Introduction
Malignant tumors constitute a threat to human
health. Traditional methods for treating tumors are surgery,
radiotherapy, chemotherapy and biological therapy; however,
monotherapy often has its own limitations. Therefore, various means
of combined therapy for tumors have become a hot topic.
On the basis of the respective characteristics of
radiotherapy and gene therapy, Weichselbaum et al proposed
the use of tumor gene-radiotherapy (coupling the regulatory
sequence of the ionizing radiation-induced gene and the
tumor-killing gene to be transferred into irradiated tumor cells),
thus inducing the expression of the tumor-killing gene and forming
a synergism of inhibiting tumors by ionizing radiation and gene
suppression (1). Early growth
response-1 (Egr1), identified by Sukhatme et al(2) is activated by ionizing radiation,
free radicals and hypoxia to induce the expression of downstream
genes (3–5). According to the characteristics of
the Egr1 gene induced by ionizing radiation, the ionizing
radiation-inducing expressive system is constructed with the
cis-acting element of the Egr1 promoter region, which controls the
temporal and spatial expression of target genes to produce the
synergistic killing effect on tumor cells and decrease the
cytotoxic effect on normal tissues. Weichselbaum et
al(6) first constructed the
expressive plasmid pEgr1-tumor necrosis factor-α (TNF-α) and
performed a series of studies on tumor gene-radiotherapy with TNF-α
as a theoretical gene. The authors demonstrated that the plasmid
with radiotherapy inhibited tumor growth and decreased the local
and systemic toxic effects (7–9).
Gene therapy is a method employed to treat tumors.
The degree of safety and high-performance of the expression vector
determines the efficacy. Conditionally replicating adenovirus
(CRAd), also known as oncolytic adenovirus, is an ideal
tumor-treatment vector since it replicates specifically in tumor
cells. Progeny virus released from the vector infects the vicinity
of tumor cells and then initiates a cascade amplification reaction,
leading to cell disruption, eventually eliminating the cell
population (10,11). Yu and Fang demonstrated that CRAd
H101 in combination with chemotherapy has favorable tolerance and
curative effects on certain tumors (12). Due to the potential of CRAd, it has
been previously tested in a clinical trial and its safety has been
confirmed (13). Gene therapy with
CRAd has become a hot spot in tumor gene therapy (14), since CRAd specifically infects and
kills tumor cells without damaging normal cells (15). There is no crossing drug tolerance
between CRAd therapeutics and traditional tumor therapeutics. In
addition, the combination of CRAd with radiotherapy and
chemotherapy achieves a superposition or synergism (16,17).
TNF-related apoptosis inducing ligand (TRAIL), cloned by Wiley
et al(18), is a member of
the TNF superfamily. TRAIL induces apoptosis in tumor, transformant
and virus-infecting cells, but does not damage normal cells
(19–22). TRAIL, not only has a killing effect
on tumor cells, but also induces the apoptosis of tumor cells when
combined with radiotherapy, which produces the synergistic effect
of killing tumor cells, as well as increasing the sensitivity of
TRAIL to irradiation. Studies (19,20,22,23)
have confirmed that ionizing radiation upregulates the expression
of TRAIL and death receptors 4 and 5 (DR4 and DR5), and promotes
the apoptosis of tumor cells. TRAIL in combination with
radiotherapy has apparent synergistic effects, producing good
curative effect on treating breast cancer in preclinical
experiments and one/two-stage clinical trials (22,23).
If the TRAIL gene is constructed downstream of the Egr1 gene
promoter, ionizing radiation induces and activates the Egr1 gene,
which regulates the expression of the downstream TRAIL gene,
increasing the curative effect of gene-radiotherapy on tumors.
In the present study, the Egr1 promoter and TRAIL
gene were inserted into a CRAd vector, which contained the original
CR2 region-deleted E1A gene controlled by the human telomere
reverse transcriptase (hTERT) promoter. The recombinant expression
system integrated three functions: i) the CRAd vector has a double
targeting effect in tumor cells, disrupting tumor cells; ii)
radiotherapy kills tumor cells and induces the transcription of the
Egr1 promoter, which triggers TRAIL; and iii) the TRAIL gene
promotes apoptosis of tumor cells. The recombinant multifunctional
expression system CRAd.pEgr1-TRAIL combined with ionizing
irradiation were used to kill human breast cancer cells.
Integration of radiotherapy, virus therapy and gene therapy may
provide a new approach to the combined therapy of malignant
tumors.
Materials and methods
Cell lines and reagents
MDA-MB-231 and MCF-7 human cancer cell lines were
obtained from the Shanghai Institute of Cell Biology, Chinese
Academy of Science (Shanghai, China). MDA-MB-231 cells were
maintained in L-15 medium (Sigma, St. Louis, MO, USA). MCF-7 cells
were cultured in complete Dulbecco’s modified Eagle’s medium (DMEM;
Sigma) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL,
Grand Island, NY, USA), 100 U/ml penicillin and streptomycin
(Gibco-BRL) and incubated at 37°C, 5% CO2. Anti-human
caspase-8 and -3 and anti-human glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) monoclonal antibodies were purchased from
Cell Signaling Technology Inc. (Boston, MA, USA). The anti-human
DR5 monoclonal antibody was purchased from Millipore (Billerica,
MA, USA). Horseradish peroxidse (HRP)-labeled rabbit anti-goat
polyclonal antibody was from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA).
Recombinant adenovirus
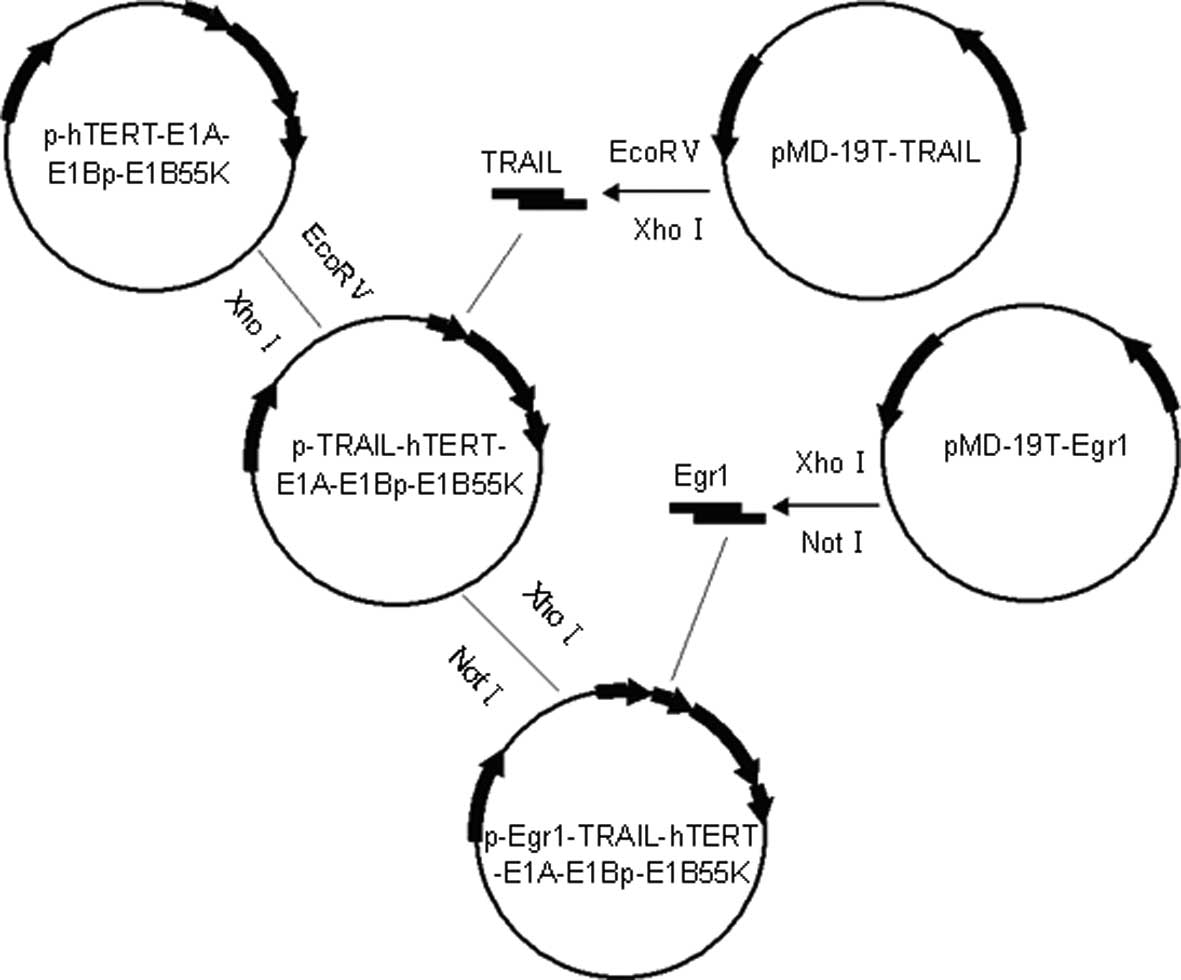
The shuttle vector
pShuttle-Egr1-TRAIL-hTERT-E1A-E1Bp-E1B55K was transformed into
BJ5183 (pAdEasy-1+) competent cells, forming CRAd.pEgr1-TRAIL
through homologous recombination (Fig.
1). The adenovirus was amplified in HEK293 cells and prepared
by a standard CsCl method. The adenovirus titers were measured by
the TCID50 method and the acquired value was recalculated as
pfu/ml.
X-ray irradiation
MDA-MB-231 cells were irradiated using X-rays
generated by a Philips X-ray machine (XSS 205FZ; settings: 180 kV,
12 mA and filters of 0.5 mm Cu and 1.0 mm Al) to deliver doses of
1–5 Gy at a dose rate of 0.387 Gy/min and a target skin distance of
60 cm.
Cell viability assay
The cytotoxicities of plasmids and CRAd in
MDA-MB-231 cells were assessed by Cell Counting Kit-8 (CCK-8;
Dojindo Molecular Technologies, Japan). Briefly, the cells were
seeded in 96-well plates at a density of 2×104
cells/well and treated with pShuttle, pShuttle-Egr1-TRAIL, CRAd.p
and CRAd.pEgr1-TRAIL, respectively. The untreated cells were used
as a control group. After 24 h, the cells were irradiated with a
dose of 2.0 Gy. Twenty-four hours after irradiation, CCK-8 was
added to each well, followed by incubation at 37°C for 1 h. The
optical absorbance value of each well was measured at 490 nm on a
microplate reader (Bio-Rad, Hercules, CA, USA).
Flow cytometry
Cell apoptosis was assessed by flow cytometry with
Annexin V and propidium iodide (PI) double-staining. Cells were
seeded in 24-well plates at a density of 3×105
cells/well one day prior to treatment. Then, the cells were treated
with CRAd.p or CRAd.pEgr1-TRAIL at a multiplicity of infection
(MOI). After 24 h, the cells were irradiated with 2.0 Gy.
Twenty-four hours after irradiation, the floating and attached
cells were collected and washed with phosphate-buffered saline
(PBS). Binding buffer (500 μl) mixed with 5 μl Annexin
V-fluorescein isothiocyanate (FITC) and subsequently with 5 μl PI
was added to 5×105 cells, followed by incubation at room
temperature in the dark for 10 min. The stained cells were then
analyzed using a FACSCalibur device (BD Biosciences, San Jose, CA,
USA).
Real-time polymerase chain reaction
(PCR)
MDA-MB-231 cells were seeded in 6-well plates at a
density of 5×105 cells/well and cultured for 24 h, and
then treated with CRAd.p or CRAd.pEgr1-TRAIL at 5 MOI. After 24 h,
the cells were irradiated with 2.0 Gy. Eight hours after
irradiation, total RNA was extracted using TRIzol reagent. The RNA
concentration and purity were determined using a spectrophotometer.
A total of 200 ng RNA was reverse-transcribed into cDNA. Real-time
fluorescent quantitative PCR was performed with the cDNA template
using the SYBR® reverse transcription (RT)-PCR kit and
the Stratagene Mx3000P Real-Time PCR System (Agilent Technologies
Inc., Santa Clara, CA, USA) according to the manufacturer’s
instructions. PCR was performed as follows: 1 cycle of 95°C for 30
sec; 40 cycles of 95°C for 20 sec and 60°C for 20 sec (fluorescence
was collected at this stage); and 1 cycle of 95°C for 1 min, 55°C
for 30 sec and 95°C for 30 sec. The primers (Table I) were synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China). For the data analysis, the
comparative threshold cycle (CT) value for GAPDH was used to
normalize the loading variations in the real-time PCR
reactions.
 | Table IPrimer sequences of TRAIL, DR5,
caspase-3 and caspase-8. |
Table I
Primer sequences of TRAIL, DR5,
caspase-3 and caspase-8.
| Gene name | Sequence no. | Primers (5′–3′) | Product length
(bp) |
|---|
| TRAIL | NM_003810 | F:
ATGGCTATGATGGAGGTCCAG
R: 5′-TTGTCCTGCATCTGCTTCAGC | 142 |
| DR5 | NM_003844.3 | F:
AAGACCCTTGTGCTCGTTGT
R: AGGTGGACACAATCCCTCTG | 133 |
| Caspase-3 | NM_032991 | F:
TTCAGGCCTGCCGTGGTACA
R: CCAAGAATAATAACCAGGTGCT | 140 |
| Caspase-8 | NM_033357 | F:
TCATCTGCTGTATCCTCTCCCAT
R: CCCTGACAAGCCTGAATAAAAAA | 154 |
Enzyme-linked immunosorbent assay (ELISA)
for TRAIL
MDA-MB-231 cells were seeded in 6-well plates at a
density of 5×105 cells/well. Following overnight
incubation, the cells were treated with CRAd.p or CRAd.pEgr1-TRAIL
at 5 MOI. After 24 h, the cells were irradiated with 2.0 Gy. Twelve
hours after irradiation, the levels of TRAIL in the supernatant of
the cell culture were determined by ELISA according to the
manufacturer’s instructions (R&D Systems, Minneapolis, MN,
USA).
Western blotting
MDA-MB-231 cells were cultured overnight in 25
cm2 culture flasks and then treated with CRAd.p or
CRAd.pEgr1-TRAIL at 5 MOI. After 24 h, the cells were irradiated
with 2.0 Gy. Twelve hours after irradiation, the cells were washed
with cold PBS and lysed in Laemmli lysis buffer. Western blot
analysis was performed as follows: A total of 60 μg denatured
protein samples were loaded onto 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels,
electroblotted onto nitrocellulose membranes and incubated with 5%
non-fat dry milk for 1 h. The membranes were then incubated with
anti-DR5, anti-caspase-3 and anti-caspase-8 antibodies overnight.
GAPDH was used as the housekeeping protein control. Following
incubation with HRP-labeled secondary antibody, the blots were
developed by enhanced chemiluminescence (ECL) procedures according
to the manufacturer’s instructions.
Statistical analysis
Differences among the groups were assessed by
analysis of variance (ANOVA) using SPSS 12.0 (SPSS Inc., Chicago,
IL, USA). The results are expressed as mean ± standard deviation
(SD). P<0.05 or P<0.01 were considered to indicate a
statistically significant difference.
Results
Recombinant adenovirus inhibits
MDA-MB-231 cells
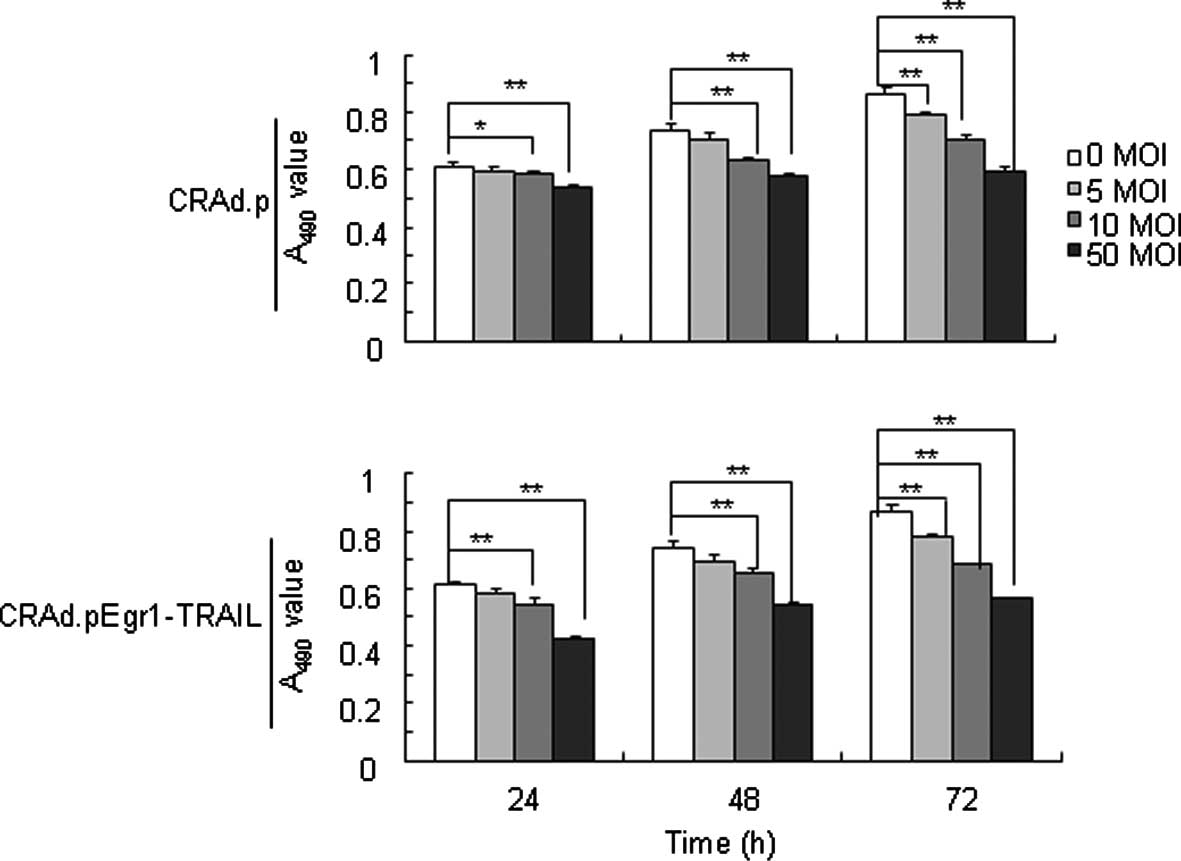
The cell-killing effect of recombinant adenovirus
was analyzed by a CCK-8 Kit. The inhibitory effect on MDA-MB-231
cells treated with CRAd.pEgr1-TRAIL and CRAd.p, respectively, at 0,
5, 10 and 50 MOIs presented a dose- and time-dependent pattern
(Fig. 2).
CRAd.pEgr1-TRAIL combined with 2.0-Gy
irradiation enhances cytotoxicity to MDA-MB-231 cells
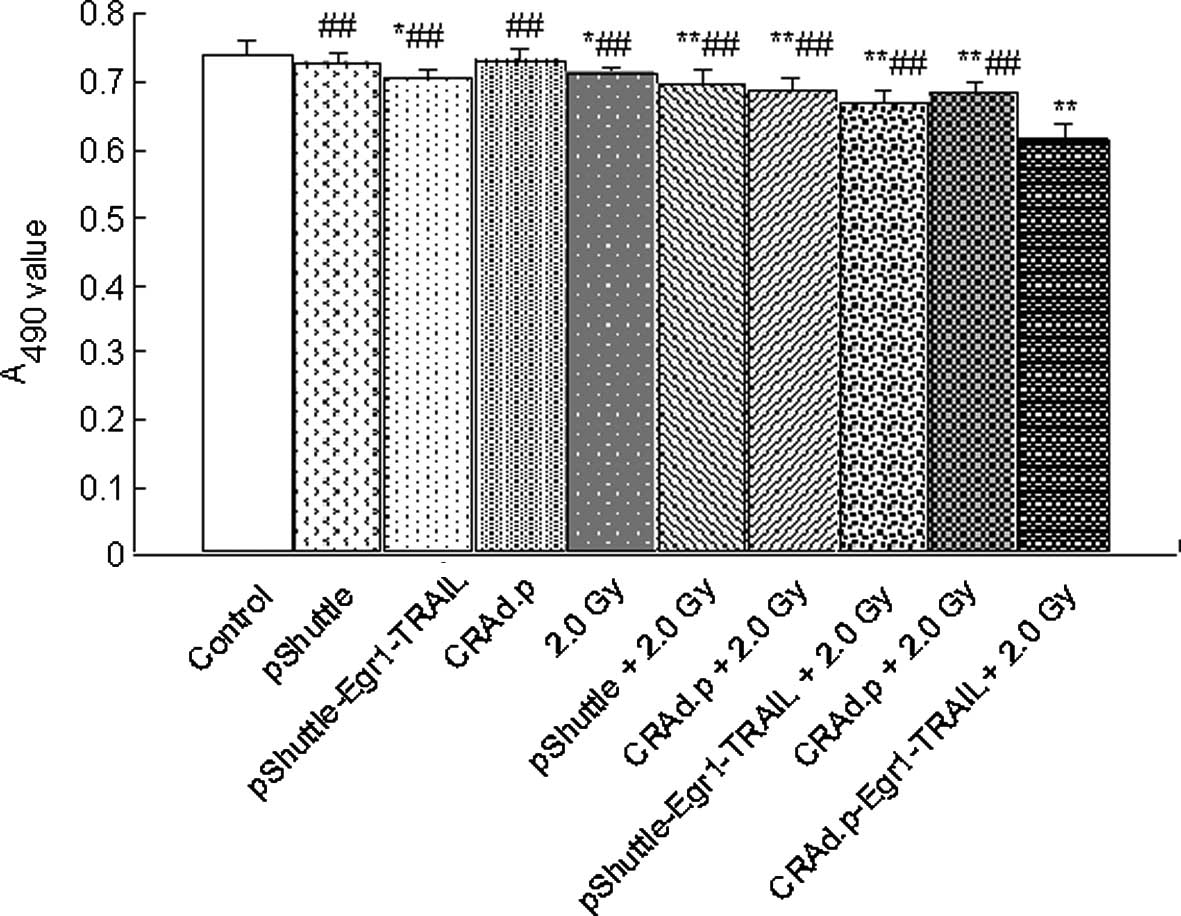
To determine whether CRAd.pEgr1-TRAIL improves
antitumor activity, MDA-MB-231 cells were treated with pShuttle,
pShuttle-Egr1-TRAIL, CRAd.p and CRAd.pEgr1-TRAIL, respectively.
Twenty-four hours after irradiation, the cell viability was
assessed by a CCK-8 Kit. MDA-MB-231 cells treated with pShuttle,
pShuttle-Egr1-TRAIL, CRAd.p and CRAd.pEgr1-TRAIL, respectively,
were inhibited compared with cells treated without virus
(P<0.01). Moreover, the cytotoxicity in the CRAd.pEgr1-TRAIL
plus 2.0-Gy irradiation group was stronger compared to that in
other groups (P<0.05 for each; Fig.
3).
Enhanced expression of TRAIL and
apoptosis in MDA-MB-231 cells induced by CRAd.pEgr1-TRAIL in
combination with 2.0-Gy irradiation
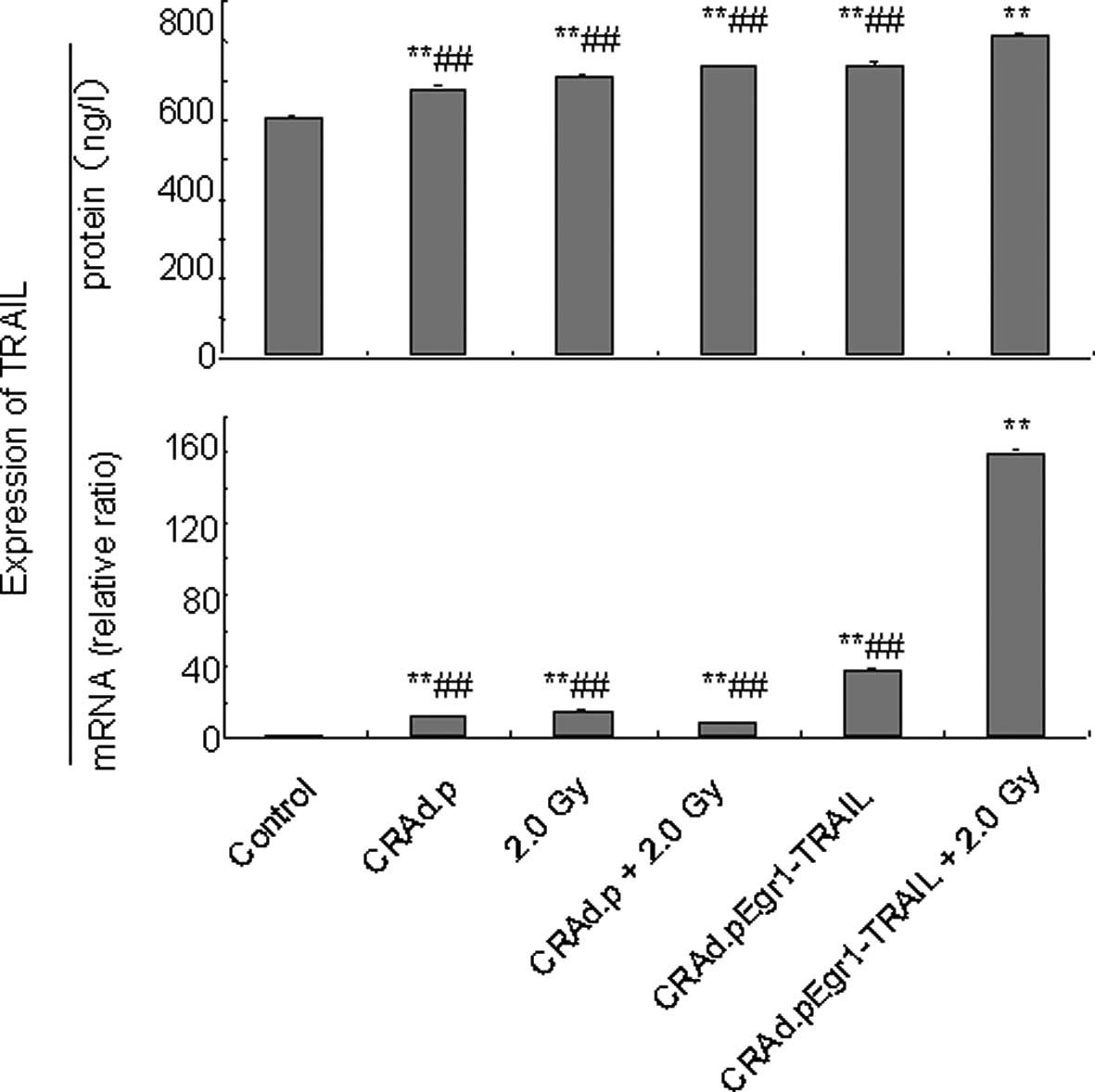
To investigate whether an enhanced expression of
TRAIL resulted in increased anticancer effects, expression of the
TRAIL gene in MDA-MB-231 cells was detected by real-time PCR and
ELISA. Fig. 4 (upper panel) shows
that the level of TRAIL mRNA expression in the CRAd.pEgr1-TRAIL
plus 2.0-Gy irradiation group was much higher compared to that in
other groups (P<0.05 for each). TRAIL mRNA expression in the
CRAd.pEgr1-TRAIL plus 2.0-Gy irradiation group was 159-fold higher
than that in the control group (P<0.01). ELISA revealed that
TRAIL protein expression increased in all groups 24 h after 2.0-Gy
irradiation and increased significantly in the CRAd.pEgr1-TRAIL
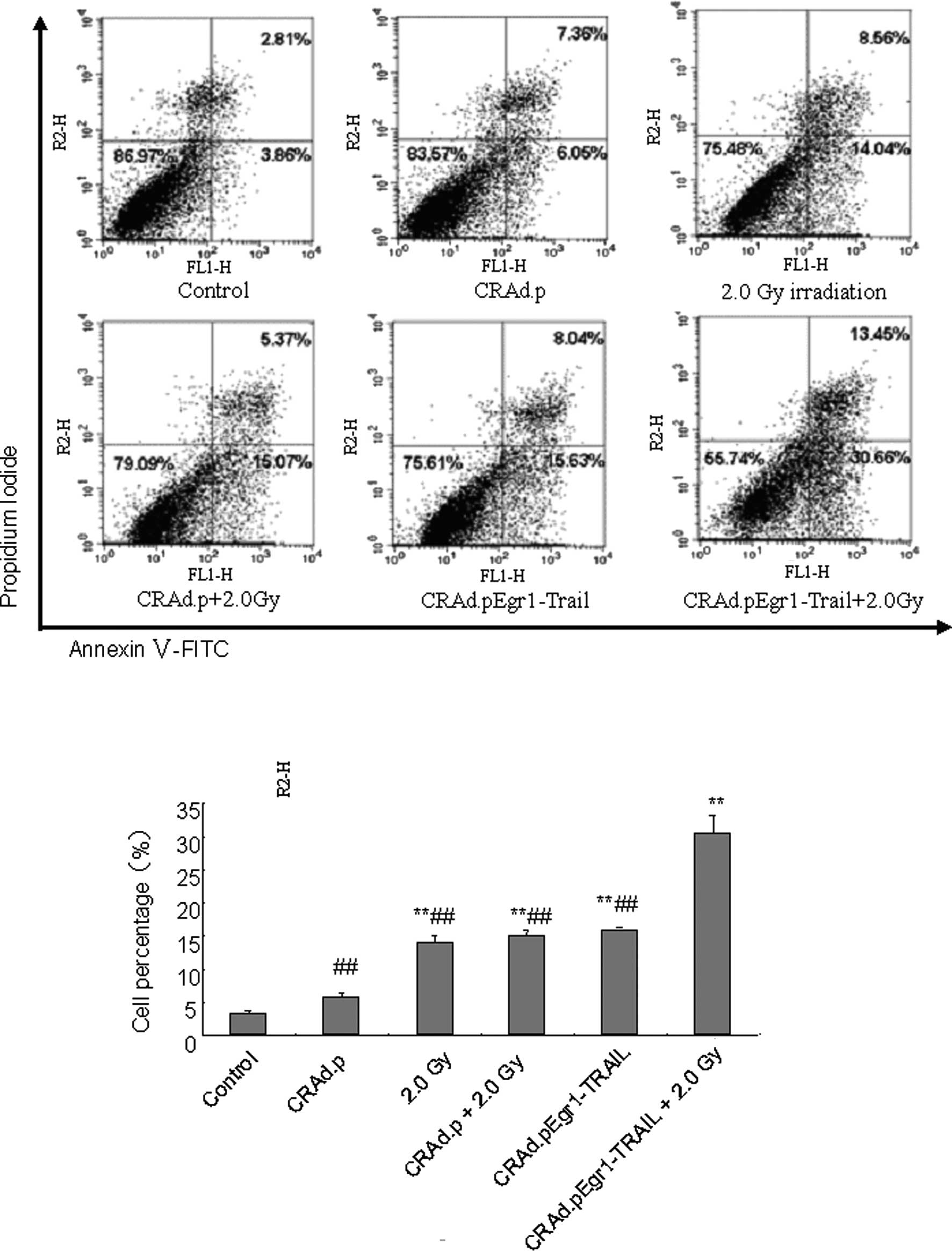
plus 2.0-Gy irradiation group (P<0.01; Fig. 4, lower panel). Cell apoptosis was
also detected by flow cytometric analysis. The percentage of cell
apoptosis significantly increased 24 h after irradiation in the
CRAd.pEgr1-TRAIL plus 2.0-Gy irradiation group (P<0.05 and
P<0.01; Fig. 5). These findings
indicate that CRAd.pEgr1-TRAIL in combination with irradiation
increases the expression of TRAIL, leading to enhanced cell
apoptosis.
Increased expression of DR5, caspase-8
and caspase-3 mRNA and protein in MDA-MB-231 cells induced by
CRAd.pEgr1-TRAIL in combination with 2.0-Gy irradiation
To elucidate the apoptotic signal transduction
pathway in MDA-MB-231 cells affected by CRAd.pEgr1-TRAIL in
combination with 2.0-Gy irradiation, real-time PCR and western blot
analysis were performed to evaluate the expression of DR5,
caspase-8 and caspase-3 mRNA and proteins in cells treated with
2.0-Gy irradiation, CRAd.p, CRAd.p plus 2.0-Gy, CRAd.pEgr1-TRAIL
and CRAd.pEgr1-TRAIL plus 2.0-Gy, respectively. Real-time PCR
analysis revealed that 8 h after treatment, the expression of DR5,
caspase-3 and caspase-8 mRNA was elevated in the irradiation
groups, particularly in the CRAd.pEgr1-TRAIL plus 2.0-Gy
irradiation group, compared to the non-irradiation groups (Fig. 6A). Western blot analysis revealed
that the expression of DR5, cleaved caspase-3 and caspase-8
proteins was upregulated following treatment with CRAd.pEgr1-TRAIL
plus 2.0-Gy irradiation (Fig. 6B).
These results suggest that CRAd.pEgr1-TRAIL in combination with
radiotherapy is involved in the apoptosis of MDA-MB-231 cells.
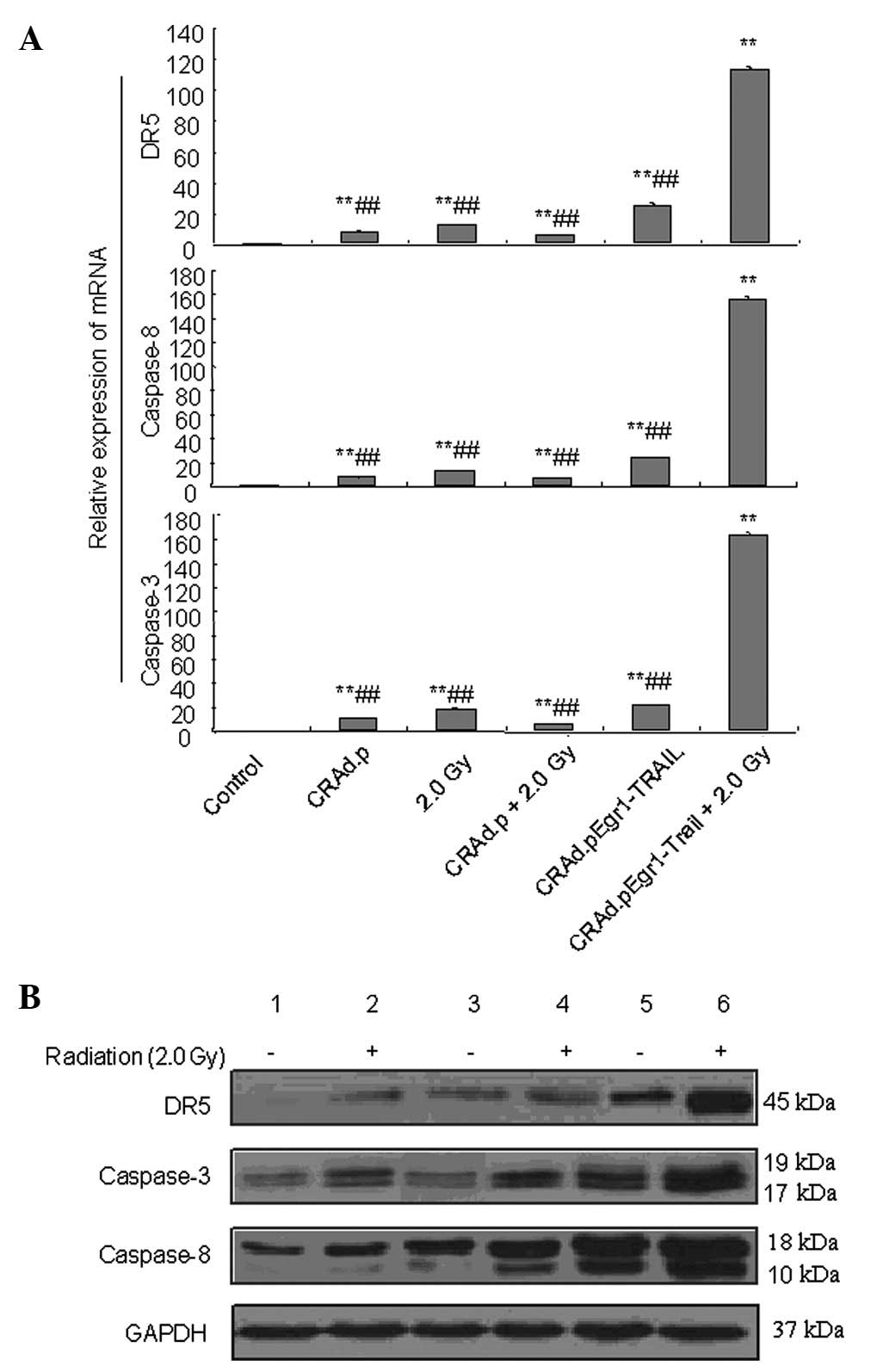 | Figure 6Expression of apoptosis-related gene
mRNA and proteins induced by CRAd.pEgr1-TRAIL and irradiation. (A)
Expression of death receptor 5 (DR5), caspase-3 and caspase-8 mRNA
in MDA-MB-231 cells treated with adenovirus 8 h after irradiation.
**P<0.01 vs. control and ##P<0.01 vs.
CRAd.pEgr1-TRAIL plus 2.0 Gy. (B) Expression of DR5, caspase-3 and
caspase-8 proteins in MDA-MB-231 cells treated with adenovirus 12 h
after irradiation. Expression of DR5, cleaved caspase-3 and
caspase-8 proteins were detected. Lanes: 1, control; 2, CRAd.p; 3,
CRAd.pEgr1-TRAIL; 4, 2.0 Gy; 5, CRAd.p plus 2.0 Gy and 6,
CRAd.pEgr1-TRAIL plus 2.0 Gy. CRAd.p, conditionally replicating
adenovirus plasmid; Egr1, early growth response-1; TRAIL, tumor
necrosis factor-related apoptosis-inducing ligand; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
Breast cancer is a type of malignant tumor that
constitutes a threat to human health (24). Traditional methods of treating
tumors are surgery, radiotherapy, chemotherapy and biological
therapy; however, single treatment often has its own limitations.
The development of molecular biology has led to the rapid
progression of gene therapy in breast cancer, resulting in good
curative effect. Tumor gene-radiotherapy implements the organic
integration of radiotherapy and gene therapy, and achieves a
superposition or synergism. Ionizing radiation has the effect of
targeting and regulating the temporal and spatial expression of
anti-oncogenes (25,26). Usually, the effect of this combined
therapy is obtained by the Egr1 promoter and the targeting effect
to kill tumor cells may be increased by CRAd. There are two main
pathways in the targeting strategy of CRAd: i) to delete the genes
unwanted in tumor cells and wanted in normal cells (27) and ii) to regulate gene expression
by the tumor-specific promoter. Additionally, the adenovirus has a
cytotoxic effect, increasing the effect of irradiation on the
breakdown of tumor cells.
In the present study, the TRAIL gene was constructed
downstream of the Egr1 promoter, which has the effect of double
targeting and replication of the CRAd vector,
pShuttle-Egr1-TRAIL-hTERT-E1A-E1Bp-E1B55K, in tumor cells. The
adenovirus with the CR2 region-deleted E1A gene only replicates
under the upregulation of telomerase activity and the mutation or
inactivation of the Rb gene in tumor cells. The broad-spectrum
hTERT promoter was placed upstream of the E1A gene to regulate the
expression of the E1A gene. The selection of target genes to induce
apoptosis of tumor cells in tumor gene-radiotherapy is extremely
important. The TRAIL gene induces the apoptosis of tumor cells by
activating the DR, but does not cause toxic effect to normal cells.
In the present study, the recombinant virus plasmid was
successfully packaged and amplified in HEK293 cells, producing a
stable CRAd.
Singh et al determined the sensitivity of
TRAIL to the apoptosis of several breast cancer cell lines,
suggesting that MDA-MB-231 cells are extremely sensitive to the
induction of TRAIL, resulting in apoptosis (28). Therefore, in the present study,
human breast cancer MDA-MB-231 cells were selected to explore the
suppressing effect of the virus in combination with irradiation. In
the present study, the tumor-killing effect in the CRAd.pEgr1-TRAIL
group was significantly higher compared to that in the pShuttle,
pShuttle-Egr1-TRAIL and CRAd.p groups. This finding suggests that
the anticancer effect of the recombinant CRAd with the Egr1
promoter and TRAIL gene is better than that of pShuttle-Egr1-TRAIL
and adenovirus vector alone.
Apoptosis is a crucial pathway of tumor cell death
following radiotherapy, chemotherapy or apoptotic gene expression.
In the present study, the rate of cell apoptosis following
irradiation increased in each group. Specifically, the rate of
apoptosis in the CRAd.pEgr1-TRAIL plus 2.0-Gy irradiation group was
significantly different to that in the other groups (P<0.05 or
P<0.01). This indicates that the apoptosis-promoting effect of
CRAd.pEgr1-TRAIL plus 2.0-Gy irradiation was better compared to
that of simple radiotherapy, simple gene therapy and radiotherapy
combined with the adenovirus vector. These results also suggest
that the TRAIL gene regulated by Egr1 induces tumor cell apoptosis,
exhibiting a synergism for killing tumor cells when combined with
ionizing irradiation.
Cell apoptosis is an intricate process, involving a
number of apoptotic signaling pathways and is regulated by a series
of genes and proteins. Furthermore, the abnormal expression of
these mRNAs and proteins are earlier than the occurrence of cell
apoptosis. In order to reveal the regulatory effect of
irradiation-induced expression and the anti-tumor mechanism of
CRAd.pEgr1-TRAIL in MDA-MB-231 cells, we detected the mRNA and
protein levels of related genes in the DR pathway. The results
revealed that the expression of TRAIL, DR5, caspase-8 and caspase-3
mRNA and protein in MDA-MB-231 cells of each group increased
following 2.0-Gy irradiation, which was significantly higher in the
CRAd.pEgr1-TRAIL plus 2.0-Gy irradiation group. The mRNA expression
occurred earlier than the protein expression. Therefore, we
hypothesized that TRAIL is a secreted protein and its secretion
process is completed after transmembrane appearance when the signal
peptide is excised with signal peptidase. Thus, the peak value of
detected TRAIL protein should be later than that of the other three
types of proteins. The expression levels of TRAIL, DR5, caspase-8
and caspase-3 mRNA and protein in the CRAd.pEgr1-TRAIL plus 2.0-Gy
irradiation group increased and was maintained constantly at a
higher level. This result indicates that CRAd.pEgr1-TRAIL combined
with radiotherapy induces apoptosis. On the basis of these results,
we consider that ionizing radiation activated the Egr1 promoter,
which induced the sustained expression of TRAIL mRNA and protein,
promoting apoptosis in tumor cells. Simultaneously, the TRAIL gene
in combination with irradiation enhances the expression of DR5,
caspase-8 and caspase-3 mRNA and protein, promoting apoptosis.
In conclusion, we constructed the
pShuttle-TRAIL-hTERT-E1A (CR2)-E1Bp-E1B55K with double targeting to
tumor cells and packaged it into CRAd with the Egr1 promoter and
TRAIL gene. This recombinant adenovirus was able to target
proliferation and radiation-enhanced gene expression in tumor
cells, as well as promote the apoptosis of MDA-MB-231 cells when
combined with ionizing radiation. These results provide
experimental basis for the clinical application of tumor
gene-radiotherapy and provides confirmation for use of combined
therapy in tumors.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (nos. 30870747 and 30970681).
References
|
1
|
Weichselbaum RR, Kufe DW, Advani SJ and
Roizman B: Molecular targeting of gene therapy and radiotherapy.
Acta Oncol. 40:735–738. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sukhatme VP, Cao XM, Chang LC, et al: A
zinc finger-encoding gene coregulated with c-fos during growth and
differentiation, and after cellular depolarization. Cell. 53:37–43.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manente AG, Pinton G, Tavian D,
Lopez-Rodas G, Brunelli E and Moro L: Coordinated sumoylation and
ubiquitination modulate EGF induced EGR1 expression and stability.
PLoS One. 6:e256762011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du N, Pei XT, Zhou JM, Zhao H, Li XS, Fu Y
and Hao YX: Transcriptional control of Flt3 ligand targeted by
fluorouracil-induced Egr-1 promoter in hematopoietic damage. J
Biomed Sci. 16:852009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marignol L, Coffey M, Hollywood D and
Lawler M: Radiation to control transgene expression in tumors.
Cancer Biol Ther. 6:1005–1012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weichselbaum RR, Hallahan DE, Beckett MA,
Mauceri HJ, Lee H, Sukhatme VP and Kufe DW: Gene therapy targeted
by radiation preferentially radiosensitizes tumor cells. Cancer
Res. 54:4266–4269. 1994.PubMed/NCBI
|
|
7
|
Weichselbaum RR, Halahan DE, Sukhatme VP
and Kufe DW: Gene therapy targeted by ionizing radiation. Int J
Radiat Oncol Biol Phys. 24:565–567. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weichselbaum RR: Growth factors alter the
therapeutic ratio in radiotherapy. Cancer J Sci Am. 1:28–29.
1995.PubMed/NCBI
|
|
9
|
Weichselbaum RR and Kufe D: Gene therapy
of cancer. Lancet. 349:51110–51112. 1997. View Article : Google Scholar
|
|
10
|
Yang SW, Chanda D, Cody JJ, Rivera AA,
Waehler R, Siegal GP, Douglas JT and Ponnazhagan S: Conditionally
replicating adenovirus expressing TIMP2 increases survival in a
mouse model of disseminated ovarian cancer. PLoS One. 6:e251312011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng FQ, Xu Y, Yang RJ, Wu B, Tan XH, Qin
YD and Zhang QW: Combination effect of oncolytic adenovirus therapy
and herpes simplex virus thymidine kinase/ganciclovir in hepatic
carcinoma animal models. Acta Pharmacol Sin. 30:617–627. 2009.
View Article : Google Scholar
|
|
12
|
Yu W and Fang H: Clinical trials with
oncolytic adenovirus in China. Curr Cancer Drug Targets. 7:141–148.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang SW, Cody JJ, Rivera AA, Waehler R,
Wang M, Kimball KJ, Alvarez RA, Siegal GP, Douglas JT and
Ponnazhagan S: Conditionally replicating adenovirus expressing
TIMP2 for ovarian cancer therapy. Clin Cancer Res. 17:538–549.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barnes MN, Coolidge CJ, Hemminki A,
Alvarez RD and Curiel DT: Conditionally replicative adenoviruses
for ovarian cancer therapy. Mol Cancer Ther. 1:435–439.
2002.PubMed/NCBI
|
|
15
|
Gómez-Navarro J and Curiel DT:
Conditionally replicative adenoviral vectors for cancer gene
therapy. Lancet Oncol. 1:148–158. 2000.
|
|
16
|
Paupoo AA, Zhu ZB, Wang M, Rein DT,
Starzinski-Powitz A and Curiel DT: A conditionally replicative
adenovirus, CRAd-S-pK7, can target endometriosis with a
cell-killing effect. Hum Reprod. 25:2068–2083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kimball KJ, Preuss MA, Barnes MN, et al: A
phase I study of a tropism-modified conditionally replicative
adenovirus for recurrent malignant gynecologic diseases. Clin
Cancer Res. 16:5277–5287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wiley SR, Schooley K, Smolak PJ, et al:
Identification and characterization of a new member of the TNF
family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Walczak H, Miller RE, Ariail K, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gores GJ and Kaufmann SH: Is TRAIL
hepatotoxic? Hepatology. 34:3–6. 2001. View Article : Google Scholar
|
|
21
|
Wang P, Song JH, Song DK, Zhang J and Hao
C: Role of death receptor and mitochondrial pathways in
conventional chemotherapy drug induction of apoptosis. Cell Signal.
18:1528–1535. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mahalingam D, Szegezdi E, Keane M, de Jong
S and Samali A: TRAIL receptor signalling and modulation: are we on
the right TRAIL? Cancer Treat Rev. 35:280–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kruyt FA: TRAIL and cancer therapy. Cancer
Lett. 263:14–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smith RA, Cokkinides V, Brooks D, Saslow
D, Shah M and Brawley OW: Cancer screening in the United States,
2011: a review of current American Cancer Society guidelines and
issues in cancer screening. CA Cancer J Clin. 61:8–30. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marples B, Greco O, Joiner MC and Scott
SD: Radiogenetic therapy: strategies to overcome tumor resistance.
Curr Pharm Des. 9:2105–2112. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahmed MM: Regulation of radiation-induced
apoptosis by early growth response-1 gene in solid tumors. Curr
Cancer Drug Targets. 4:43–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Crompton AM and Kim DH: From ONYX-015 to
armed vaccinia viruses: the education and evolution of oncolytic
virus development. Curr Cancer Drug Targets. 7:133–139. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh TR, Shankar S, Chen X, Asim M and
Srivastava RK: Synergistic interactions of chemotherapeutic drugs
and tumor necrosis factor-related apoptosis-inducing ligand/Apo-2
ligand on apoptosis and on regression of breast carcinoma in vivo.
Cancer Res. 63:5390–5400. 2003.
|