analysis of eutopic endometrial cells in
menstrual blood collected from three controls and three patients
with advanced endometriosis. Western blot results demonstrated that
CRMP2 protein levels were 2.5-fold higher in the controls compared
to that of the endometriosis patients (Fig. 4C and D).
Discussion
Stem cells are undifferentiated cells capable of
self-renewal, proliferation and production of a large number of
differentiated daughter cells (1–4). The
menstrual cycle is characterized by the growth of endometrial
tissues and blood vessels. After menstruation, the proliferative
stage of endometrial tissues is initiated by increasing levels of
circulating estrogen (25). The
isolated small pluripotent stem cells (2–3 min diameter)
co-expressing embryonic stem cell markers, including Oct-4 and
SOX2, are from human umbilical cord blood (26). The small round cells obtained by
ovarian surface epithelium isolation express early embryonic
developmental markers, including stage-specific embryonic antigen-4
(SSEA-4) surface antigen, as well as Oct-4, NANOG, SOX2 and c-kit
(27). By contrast, pluripotent
stem cells expressing Oct-4, SSEA-4, CXCR4 and MET are present in
human and mice bone marrow (28).
In the present study, results demonstrated that the
mRNA expression of stem cell marker genes (Oct-4, CXCR4, SOX2 and
c-MET) was higher in eutopic endometrial cells from the menstrual
blood of patients with endometriosis compared to those from the
controls (Figs. 1 and 3). The proliferation of stem cells during
endometriosis may play a role in endometriotic implantation.
Results indicated that the menstrual blood-derived endometrial
cells we isolated express stem cell markers and possess
characteristics of stem cells. Proteomic studies have helped
elucidate the function of specific proteins at the tissue, organ
and cellular levels. Using a 2-DE method to compare healthy and
pathological states is likely to not only increase our general
understanding of a disease at the molecular level, but also provide
a driving force to accelerate the identification of biomarkers for
the prediction, diagnosis and treatment of diseases. Moreover,
microarray and 2-DE analyses have demonstrated that the gene and
protein expression profiles of ectopic endometrial implants differ
from those of the eutopic endometrium (29,30).
Three proteins were selected as candidate biomarkers
of endometriosis based on the first round of 2-DE analysis. In
addition, the 2-DE analysis of the second round demonstrated that
the CRMP2 protein expression was 2.21-fold higher in healthy
individuals compared to endometriosis patients. This significantly
higher expression of CRMP2 was confirmed by western blotting
(Fig. 4). CRMP2 is a member of the
CRMP family with five isoforms, and develops in the neuronal
system. This protein does not have enzymatic activity; however, it
is involved in neuronal differentiation, axonal guidance and
neuronal polarity (31).
Overexpression of CRMP2 induces the growth of axons, neurites and
dendrites (32,33). At the same time, CRMP2 promotes the
elongation and branching of axons by stimulating microtubule
assembly after forming a complex with tubulin heterodimers
(34). In addition, CRMP2 inhibits
axonal growth by regulating phosphorylation via glycogen synthase
kinase (GSK)-3β signaling (31).
It was shown that CRMP2 phosphorylation is directly and indirectly
regulated by CRMP2 directly binding to the C-terminal of
neurofibromin in PC12 cells (34).
Neurofibromin, a protein produced by the tumor suppressor gene
NF1, also acts as a negative regulator of Ras via the
Ras-GTPase-activating protein (Ras-GAP) pathway. Neurofibromin
directly regulates CRMP2 by forming a complex to control its
phosphorylation and indirectly regulates CRMP2 by inhibiting CRMP2
phosphorylation via its function in the Ras-GAP pathway (34). Another study demonstrated that the
protein expression is affected by the phosphorylation of CRMP2
(35). Therefore, higher levels of
phosphorylated CRMP2 result in higher total CRMP2 protein
expression since the phosphorylation of CRMP2 induces protein
synthesis and/or inhibits protein degradation. An NF1 gene
mutation in mast cells was shown to stimulate angiogenesis
(35,36). These mast cells may be involved in
the progression of endometriosis. According to Kempuraj et
al, the number of mast cells is significantly increased in
endometriosis patients (37). Stem
cell factor (SCF) is a mast cell growth factor. SCF levels are
increased in the peritoneal fluid of endometriosis patients
(38). This finding suggests that
CRMP2 may increase the proliferation of mast cells in the
peritoneum (37). SCF activates
mast cells and SCF receptors have been identified in the tissues of
endometriosis patients (38).
Taken together, these findings indicate that CRMP2
is not directly involved in the regulation of endometriosis.
However, this factor may be involved in the regulation of
endometriosis indirectly due to its signals and mediators,
including NF1. In conclusion, CRMP2 may be a protein associated
with endometriosis and may play an important role in the
pathogenesis of this condition.
Acknowledgements
This study was supported by Medical Research
Institute Grant (2010–12), Pusan National University Hospital.
References
|
1
|
Sasson IE and Taylor HS: Stem cells and
the pathogenesis of endometriosis. Ann NY Acad Sci. 1127:106–115.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fox H: The pathology of endometriosis. Ir
J Med Sci. 152(Suppl 2): 9–13. 1983. View Article : Google Scholar
|
|
3
|
Gargett CE: Uterine stem cells: what is
the evidence? Hum Reprod Update. 13:87–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Figueira PG, Abrão MS, Krikun G and Taylor
HS: Stem cells in endometrium and their role in the pathogenesis of
endometriosis. Ann NY Acad Sci. 1221:10–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Hooghe TM, Bambra CS, Raeymaekers BM, De
Jonge I, Lauweryns JM and Koninckx PR: Intrapelvic injection of
menstrual endometrium causes endometriosis in baboons (Papio
cynocephalus and Papio anubis). Am J Obstet Gynecol.
173:125–134. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Du H and Taylor HS: Contribution of bone
marrow-derived stem cells to endometrium and endometriosis. Stem
Cells. 25:2082–2086. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Du H and Taylor HS: Stem cells and female
reproduction. Reprod Sci. 16:126–139. 2009. View Article : Google Scholar
|
|
8
|
Murk W, Atabekoglu CS, Cakmak H, Heper A,
Ensari A, Kayisli UA and Arici A: Extracellularly signal-regulated
kinase activity in the human endometrium: possible roles in the
pathogenesis of endometriosis. J Clin Endocrinol Metab.
93:3532–3540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim CM, Oh YJ, Cho SH, Chung DJ, Hwang JY,
Park KH, Cho DJ, Choi YM and Lee BS: Increased telomerase activity
and human telomerase reverse transcriptase mRNA expression in the
endometrium of patients with endometriosis. Hum Reprod. 22:843–849.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Götte M, Wolf M, Staebler A, Buchweitz O,
Kelsch R, Schüring AN and Kiesel L: Increased expression of the
adult stem cell marker Musashi-1 in endometriosis and endometrial
carcinoma. J Pathol. 215:317–329. 2008.PubMed/NCBI
|
|
11
|
Ruiz A, Salvo VA, Ruiz LA, Báez P, García
M and Flores I: Basal and steroid hormone-regulated expression of
CXCR4 in human endometrium and endometriosis. Reprod Sci.
17:894–903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim JB, Greber B, Araúzo-Bravo MJ, Meyer
J, Park KI, Zaehres H, Zaehres H and Schöler HR: Direct
reprogramming of human neural stem cells by OCT4. Nature.
461:649–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huangfu D, Osafune K, Maehr R, Guo W,
Eijkelenboom A, Chen S, Muhlestein W and Melton DA: Induction of
pluripotent stem cells from primary human fibroblasts with only
Oct4 and Sox2. Nat Biotechnol. 26:1269–1275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Götte M, Wolf M, Staebler A, Buchweitz O,
Kiesel L and Schüring AN: Aberrant expression of the pluripotency
marker SOX-2 in endometriosis. Fertil Steril. 95:338–341.
2011.PubMed/NCBI
|
|
15
|
Pacchiarotti A, Caserta D, Sbracia M and
Moscarini M: Expression of oct-4 and c-kit antigens in
endometriosis. Fertil Steril. 95:1171–1173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanash S: Disease proteomics. Nature.
422:226–232. 2003. View Article : Google Scholar
|
|
17
|
Ahram M and Petricoin EF: Proteomics
discovery of disease biomarkers. Biomark Insights. 3:325–333.
2008.
|
|
18
|
Poliness AE, Healey MG, Brennecke SP and
Moses EK: Proteomic approaches in endometriosis research.
Proteomics. 4:1897–1902. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gupta S, Agarwal A, Sekhon L, Krajcir N,
Cocuzza M and Falcone T: Serum and peritoneal abnormalities in
endometriosis: potential use as diagnostic markers. Minerva
Ginecol. 58:527–551. 2006.PubMed/NCBI
|
|
20
|
Ferrero S, Gillott DJ, Remorgida V,
Anserini P, Leung KY, Ragni N and Grudzinskas JG: Proteomic
analysis of peritoneal fluid in women with endometriosis. J
Proteome Res. 6:3402–3411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H, Niu Y, Feng J, Guo H, Ye X and
Cui H: Use of proteomic analysis of endometriosis to identify
different protein expression in patients with endometriosis versus
normal controls. Fertil Steril. 86:274–282. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2[-Delta Delta C(T)] Method. Methods. 25:402–408. 2001.
|
|
23
|
Desrivières S, Prinz T, Castro-Palomino
Laria N, Meyer M, Boehm G, Bauer U, Schäfer J, Neumann T, Shemanko
C and Groner B: Comparative proteomic analysis of proliferating and
functionally differentiated mammary epithelial cells. Mol Cell
Proteomics. 2:1039–1054. 2003.PubMed/NCBI
|
|
24
|
Jin YC, Lee HG, Xu CX, et al: Proteomic
analysis of endogenous conjugated linoleic acid biosynthesis in
lactating rats and mouse mammary gland epithelia cells (HC11).
Biochim Biophys Acta. 1804:745–751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Patel AN, Park E, Kuzman M, Benetti F,
Silva FJ and Allickson JG: Multipotent menstrual blood stromal stem
cells: isolation, characterization, and differentiation. Cell
Transplant. 17:303–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McGuckin C, Jurga M, Ali H, Strbad M and
Forraz N: Culture of embryonic-like stem cells from human umbilical
cord blood and onward differentiation to neural cells in vitro. Nat
Protoc. 3:1046–1055. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Virant-Klun I, Rozman P, Cvjeticanin B,
Vrtacnik-Bokal E, Novakovic S, Rülicke T, Dovc P and Meden-Vrtovec
H: Parthenogenetic embryo-like structures in the human ovarian
surface epithelium cell culture in postmenopausal women with no
naturally present follicles and oocytes. Stem cells Dev.
18:137–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kucia M, Reca R, Campbell FR, Zuba-Surma
E, Majka M, Ratajczak J and Ratajczak MZ: A population of very
small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells
identified in adult bone marrow. Leukemia. 20:857–869. 2006.
|
|
29
|
Filigheddu N, Gregnanin I, Porporato PE,
Surico D, Perego B, Galli L, Patrignani C, Graziani A and Surico N:
Differential expression of microRNAs between eutopic and ectopic
endometrium in ovarian endometriosis. J Biomed Biotechnol.
369549–369578. 2010.PubMed/NCBI
|
|
30
|
Chehna-Patel N, Sachdeva G, Gajbhiye R,
Warty N and Khole V: ‘Spot’-ting differences between the ectopic
and eutopic endometrium of endometriosis patients. Fertil Steril.
94:1964–1971. 2010.
|
|
31
|
Yoshimura T, Kawano Y, Arimura Y, Kawabata
S, Kikuchi A and Kaibuchi K: GSK-3beta regulates phosphorylation of
CRMP-2 and neuronal polarity. Cell. 120:137–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arimura N, Menager C, Fukata Y and
Kaibuchi K: Role of CRMP-2 in neuronal polarity. J Neurobiol.
58:34–47. 2004. View Article : Google Scholar
|
|
33
|
Fukata Y, Itoh TJ, Kimura T, Ménager C,
Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani
H and Kaibuchi K: CRMP-2 binds to tubulin heterodimers to promote
microtubule assembly. Nat Cell Biol. 4:583–591. 2002.PubMed/NCBI
|
|
34
|
Patrakitkomjorn S, Kobayashi D, Morikawa
T, Wilson MM, Tsubota N, Irie A, Ozawa T, Aoki M, Arimura N,
Kaibuchi K, Saya H and Araki N: Neurofibromatosis type 1 (NF1)
tumor suppressor, neurofibromin, regulates the neuronal
differentiation of PC12 cells via its associating protein, CRMP-2.
J Biol Chem. 283:9399–9413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang FC, Chen S, Clegg T, et al: Nf1+/−
mast cells induce neurofibroma like phenotypes through secreted
TGF-beta signaling. Hum Mol Genet. 15:2421–2437. 2006.
|
|
36
|
Le LQ and Parada LF: Tumor
microenvironment and neurofibromatosis type I: connecting the GAPs.
Oncogene. 26:4609–4016. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kempuraj D, Papadopoulou N, Stanford EJ,
Christodoulou S, Madhappan B, Sant GR, Solage K, Adams T and
Theoharides TC: Increased numbers of activated mast cells in
endometriosis lesions positive for corticotropin-releasing hormone
and urocortin. Am J Reprod Immunol. 52:267–275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Osuga Y, Koga K, Tsutsumi O, Igarashi T,
Okagaki R, Takai Y, Matsumi H, Hiroi H, Fujiwara T, Momoeda M, Yano
T and Taketani Y: Stem cell factor (SCF) concentrations in
peritoneal fluid of women with or without endometriosis. Am J
Reprod Immunol. 44:231–235. 2000. View Article : Google Scholar : PubMed/NCBI
|