Introduction
Osteosarcoma is the most common form of primary
malignant bone tumor and exhibits a high degree of malignancy.
Osteosarcoma is most common in young individuals aged between 10
and 25 years. Single amputation treatment has been associated with
a number of limitations, including a high disability rate and a low
5-year survival rate. Recently, the clinical application of
neoadjuvant chemotherapy and surgical staging systems has been
successfully used in the majority of patients with stage IIB
osteosarcoma while performing limb salvage surgery; as a result of
this, the 5-year survival rate has increased to 60–70% (1,2).
However, patients who are insensitive to chemotherapy continue to
have a poor prognosis. Based on the results of advanced studies on
tumor molecular biology and gene function, we hypothesized that
differences in tumor-associated gene expression are the main cause
of differences in the sensitivity to identical chemotherapeutic
drugs observed between patients (3).
According to a recent study, 70% of human tumor
cells exhibit c-myc overexpression, which stimulates cell
proliferation, migration and invasion (4). Hattinger et al(5) confirmed that varying degrees of c-myc
amplification were observed in doxorubicin and the human
osteosarcoma cell lines U2OS and SAOS-2, which demonstrated
methotrexate (MTX) resistance. Bmi-1 is one of the core members of
the Polycomb-group (PcG) gene family. It is involved in the
regulation of transcriptional repression associated with the cell
cycle and cell proliferation. A reduction in Bmi-1 gene expression
in glioma cells and multiple myeloma is capable of inhibiting the
proliferation of tumor cells (6,7).
Bmi-1 is highly expressed in Ewing's sarcoma cells. By knocking
down the expression of Bmi-1, the expression of multiple downstream
genes associated with the cell cycle may be regulated (8). Several studies have shown that c-myc
and Bmi-1 are involved in the regulation of cell growth and the
apoptotic signaling pathway. Thus, the expression of these genes
determines the sensitivity of tumors to chemotherapy (9,10). A
previous study investigated genes associated with the drug
resistance of osteosarcoma in different patients. It was observed
that reducing c-myc expression in osteosarcoma cells significantly
reduced cell resistance to MTX (11). Knocking down Bmi-1 gene expression
in SAOS-2 osteosarcoma cells caused inhibition of the PI3K/AKT
signaling pathway, leading to the enhancement of tumor sensitivity
to chemotherapy (12). In our
previous study, we reported that reduced c-myc expression in MG-63
osteosarcoma cells was capable of increasing the chemosensitivity
of these cells to cisplatin (CDDP) in order to induce cell
apoptosis (13). Therefore,
coupling of the c-myc and Bmi-1 genes may provide suitable
therapeutic targets for enhancing osteosarcoma sensitivity to
chemotherapy. However, it is necessary to further investigate this
hypothesis, since it has not been explored by previous studies.
In the present study, MG-63 cells were transfected
with the small interfering RNAs (siRNAs) c-myc and Bmi-1 separately
or in combination, and the chemosensitivity to CDDP and cell
proliferation and apoptotic rates were detected. The aim of this
study was to investigate the combinational effects of c-myc and
Bmi-1 siRNAs on osteosarcoma cell growth and chemosensitivity.
These data may provide novel insights to improve our understanding
of the underlying mechanisms involved in chemotherapeutic
sensitivity, and aid in the development of future therapeutic
strategies for the treatment of osteosarcoma.
Materials and methods
Cell culture
The human osteosarcoma MG-63 cell line was purchased
from Shanghai Institute of Cell Biology (Shanghai, China). The
cells were maintained in DMEM (Gibco-BRL, Carlsbad, CA, USA)
supplemented with 10% FBS (Hyclone, Thermo Fisher Scientific Inc.,
Logan, UT, USA). The cell culture was stored at 37°C in a 5%
CO2 incubator. The study was approved by the ethics
committee of Second Affiliated Hospital College of Medicine,
Zhejiang University, Hangzhou, China.
Transfection of c-myc and Bmi-1
siRNAs
c-myc and Bmi-1 siRNAs were purchased from Abcam
Inc. (Cambridge, MA, USA). Two types of siRNA were diluted in
serum-free DMEM, individually and in combination. Lipofectamine
2000 (Gibco-BRL) was diluted in RPMI-1640 medium, following
incubation at room temperature for 5 min. To facilitate the
formation of a siRNA/liposome complex, the two reactant solutions
were mixed thoroughly and incubated at room temperature for 20 min.
The dose of the siRNA/liposome complex medium was 250 μl, while the
strength of siRNA was 100 pmol and that of the liposome was 5 μl.
According to the experimental conditions, there were four groups:
i) The c-myc siRNA group; ii) the Bmi-1 siRNA group; iii) the
c-myc/Bmi-1 siRNA combination group and iv) the empty liposome
group. A blank control group only contained DMEM. Synchronous
transfection was conducted until the MG-63 cells had grown to an
appropriate density. Three duplicate wells were prepared for each
group.
RT-PCR analysis
MG-63 cells from each group were collected at 0, 24,
48 and 72 h following transfection. Total RNA was extracted using
the TRIzol kit (Takara Bio, Inc., Shiga, Japan) according to the
manufacturer's instructions. The concentration and purity of total
RNA were detected using an UV spectrophotometer, followed by
two-step RT-PCR. The amplification products of β-actin were used as
an internal control. RT reaction conditions were as follows: 42°C
for 60 min followed by 75°C for 10 min. PCR reaction conditions
were as follows: denaturation at 94°C for 5 min; cycles were
started at 94°C for 40 sec and continued at 58°C for 35 sec and
72°C for 50 sec; a total of 34 cycles were performed and the final
extension cycle was at 72°C for 10 min. Electrophoresis of PCR
products was conducted on 2% agarose gel. Images were captured
using the Imagemaster VDS gel imaging system (Amersham Pharmacia
Biotech, UK).
Western blot analysis
MG-63 cells from each group were collected 72 h
after transfection. Cells were lysed with cell lysis buffer and the
total protein concentration was detected using the BCA protein
quantification kit (BCA Protein Assay Kit, Thermo Fisher Scientific
Inc., USA). Protein (20 μg) was separated by electrophoresis on 10%
polyacrylamide gel. Electrophoretic transfer was conducted for 2 h
at a constant voltage of 120 V. Thereafter, the protein medium was
kept at room temperature for 1 h and blocked overnight at 4°C.
c-myc primary antibody at a 1:600 dilution, Bmi-1 primary antibody
at a 1:800 dilution and β-actin primary antibody at a 1:1000
dilution (Invitrogen Life Technologies, Carlsbad, CA, USA) were
added sequentially to the medium prior to overnight incubation at
4°C. The primary antibodies were then hybridized with secondary
antibodies at room temperature for 1 h following washing of the
membrane with TBST buffer. The membrane was developed using the ECL
system (Amersham Pharmacia Biotech). Protein expression levels were
analyzed using a gel image analysis system. Using the expression
levels of β-actin as a reference, we compared the relative
expression levels of c-myc and Bmi-1 proteins in all groups.
Cell growth assay
MG-63 cells were transfected with c-myc and Bmi-1
siRNAs either individually or in combination. Following 48 h of
transfection, the MG-63 cells of each group were mixed with 1.0,
2.0 and 5.0 μg/ml CDDP for 2 h. Absorptiometry values
(An) for each group were detected at a wavelength of 490
nm. Cell growth curves were created and the growth inhibition rate
of cells was calculated using the measured An values.
The cell growth inhibition rate was calculated using the following
formula: Cell growth inhibition rate =
[(Ac-Ae)/Ac] × 100%
(Ac, absorptiometry value of the control group;
Ae, absorptiometry value of the experimental group).
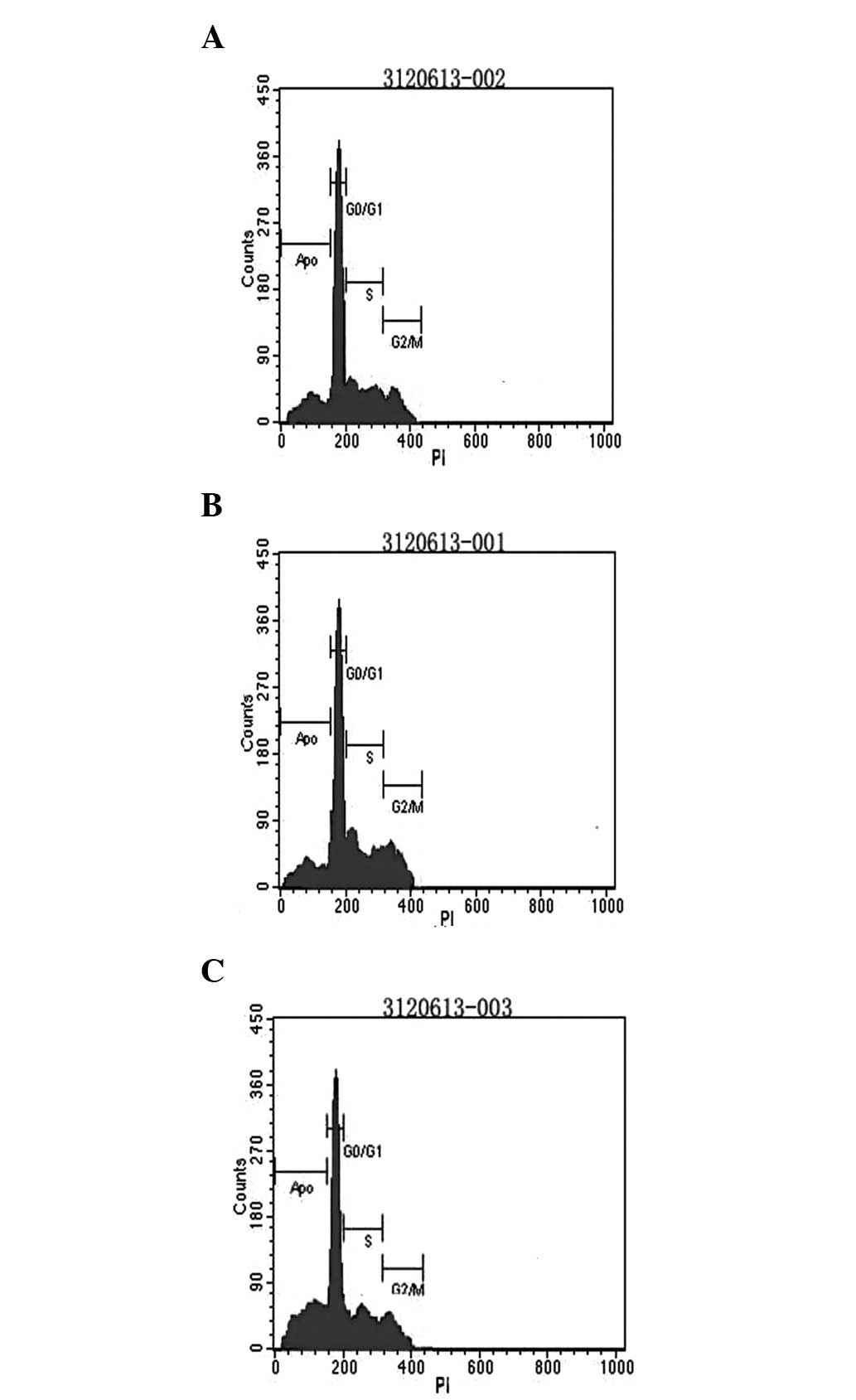
Flow cytometric analysis
To detect cell apoptosis, fluorescent staining was
performed using the Annexin V-FITC/propidium iodide (PI)
double-labeling method. Cells were treated with c-myc and Bmi-1
siRNAs for 24 h, either separately or in combination. Thereafter, 5
μg/ml CDDP was added to the cell culture and incubated for 48 h.
Cells were collected and centrifuged at 300 × g for 5 min.
Following removal of the supernatant, cells were resuspended in
PBS. Then, 5 μl Annexin V-FITC and 5 μl PI dye (Sigma, St. Louis,
MO, USA) were added to the medium. Oscillation, mixing and cold
staining of this medium were then performed at 4°C for 10 min.
Next, 25 μl DNA-Prep LPR (Coulter Electronics Health, USA) was
added to the aforementioned double-labeled staining samples for a
10 min incubation in the dark, which were then centrifuged at 1,000
× g for 5 min. The supernatant was removed, 500 μl DNA-Prep stain
(Coulter Electronics Health) was added and the staining was
conducted for 15 min at room temperature away from light. Cell
apoptosis and the cell cycle distribution of each group were
detected by flow cytometry.
Statistical analysis
Quantitative data were expressed as the mean ± SD.
Comparisons among the groups were conducted using single-factor
analysis of variance. Comparisons between two groups were examined
using Student's t-test. P<0.05 was considered to indicate a
statistically significant difference. All data were analyzed using
SPSS 12.0 software (SPSS Inc., USA).
Results
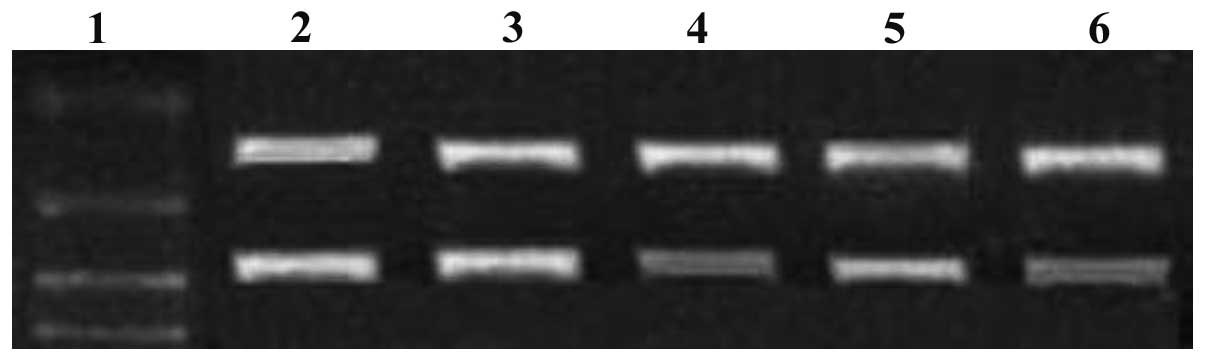
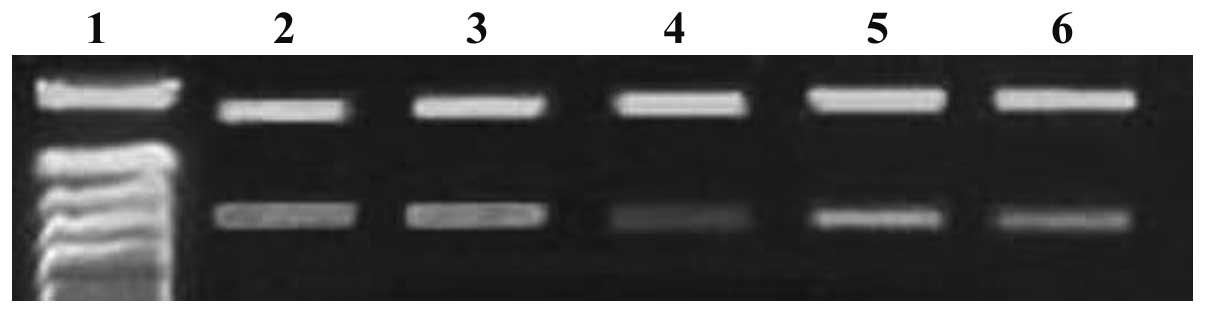
siRNA downregulates c-myc and Bmi-1 gene
expression
When MG-63 cells were transfected with c-myc and
Bmi-1 siRNAs (either individually or in combination), c-myc and
Bmi-1 mRNA expression levels gradually decreased within 72 h, as
demonstrated by the RT-PCR assay. Compared with c-myc and Bmi-1
mRNA expression levels in the single siRNA groups, levels were
significantly decreased in the combination siRNA group (P<0.05;
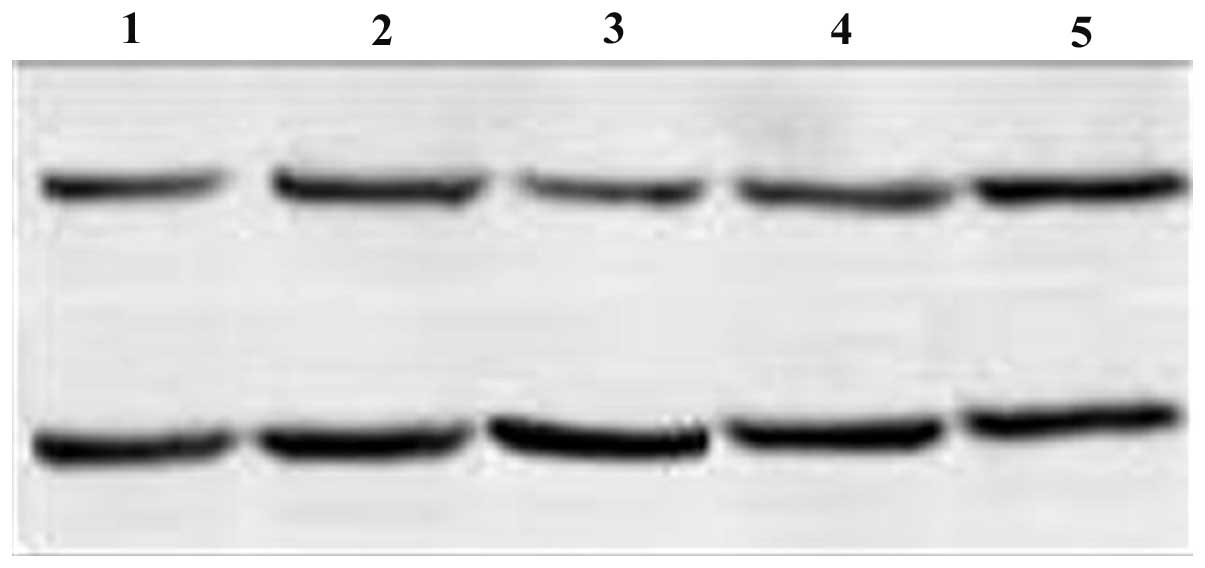
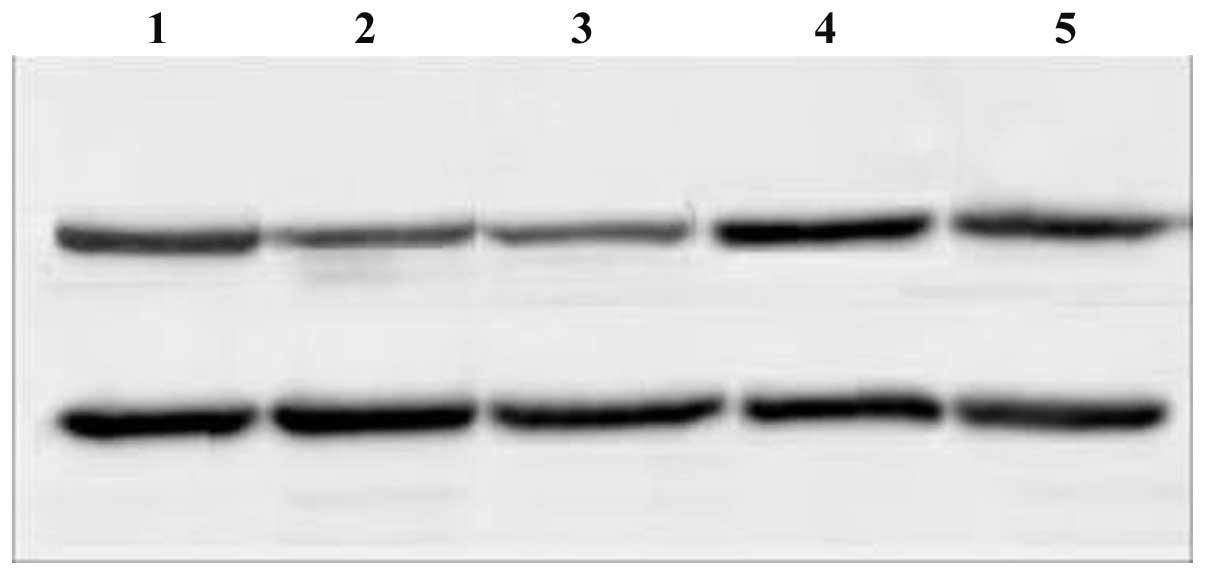
Figs. 1 and 2). c-myc protein expression levels
decreased in the c-myc siRNA and combination groups 72 h after
transfection; the decrease in expression levels was more evident in
the combination group (P<0.05). By contrast, no distinct
decrease in c-myc protein expression levels was detected in the
Bmi-1 siRNA and control groups (Fig.
3). Compared with the control group, Bmi-1 protein expression
levels decreased in all siRNA groups (P<0.05). The most marked
decrease in Bmi-1 protein expression levels was observed in the
combination siRNA group. Bmi-1 protein expression levels were
decreased to a greater extent in the Bmi-1 siRNA group compared
with the c-myc siRNA group; a significant difference was observed
between the two groups (P<0.05; Fig. 4).
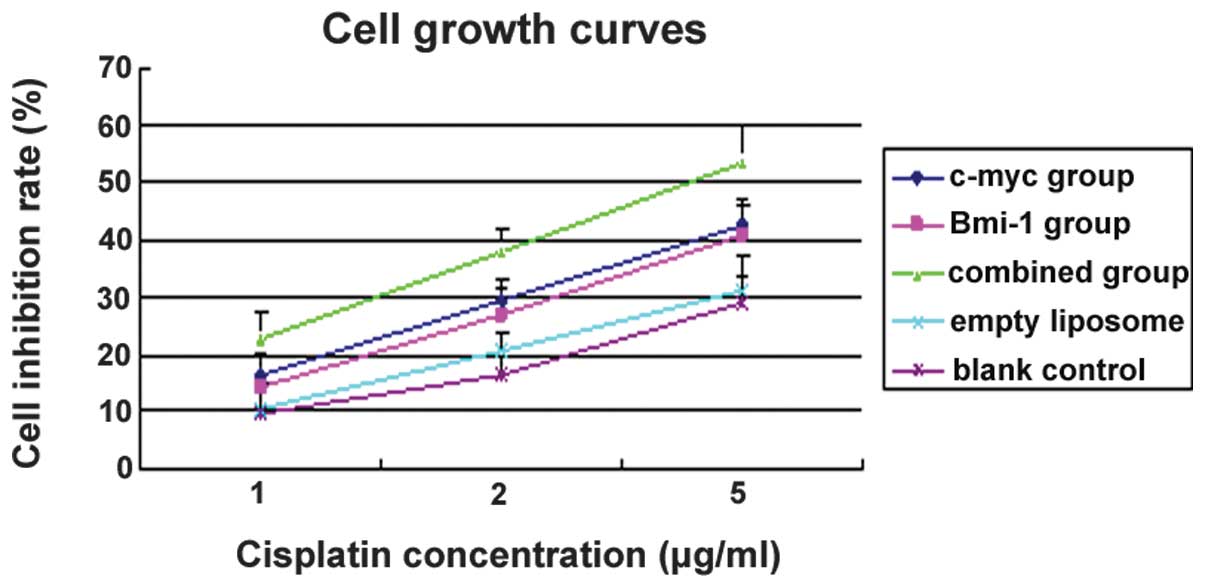
Cell growth inhibitory effects
Compared with the empty liposome and blank control
groups, the cell growth inhibition rates of MG-63 cells gradually
increased with increasing concentrations of CDDP. This observation
was consistent for the single and combined siRNA groups. The growth
inhibition rate in the combination siRNA group was significantly
higher than that of the single siRNA groups (P<0.05). However,
no significant differences were detected between the c-myc and
Bmi-1 siRNA groups (P>0.05). At a concentration of 5.0 μg/ml
CDDP, the growth inhibition rates were 53.3±5.2, 42.7±6.3 and
40.9±4.7% for the combined, c-myc and Bmi-1 siRNA groups,
respectively (Fig. 5). The cell
growth inhibitory effects observed in the combined siRNA group were
greater than those observed in the two single siRNA groups
(P<0.05), indicating a significant increase in the
chemosensitivity of MG-63 cells to CDDP.
Flow cytometric analysis of MG-63 cell
apoptosis
The apoptotic rate and cell cycle distribution of
cells were detected by flow cytometry. The apoptotic rates were
37.3±4.9, 24.8±5.6 and 22.7±6.1% in the combined, c-myc and Bmi-1
siRNA groups, respectively. The apoptotic rates of cells in these
groups were markedly higher than that of the control group
(P<0.05). In addition, compared with the two single siRNA
groups, MG-63 cell apoptosis increased considerably in the combined
siRNA group (P<0.05; Fig. 6).
However, there were no significant differences in the cellular
apoptotic rates between the two single siRNA groups
(P>0.05).
Discussion
Tumor chemosensitivity is correlated with three
important parameters; the tumor cell proliferation ratio, the cell
cycle and doubling time of cell proliferation. In addition, several
factors affect the sensitivity of tumor cells to chemotherapeutic
agents, including disorders in drug uptake and transport, drug
activation barriers, enhancement of DNA damage and repair capacity
and obstacles in the apoptotic pathway (14). The effects of ATP-binding cassette
(ABC) transporter proteins on tumor chemosensitivity were studied
extensively in recent studies. ABC transporter proteins release
energy through the hydrolysis of ATP; this energy is used to
transport chemotherapeutic drugs from the inside to the outside of
tumor cells. This significantly reduces the intracellular
chemotherapeutic drug concentration, resulting in the resistance of
tumor cells (15). However, there
is currently no consensus on whether the overexpression of ABC
transporter proteins in osteosarcoma may be used as an indicator to
predict the effects of chemotherapy and its long-term efficacy.
Previous studies have also reported that chemotherapy sensitivity
or resistance is caused by the interaction of various factors
(5,12,15).
Therefore, identifying phenotypic indicators that determine cell
chemosensitivity may provide novel insights into the underlying
mechanisms of chemosensitivity. c-myc, an upstream signal, is
capable of regulating the expression of ABC transporter proteins
that affect the active transport of chemotherapeutic drugs from the
inside to the outside of tumor cells. In addition, it may also
stimulate downstream signaling pathways by regulating the
transcriptional expression of downstream genes, thereby decreasing
the sensitivity of tumor cells to chemotherapy (16). According to previous studies,
recombinant adenovirus (Myc-AS) combined with caffeine is capable
of enhancing the induction of apoptosis and the chemotherapeutic
effects of CDDP on MG-63 osteosarcoma cells (13). Qin et al(17) also revealed that a decrease in the
gene expression of Bmi-1 in nasopharyngeal carcinoma enhanced
5-fluorouracil-mediated apoptosis. In the present study, we
demonstrated that the effects of c-myc and Bmi-1 siRNAs in
combination significantly improve the chemosensitivity of MG-63
cells to CDDP compared with the single siRNA groups (P<0.05).
The cell growth inhibition rates for the combined, c-myc and Bmi-1
siRNA groups were 53.3±5.2, 42.7±6.3 and 40.9±4.7%, respectively.
The cell growth inhibitory effects observed in the combined siRNA
group were greater than those in the two single siRNA groups
(P<0.05). This indicates that the chemosensitivity of MG-63
cells to CDDP may be significantly increased.
A number of studies have reported that c-myc is
important in regulating cell proliferation, differentiation and
apoptosis. As an early response gene, c-myc is important in
regulating the transcription of a series of target genes in a
number of signal transduction pathways (18,19).
Previous studies have reported that high expression levels of c-myc
are able to induce osteosarcoma in mouse models. This suggests that
the inhibition of c-myc expression may induce the differentiation
of osteosarcoma cells into mature bone cells and significantly
inhibit tumor growth (20). The
Bmi-1 gene is located at 10p11.23; it contains a RING finger (RF)
motif in the RF domain at the N-terminal end. The Bmi-1 gene is
important in cell proliferation and tumor formation in
co-ordination with c-myc. In normal cells, Bmi-1 uses different
promoters to regulate the gene expression levels of p16Ink4a and
p19Arf, which play important regulatory roles through the tumor
suppressor protein pRb and the transcription factor p53-related
cell cycle pathways, respectively (21). When the expression of Bmi-1 is
inhibited, p16Ink4a expression levels increase, leading to pRB
dephosphorylation. Dephosphorylated pRB inhibits E2F-mediated gene
transcription as a result of combining with E2F, thereby causing
cell cycle arrest, which promotes apoptosis. Overexpression of
Bmi-1 is able to inhibit the transcription of p19Arf, thereby
increasing p53 degradation. This ultimately prevents p53-mediated
apoptosis (22). P53 gene mutation
and deletion is often observed in osteosarcoma cells. Bmi-1 is
capable of regulating tumor cell proliferation and apoptosis
through several signaling pathways. In the present study, we
demonstrated that c-myc and Bmi-1 mRNA expression levels decreased
significantly in the combination siRNA group compared with the
single siRNA groups (P<0.05). Compared with the control group,
Bmi-1 protein expression levels decreased in all siRNA groups
(P<0.05), with the most marked decrease being observed in the
combination siRNA group. Furthermore, expression levels in the
Bmi-1 siRNA group decreased to a greater extent compared with the
c-myc siRNA group (P<0.05). The data demonstrated that Bmi-1 is
downregulated by c-myc via an unknown mechanism. Cellular apoptotic
rates for the combined, c-myc and Bmi-1 siRNA groups were 37.3±4.9,
24.8±5.6 and 22.7±6.1%, respectively. Notably, the cellular
apoptotic rates of these three groups were significantly higher
than that of the control group (P<0.05). In addition, compared
with the two single siRNA groups, MG-63 cell apoptosis
significantly increased in the combined siRNA group (P<0.05).
However, there were no significant differences in cellular
apoptotic rates between the two single siRNA groups
(P>0.05).
In the present study, we compared the effects of
single and combined c-myc and Bmi-1 siRNAs on MG-63 cells. We
demonstrated that the chemosensitivity of MG-63 cells to CDDP was
markedly enhanced in the siRNA combination group. A declined
proliferative capacity of MG-63 cells and increased apoptosis were
also observed in the siRNA combination group. This study may
provide novel insights to further elucidate the pathogenesis and
drug resistance mechanisms involved in osteosarcoma. It may also
improve our understanding of the underlying mechanisms involved in
chemotherapeutic sensitivity and aid in the development of future
therapeutic strategies for the treatment of osteosarcoma.
Acknowledgements
The authors would like to thank colleagues at the
Department of Orthopedics, Second Affiliated Hospital College of
Medicine, Zhejiang University, for their technical assistance and
helpful comments. This study was supported by a medical scientific
project grant from the Bureau of Health, Zhejiang Province, China
(no. 2011RCB023).
References
|
1
|
Poletajew S, Fus L and Wasiutyński A:
Current concepts on pathogenesis and biology of metastatic
osteosarcoma tumors. Ortop Traumatol Rehabil. 13:537–545. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bernthal NM, Federman N, Eilber FR, Nelson
SD, Eckardt JJ, Eilber FC and Tap WD: Long-term results (>25
years) of a randomized, prospective clinical trial evaluating
chemotherapy in patients with high-grade, operable osteosarcoma.
Cancer. 118:5888–5893. 2012.
|
|
3
|
Broadhead ML, Clark JC, Myers DE, Dass CR
and Choong PF: The molecular pathogenesis of osteosarcoma: a
review. Sarcoma. 2011:9592482011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dang CV: MYC on the path to cancer. Cell.
149:22–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hattinger CM, Stoico G, Michelacci F,
Pasello M, Scionti I, Remondini D, Castellani GC, Fanelli M,
Scotlandi K, Picci P and Serra M: Mechanisms of gene amplification
and evidence of coamplification in drug-resistant human
osteosarcoma cell lines. Genes Chromosomes Cancer. 48:289–309.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Godlewski J, Nowicki MO, Bronisz A,
Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca
EA and Lawler S: Targeting of the Bmi-1 oncogene/stem cell renewal
factor by microRNA-128 inhibits glioma proliferation and
self-renewal. Cancer Res. 68:9125–9130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jagani Z, Wiederschain D, Loo A, He D,
Mosher R, Fordjour P, Monahan J, Morrissey M, Yao YM, Lengauer C,
Warmuth M, Sellers WR and Dorsch M: The Polycomb group protein
Bmi-1 is essential for the growth of multiple myeloma cells. Cancer
Res. 70:5528–5538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Douglas D, Hsu JH, Hung L, Cooper A,
Abdueva D, van Doorninck J, Peng G, Shimada H, Triche TJ and Lawlor
ER: BMI-1 promotes ewing sarcoma tumorigenicity independent of
CDKN2A repression. Cancer Res. 68:6507–6515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Savage KJ, Johnson NA, Ben-Neriah S,
Connors JM, Sehn LH, Farinha P, Horsman DE and Gascoyne RD: MYC
gene rearrangements are associated with a poor prognosis in diffuse
large B-cell lymphoma patients treated with R-CHOP chemotherapy.
Blood. 114:3533–3537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qin L, Zhang X, Zhang L, Feng Y, Weng GX,
Li MZ, Kong QL, Qian CN, Zeng YX, Zeng MS, Liao DF and Song LB:
Downregulation of BMI-1 enhances 5-fluorouracil-induced apoptosis
in nasopharyngeal carcinoma cells. Biochem Biophys Res Commun.
371:531–535. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scionti I, Michelacci F, Pasello M,
Hattinger CM, Alberghini M, Manara MC, Bacci G, Ferrari S,
Scotlandi K, Picci P and Serra M: Clinical impact of the
methotrexate resistance-associated genes C-MYC and dihydrofolate
reductase (DHFR) in high-grade osteosarcoma. Ann Oncol.
19:1500–1508. 2008. View Article : Google Scholar
|
|
12
|
Wu Z, Min L, Chen D, Hao D, Duan Y, Qiu G
and Wang Y: Overexpression of BMI-1 promotes cell growth and
resistance to cisplatin treatment in osteosarcoma. PLoS One.
6:e146482011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie XK, Yang DS, Ye ZM and Tao HM:
Enhancement effect of adenovirus mediated antisense C-myc and
caffeine on the cytotoxicity of cisplatin in osteosarcoma cell
lines. Chemotherapy. 55:433–440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lonning PE: Molecular basis for therapy
resistance. Mol Oncol. 4:284–300. 2010. View Article : Google Scholar
|
|
15
|
Li YT, Chua MJ, Kunnath AP and Chowdhury
EH: Reversing multidrug resistance in breast cancer cells by
silencing ABC transporter genes with nanoparticle-facilitated
delivery of target siRNAs. Int J Nanomedicine. 7:2473–2481.
2012.PubMed/NCBI
|
|
16
|
Guney I, Wu S and Sedivy JM: Reduced c-Myc
signaling triggers telomere- independent senescence by regulating
Bmi-1 and p16INK4a. Proc Natl Acad Sci USA. 103:3645–3650. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin L, Zhang X, Zhang L, Feng Y, Weng GX,
Li MZ, Kong QL, Qian CN, Zeng YX, Zeng MS, Liao DF and Song LB:
Downregulation of BMI-1 enhances 5-fluorouracil-induced apoptosis
in nasopharyngeal carcinoma cells. Biochem Biophys Res Commun.
371:531–535. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guney I and Sedivy JM: Cellular
senescence, epigenetic switches and c-Myc. Cell Cycle. 5:2319–2323.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lüscher B and Vervoorts J: Regulation of
gene transcription by the oncoprotein MYC. Gene. 494:145–160.
2012.PubMed/NCBI
|
|
20
|
Jain M, Arvanitis C, Chu K, Dewey W,
Leonhardt E, Trinh M, Sundberg CD, Bishop JM and Felsher DW:
Sustained loss of a neoplastic phenotype by brief inactivation of
MYC. Science. 297:102–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu YL, Jiang SX, Yang YM, Xu H, Liu JL
and Wang XS: USP22 acts as an oncogene by the activation of
BMI-1-mediated INK4a/ARF pathway and Akt pathway. Cell Biochem
Biophys. 62:229–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Datta S, Hoenerhoff MJ, Bommi P, Sainger
R, Guo WJ, Dimri M, Band H, Band V, Green JE and Dimri GP: Bmi-1
cooperates with H-Ras to transform human mammary epithelial cells
via dysregulation of multiple growth-regulatory pathways. Cancer
Res. 67:10286–10295. 2007. View Article : Google Scholar
|