Introduction
Prostate cancer is the most common type of cancer
and the third leading cause of cancer-related mortalities in males
worldwide, according to the World Cancer Report 2003 (1). Conventional management of prostate
cancer includes surgery, radiotherapy and androgen deprivation
(2). Adjuvant hormone therapy
following radiotherapy or surgery is a treatment option frequently
offered to males with localized or locally advanced prostate
cancer. In males with metastatic androgen-dependent prostate
cancer, androgen blockade is the most frequently used treatment.
The above-mentioned treatments may be effective and have the
potential to provide a high life expectancy by inducing tumor
suppression (3). However, patients
who initially respond to these therapies often develop a refractory
aggressive androgen-independent cancer (4). Therefore, new treatments for prostate
cancer patients are required, particularly for patients with
androgen-independent cancer.
Based on clinical and pre-clinical studies, the
concept of molecular targeting is becoming increasingly promising
as a tool to treat prostate cancer. Specificity protein 1 (Sp1) is
a sequence-specific transcription factor that binds to the GC box
and activates a host of viral and cellular genes. The
overexpression or higher binding activity of Sp1 has been found in
various types of human cancer, including pancreatic, breast,
gastric, thyroid and prostate cancer (5). Several studies have also reported
that the overexpression of Sp1 increases its ability to upregulate
vascular endothelial growth factor (VEGF) and survivin (5,6). Our
previous studies have demonstrated that Sp1 protein is
significantly overexpressed in prostate cancer cells, and that the
inhibition of Sp1 protein potently induces apoptosis through the
downregulation of survivin and Mcl-1 proteins (7,8).
HDAC inhibitors are a class of agents that function
via blocking histone deacetylation, thereby modifying chromatin
structure and gene transcription (9). According to various studies, HDAC
inhibitors are potent anticancer agents that induce cell growth
arrest, differentiation and apoptosis in human bladder, breast,
prostate, lung, ovary and colon cancer and acute myelogenous
leukemia (10). Several clinical
trials, particularly for cancer therapies, are currently being
carried out to examine the therapeutic benefits of HDAC inhibitors.
It has recently been demonstrated that HDAC inhibitors markedly
reduce prostate cancer growth and metastatic dissemination
(11). The HDAC inhibitor
Vorinostat (suberoylanilde hydroxamic acid, SAHA) has been approved
for the treatment of breast cancer. Additionally, HDAC inhibitors,
including TSA, FK228 and LBH589, are also used as anticancer agents
(12).
Therefore, the aim of the present study was to
investigate the cytotoxic activity and molecular target of A248, a
newly synthesized HDAC inhibitor, in androgen-independent human
prostate cancer cell lines.
Materials and methods
Reagents
Sp1 and β-actin antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Mcl-1 and survivin
antibodies were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). 4′-6-Diamidino-2-phenylindole (DAPI) and
propidium iodide (PI) were acquired from Sigma-Aldrich (St. Louis,
MO, USA). Mcl-1 and survivin antibodies used for
immunocytochemistry were purchased from Abcam (Cambridge, MA, USA).
IgG antibody was purchased from BD Pharmingen (Franklin Lakes, NJ,
USA). A248 was synthesized by the Gachon Institute of
Pharmaceutical Sciences, Gachon University (Incheon, Republic of
Korea).
Cell culture and chemical treatment
DU145 and PC3 cells were kindly provided by
Professor Hwan Mook Kim and were maintained in RPMI-1640 medium
containing 10% fetal bovine serum (FBS) and 100 U/ml penicillin and
streptomycin in a 5% CO2 atmosphere. Equal numbers of
cells were seeded and allowed to attach to the cell culture dishes
or plates. When 50–60% confluence was reached, DU145 and PC3 cells
were treated with DMSO or various concentrations of A248 (1, 2 and
4 μM) for 72 h. A248 was dissolved in 0.1% DMSO (vehicle
control).
MTS assay
The effect of A248 on cell viability was evaluated
using the CellTiter 96® AQueous One Solution Cell
Proliferation Assay kit (Promega, Madison, WI, USA). DU145 and PC3
cells were seeded in 96-well plates and incubated with various
concentrations of A248 for 24, 48, and 72 h. Following treatment,
3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethonyphenol)-2-(4-sulfophenyl)-2H-tetra
zolium (MTS) solution was added to each well (30 μl) and incubated
at 37°C. The absorbance was measured at 490 nm using an ELISA
reader (Bio-Tek Instruments, Inc., Madison, WI, USA). The data were
expressed as the percentage cell viability compared with that of
control cells.
FACS analysis for sub-G1 DNA
determination
The effect of A248 on the cell cycle was
investigated by FACS analysis. For PI staining, the cells were
seeded in 60-mm2 dishes. After attachment, DU145 and PC3
cells were treated with various concentrations of A248 for 72 h.
Following treatment, floating and attached cells were harvested,
washed with phosphate-buffered saline (PBS) and fixed in ice-cold
70% ethanol. The cells were then washed with PBS and suspended in
PI (0.02 mg/ml in PBS). All the measurements were performed in
triplicate and results were expressed as a fold induction with
respect to DMSO-treated cells.
DAPI staining
Apoptosis was determined morphologically using the
fluorescent nuclear dye, DAPI. Following treatment with A248 for 72
h, DU145 and PC3 cells were harvested by trypsinization and fixed
in 100% ethanol overnight at −20°C. The following day, the cells
were stained with DAPI (2 mg/ml in PBS), deposited onto slides and
observed using a fluorescence microscope to detect apoptotic
characteristics.
Western blot analysis
DU145 and PC3 cells were seeded in 60-mm2
dishes and treated with DMSO or A248. Whole-cell lysates were
extracted with lysis buffer and protein concentrations were
measured using a DC Protein Assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Samples containing equal amounts of protein
were separated by SDS-PAGE and then transferred to Immun-Blot™ PVDF
membranes (Bio-Rad Laboratories, Inc.). The membranes were blocked
with 5% skim milk in TBST at room temperature (RT) for 1 h and were
then incubated with the primary antibodies overnight at 4°C,
followed by incubation with the horseradish peroxidase
(HRP)-conjugated secondary antibodies for 90 min at RT.
Antibody-bound proteins were detected using ECL Western Blotting
Luminol reagent (Santa Cruz Biotechnology, Inc.) and then exposed
to film.
Immunocytochemical analysis
Cells were seeded on 6-well tissue culture plates.
The cells were incubated for 72 h with A248 and fixed and
permeabilized with a fixation and permeabilization solution (BD
Biosciences, Franklin Lakes, NJ, USA) for 30 min. The cells were
then blocked with 1% bovine serum albumin (BSA) and incubated with
the indicated antibodies at 4°C. Subsequently, the cells were
exposed to the FITC-conjugated secondary antibodies for 2 h at RT
and were covered with aluminum foil and were visualized using a
fluorescence microscope equipped with the appropriate filters for
DAPI and FITC dyes.
Statistical analysis
Statistical analyses of the experimental data were
performed using a two-sided Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
A248 inhibits cell viability of DU145 and
PC3 human prostate cancer cells
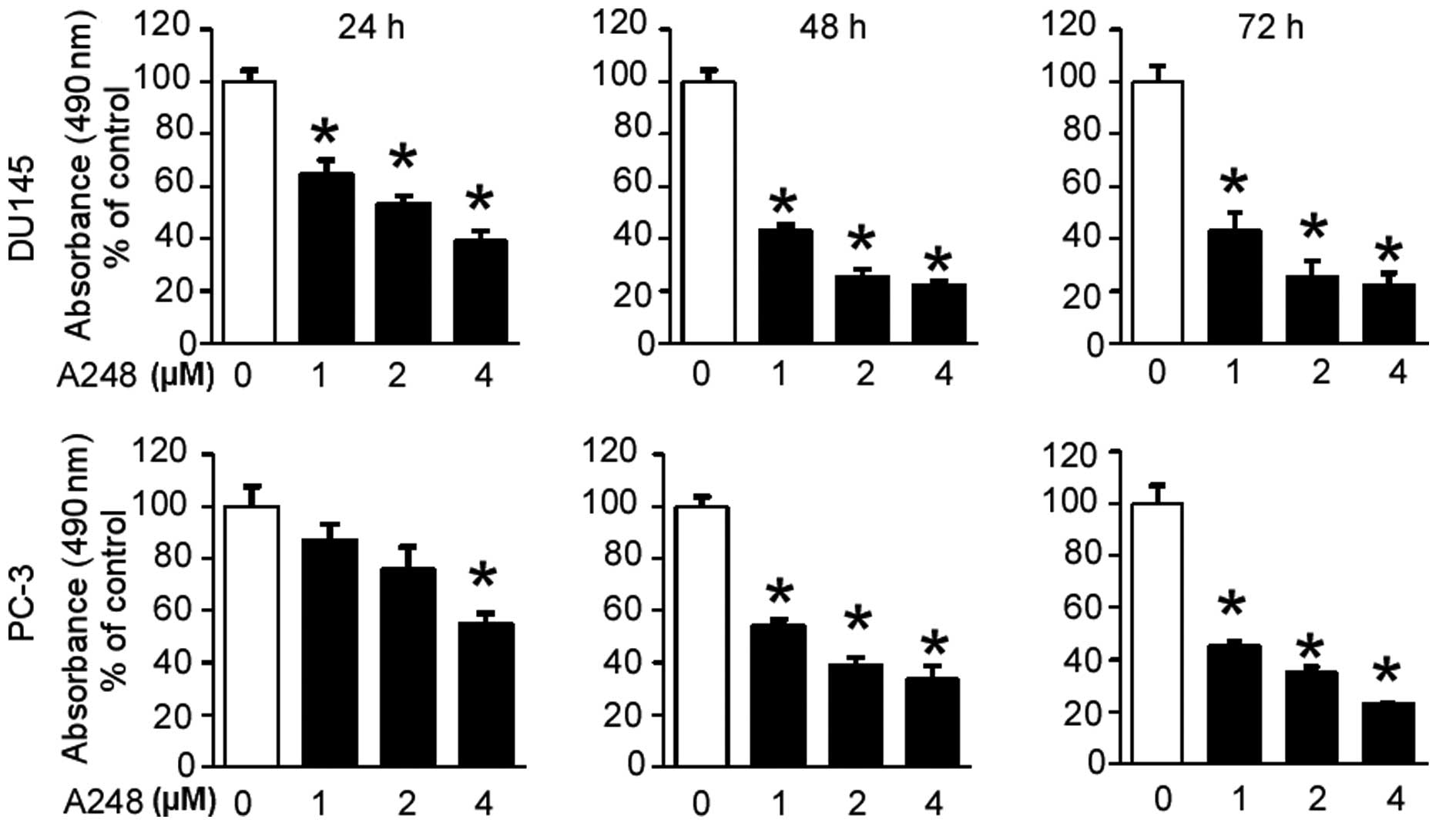
To investigate the effect of A248 on the cell
viability of DU145 and PC3 prostate cancer cells, an MTS assay was
initially performed. The cell viability of both cell lines was
shown to be significantly decreased by A248 in a concentration- and
time-dependent manner (Fig.
1).
A248 induces apoptosis in DU145 and PC3
cells
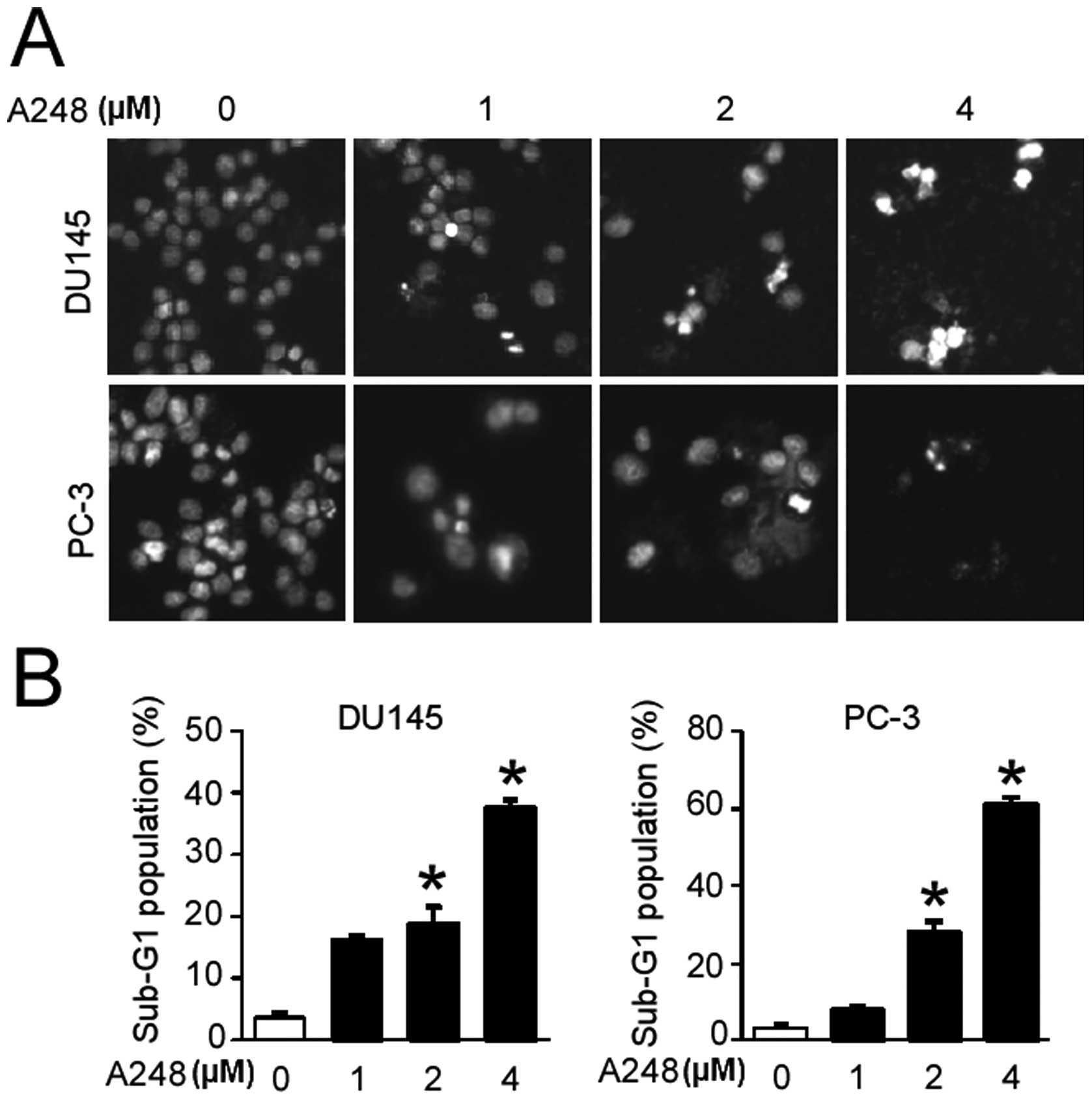
We then examined whether A248 inhibits cell
viability through inducing apoptosis. Cells treated with A248 for
72 h were stained by DAPI solution, and staining was observed using
a fluorescence microscope. The results showed that A248
significantly induced nuclei condensation and fragmentation in
DU145 and PC3 cells compared with the DMSO (vehicle
control)-treated cells (Fig. 2A).
We also determined the cell population in the sub-G1 phase of the
cell cycle using PI staining in both cell lines. A248 caused an
accumulation of cells in the sub-G1 phase of the cell cycle in
DU145 and PC3 cells (Fig. 2B).
These results suggest that apoptotic cell death may contribute to
the growth-inhibitory effect of A248 in DU145 and PC3 cells.
A248 inhibits Sp1 protein expression in
DU145 and PC3 cells
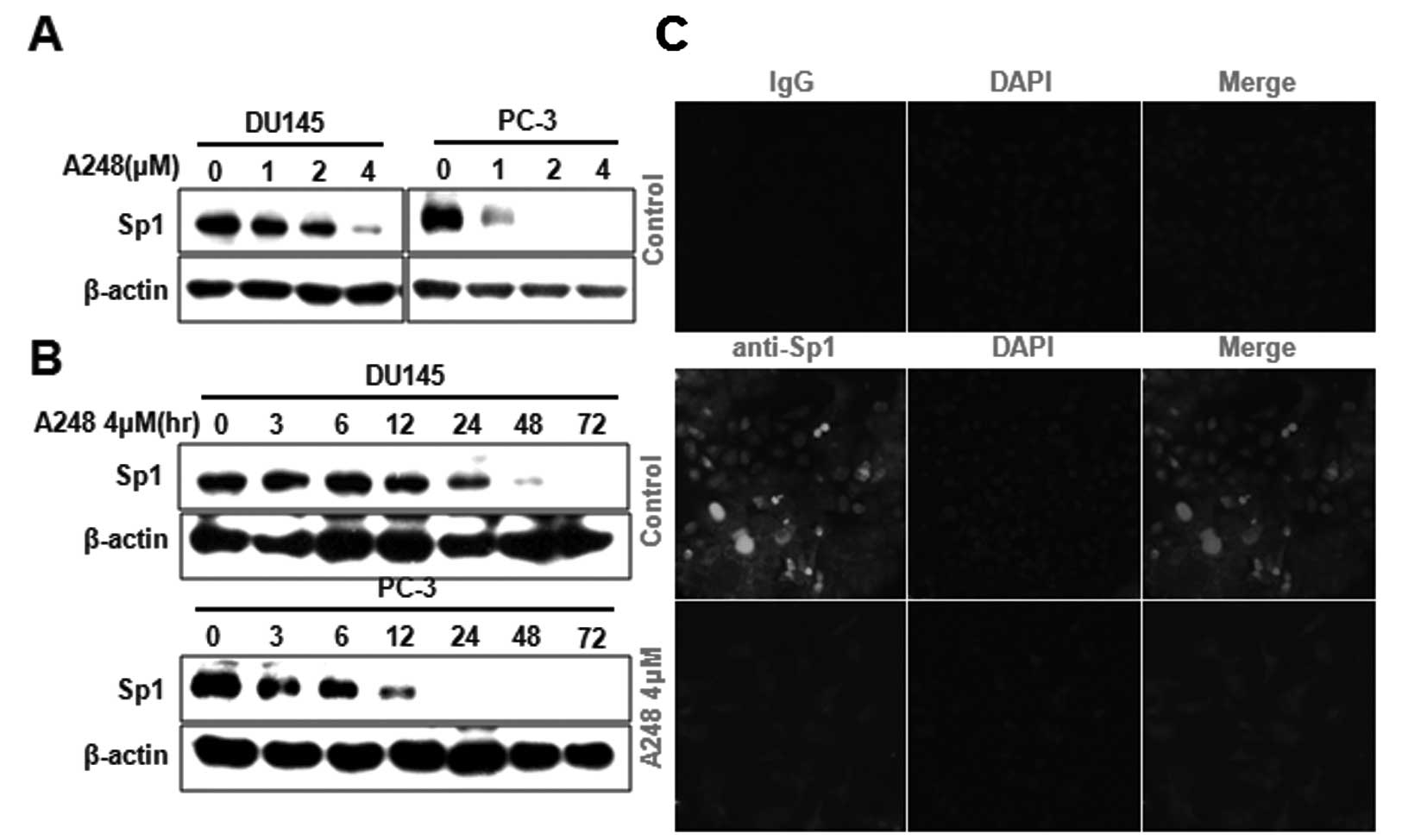
To determine the mechanism underlying A248-induced
apoptosis, we examined whether A248 affects the expression of Sp1
protein in DU145 and PC3 cells. A248 was shown to significantly
decrease Sp1 protein in a concentration- and time-dependent manner
as determined by western blot analysis (Fig. 3A and B). The expression level of
Sp1 protein in DU145 cells was also determined using
immunocytochemical analysis. The results showed that Sp1
immunostaining was observed in DU145 cells treated with DMSO
(vehicle control); however, A248 significantly decreased Sp1
staining (Fig. 3C).
A248 inhibits survivin and Mcl-1
expression through Sp1 downregulation in DU145 and PC3 cells
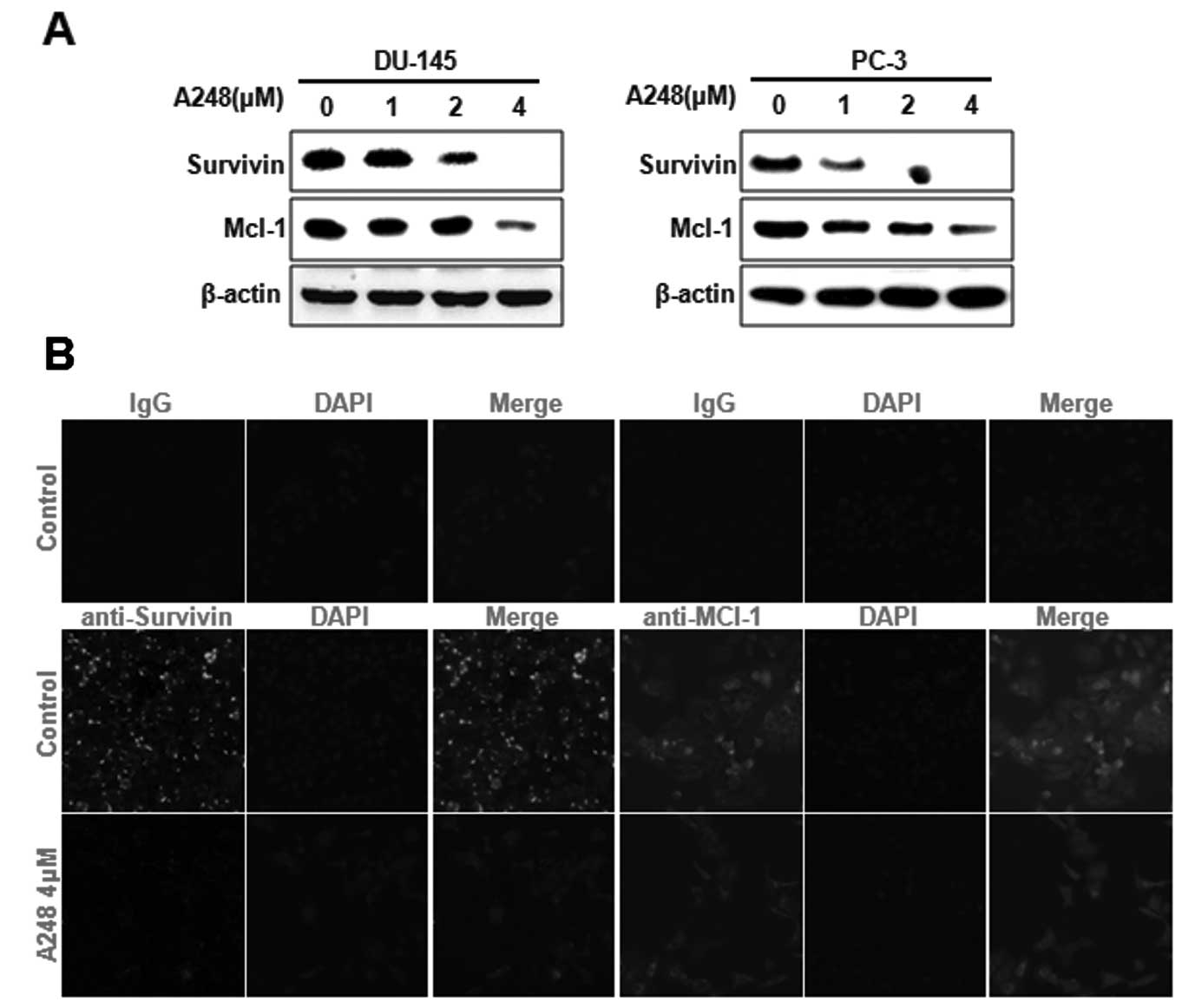
We have previously demonstrated that the
downregulation of Sp1 inhibits cell proliferation, induces
apoptosis and affects the expression of survivin and Mcl-1 proteins
(7). Thus, we next investigated
whether survivin and Mcl-1 are involved in A248-induced apoptosis
in DU145 and PC3 cells. The results showed that survivin and Mcl-1
protein expression was significantly decreased in DU145 and PC3
cells treated with A248 (Fig. 4A).
Immunocytochemical analysis clearly confirmed that survivin and
Mcl-1 were markedly decreased in DU145 cells treated with A248
(Fig. 4B).
Discussion
HDAC inhibitors have been focused on in numerous
studies on anticancer agents (9,12,13);
from a clinical perspective, there are a large number of completed
or ongoing clinical trials on these agents (14,15).
Despite the increasing interest in HDAC inhibitor therapies, the
fundamental mechanisms via which these agents exert their
anticancer effects remain unclear. An improved understanding of the
association between various target genes and types of cancer have
led several researchers to consider HDAC inhibitors as potent
agents that may be able to interfere with cancer cell proliferation
and/or survival. Recently, numerous studies have shown that HDAC
inhibitors significantly reduce prostate cancer cell growth
(11,16–18).
In the present study, we assessed the inhibitory effect of A248, a
novel synthetic HDAC inhibitor, in prostate cancer lines. A248 was
shown to strongly inhibit DU145 and PC3 prostate cancer cell
viability. It has also been reported that various HDAC inhibitors
arrest cell cycle progression and induce apoptosis in prostate
cancer cells (11,17,19–22).
Thus, we next investigated the effect of A248 on apoptosis in
prostate cancer cell lines using DAPI staining and via
determination of the cell population in the sub-G1 phase of the
cell cycle. We found that A248 caused an accumulation of cells in
the sub-G1 phase of the cell cycle; nuclei condensation and
fragmentation of DU145 and PC3 cells by A248 was observed,
suggesting that A248 is important in apoptotic signaling, including
cell-cycle arrest in the sub-G1 phase, nuclei condensation and
fragmentation. Therefore, A248 is suggested to be associated with
apoptotic cell death.
Notably, although Sp1 is an essential transcription
factor for a number of genes, it is overexpressed in numerous human
cancer cells (23–29). Several studies have demonstrated
that Sp1 is important in the pathogenesis of human cancer and that
it constitutes a promising therapeutic target (2,5,8,24,30–34).
Our previous studies also found that Sp1 is overexpressed in
prostate cancer cells compared with normal cells (7,8,35).
Yu et al(36) reported that
butyrate, a HDAC inhibitor, induces the downregulation of Sp1 in
colon cancer cells, suggesting that Sp1 protein may be modulated by
this HDAC inhibitor (36). Thus,
we examined the effects of A248 on the expression of Sp1 protein in
DU145 and PC3 prostate cancer cells. A248 was shown to
significantly decrease the expression of Sp1 protein in a
concentration- and time-dependent manner. We also demonstrated that
the overexpression of Sp1 protein in DU145 cells was clearly
inhibited by A248, indicating that A248 targets Sp1 to induce
apoptosis in prostate cancer cells. Sp1 is a mammalian
transcription factor (27,37) that binds to GC-rich sequences to
regulate gene expression (38) and
directly binds to the survivin and Mcl-1 promoters (39–42).
Our previous studies have demonstrated that Sp1 is closely
associated with the upregulation of survivin and Mcl-1 proteins,
which are known to be important in cancer cell survival (7,8,35).
In the present study, our results showed that the treatment of
DU145 and PC3 cells with A248 resulted in a decrease in the protein
levels of survivin and Mcl-1. Using immunocytochemical analysis,
survivin and Mcl-1 proteins were confirmed to be significantly
inhibited by A248 in DU145 cells, indicating that A248 inhibits
Mcl-1 and survivin through the downregulation of Sp1 to induce
apoptotic cell death in prostate cancer.
In conclusion, to the best of our knowledge, we
showed for the first time that A248 inhibits the growth of prostate
cancer cells and that the growth inhibitory effects of A248 may be
mediated by apoptosis. We also demonstrated that A248 treatment
resulted in the downregulation of Sp1, which affected the
expression levels of survivin and Mcl-1 proteins. Therefore, our
findings suggest that A248 targeting Sp1 protein may be a potential
therapeutic agent for prostate cancer.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(nos. 2012002481 and 2012003731).
References
|
1
|
Alshatwi AA, Hasan TN, Shafi G, Syed NA,
Al-Assaf AH, Alamri MS and Al-Khalifa AS: Validation of the
antiproliferative effects of organic extracts from the green husk
of Juglans regia L. on PC3 human prostate cancer cells by
assessment of apoptosis-related genes. Evid Based Complement
Alternat Med. 2012:Feb 6–2012.(Epub ahead of print).
|
|
2
|
Malek A, Núñez LE, Magistri M, et al:
Modulation of the activity of Sp transcription factors by
mithramycin analogues as a new strategy for treatment of metastatic
prostate cancer. PLoS One. 7:e351302012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soriano-Hernández AD, Galvan-Salazar HR,
Montes-Galindo DA, et al: Antitumor effect of meclofenamic acid on
human androgen-independent prostate cancer: a preclinical
evaluation. Int Urol Nephrol. 44:471–477. 2012.PubMed/NCBI
|
|
4
|
Sankpal UT, Abdelrahim M, Connelly SF, et
al: Small molecule tolfenamic acid inhibits PC3 cell proliferation
and invasion in vitro, and tumor growth in orthotopic mouse model
for prostate cancer. Prostate. 72:1648–1658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lou Z, O’Reilly S, Liang H, Maher VM,
Sleight SD and McCormick JJ: Down-regulation of overexpressed sp1
protein in human fibrosarcoma cell lines inhibits tumor formation.
Cancer Res. 65:1007–1017. 2005.PubMed/NCBI
|
|
6
|
Chintharlapalli S, Papineni S, Ramaiah SK
and Safe S: Betulinic acid inhibits prostate cancer growth through
inhibition of specificity protein transcription factors. Cancer
Res. 67:2816–2823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi ES, Shim JH, Jung JY, et al:
Apoptotic effect of tolfenamic acid in androgen
receptor-independent prostate cancer cell and xenograft tumor
through specificity protein 1. Cancer Sci. 102:742–748. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shim JH, Shin JA, Jung JY, et al:
Chemopreventive effect of tolfenamic acid on KB human cervical
cancer cells and tumor xenograft by downregulating specificity
protein 1. Eur J Cancer Prev. 20:102–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang CZ, Pan Y, Cao Y, Lai PB, Liu L,
Chen GG and Yun J: Histone deacetylase inhibitors facilitate
dihydroartemisinin-induced apoptosis in liver cancer in vitro and
in vivo. PLoS One. 7:e398702012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roh MS, Kim CW, Park BS, et al: Mechanism
of histone deacetylase inhibitor Trichostatin A induced apoptosis
in human osteosarcoma cells. Apoptosis. 9:583–589. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vallo S, Mani J, Stastny M, et al: The
prostate cancer blocking potential of the histone deacetylase
inhibitor LBH589 is not enhanced by the multi receptor tyrosine
kinase inhibitor TKI258. Invest New Drugs. 31:265–272. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rikiishi H: Autophagic and apoptotic
effects of HDAC inhibitors on cancer cells. J Biomed Biotechnol.
2011:May 18–2011.(Epub ahead of print).
|
|
13
|
Perego P, Zuco V, Gatti L and Zunino F:
Sensitization of tumor cells by targeting histone deacetylases.
Biochem Pharmacol. 83:987–994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khan O and La Thangue NB: HDAC inhibitors
in cancer biology: emerging mechanisms and clinical applications.
Immunol Cell Biol. 90:85–94. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marks PA: The clinical development of
histone deacetylase inhibitors as targeted anticancer drugs. Expert
Opin Investig Drugs. 19:1049–1066. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou YW, Chaturvedi NK, Ouyang S, et al:
Histone deacetylase inhibitor valproic acid suppresses the growth
and increases the androgen responsiveness of prostate cancer cells.
Cancer Lett. 311:177–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hudak L, Tezeeh P, Wedel S, et al: Low
dosed interferon alpha augments the anti-tumor potential of histone
deacetylase inhibition on prostate cancer cell growth and invasion.
Prostate. 72:1719–1735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim NH, Kim SN and Kim YK: Involvement of
HDAC1 in E-cadherin expression in prostate cancer cells; its
implication for cell motility and invasion. Biochem Biophys Res
Commun. 404:915–921. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gravina GL, Marampon F, Giusti I, et al:
Differential effects of PXD101 (belinostat) on androgen-dependent
and androgen-independent prostate cancer models. Int J Oncol.
40:711–720. 2012.PubMed/NCBI
|
|
20
|
Zhou X, Yang XY and Popescu NC:
Preclinical evaluation of combined antineoplastic effect of DLC1
tumor suppressor protein and suberoylanilide hydroxamic acid on
prostate cancer cells. Biochem Biophys Res Commun. 420:325–330.
2012. View Article : Google Scholar
|
|
21
|
Lai MT, Yang CC, Lin TY, Tsai FJ and Chen
WC: Depsipeptide (FK228) inhibits growth of human prostate cancer
cells. Urol Oncol. 26:182–189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bjorkman M, Iljin K, Halonen P, Sara H,
Kaivanto E, Nees M and Kallioniemi OP: Defining the molecular
action of HDAC inhibitors and synergism with androgen deprivation
in ERG-positive prostate cancer. Int J Cancer. 123:2774–2781. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abdelrahim M, Smith R 3rd, Burghardt R and
Safe S: Role of Sp proteins in regulation of vascular endothelial
growth factor expression and proliferation of pancreatic cancer
cells. Cancer Res. 64:6740–6749. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abdelrahim M, Samudio I, Smith R 3rd,
Burghardt R and Safe S: Small inhibitory RNA duplexes for Sp1 mRNA
block basal and estrogen-induced gene expression and cell cycle
progression in MCF-7 breast cancer cells. J Biol Chem.
277:28815–28822. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Safe S and Abdelrahim M: Sp transcription
factor family and its role in cancer. Eur J Cancer. 41:2438–2448.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yao JC, Wang L, Wei D, et al: Association
between expression of transcription factor Sp1 and increased
vascular endothelial growth factor expression, advanced stage, and
poor survival in patients with resected gastric cancer. Clin Cancer
Res. 10:4109–4117. 2004. View Article : Google Scholar
|
|
27
|
Wang L, Wei D, Huang S, et al:
Transcription factor Sp1 expression is a significant predictor of
survival in human gastric cancer. Clin Cancer Res. 9:6371–6380.
2003.PubMed/NCBI
|
|
28
|
Zannetti A, Del Vecchio S, Carriero MV, et
al: Coordinate up-regulation of Sp1 DNA-binding activity and
urokinase receptor expression in breast carcinoma. Cancer Res.
60:1546–1551. 2000.PubMed/NCBI
|
|
29
|
Hosoi Y, Watanabe T, Nakagawa K, et al:
Up-regulation of DNA-dependent protein kinase activity and Sp1 in
colorectal cancer. Int J Oncol. 25:461–468. 2004.PubMed/NCBI
|
|
30
|
Ishibashi H, Nakagawa K, Onimaru M, et al:
Sp1 decoy transfected to carcinoma cells suppresses the expression
of vascular endothelial growth factor, transforming growth factor
beta1, and tissue factor and also cell growth and invasion
activities. Cancer Res. 60:6531–6536. 2000.
|
|
31
|
Chadalapaka G, Jutooru I, Chintharlapalli
S, Papineni S, Smith R 3rd, Li X and Safe S: Curcumin decreases
specificity protein expression in bladder cancer cells. Cancer Res.
68:5345–5354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jutooru I, Chadalapaka G, Lei P and Safe
S: Inhibition of NFkappaB and pancreatic cancer cell and tumor
growth by curcumin is dependent on specificity protein
down-regulation. J Biol Chem. 285:25332–25344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chu S and Ferro TJ: Sp1: regulation of
gene expression by phosphorylation. Gene. 348:1–11. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deniaud E, Baguet J, Chalard R, et al:
Overexpression of transcription factor Sp1 leads to gene expression
perturbations and cell cycle inhibition. PLoS One. 4:e70352009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi KH, Shim JH, Huong LD, Cho NP and Cho
SD: Inhibition of myeloid cell leukemia-1 by tolfenamic acid
induces apoptosis in mucoepidermoid carcinoma. Oral Dis.
17:469–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu DC, Waby JS, Chirakkal H, Staton CA and
Corfe BM: Butyrate suppresses expression of neuropilin I in
colorectal cell lines through inhibition of Sp1 transactivation.
Mol Cancer. 9:2762010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu J, Zhou JY, Wei WZ, Philipsen S and Wu
GS: Sp1-mediated TRAIL induction in chemosensitization. Cancer Res.
68:6718–6726. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kadonaga JT, Courey AJ, Ladika J and Tjian
R: Distinct regions of Sp1 modulate DNA binding and transcriptional
activation. Science. 242:1566–1570. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pietrzak M and Puzianowska-Kuznicka M:
p53-dependent repression of the human MCL-1 gene encoding an
anti-apoptotic member of the BCL-2 family: the role of Sp1 and of
basic transcription factor binding sites in the MCL-1 promoter.
Biol Chem. 389:383–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li F and Altieri DC: Transcriptional
analysis of human survivin gene expression. Biochem J. 344:305–311.
1999. View Article : Google Scholar
|
|
41
|
Li Y, Xie M, Yang J, Yang D, Deng R, Wan Y
and Yan B: The expression of antiapoptotic protein survivin is
transcriptionally upregulated by DEC1 primarily through multiple
sp1 binding sites in the proximal promoter. Oncogene. 25:3296–3306.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu J, Ling X, Pan D, et al: Molecular
mechanism of inhibition of survivin transcription by the GC-rich
sequence-selective DNA binding antitumor agent, hedamycin: evidence
of survivin down-regulation associated with drug sensitivity. J
Biol Chem. 280:9745–9751. 2005. View Article : Google Scholar
|