Introduction
Cardiovascular disease (CVD) is a major cause of
premature mortality, accounting for approximately one-third of
mortalities (1,2). Each year, 17 million individuals
succumb to CVD (3–5). Osteoporosis is a serious public
health problem with an estimated worldwide incidence of >200
million (4,6). CVD and osteoporosis are the main
causes of mortality in the elderly (7,8).
Vascular calcification, as an independent risk factor for CVD, was
originally considered to be a passive and unregulated process;
however, this process is now known to be an active and tightly
regulated phenomenon, in which a variety of osteogenic regulatory
factors are involved (9,10). Clinical studies have demonstrated
that the majority of patients with osteoporosis have vascular
calcification (11), and that
vascular calcification is alleviated following treatment with
bisphosphonates (3,12–14).
These studies are indicative of a correlation between arterial and
bone pathologies (15).
Alendronate, a bisphosphonate, is widely used as a
treatment for osteoporosis and other pathological conditions
associated with bone loss (16–18).
In addition, a number of studies have shown that alendronate is not
only suitable for the treatment of the aforementioned diseases, but
also for vascular calcification (3). Analysis of the mechanism by which
alendronate inhibits vascular calcification is likely to clarify
the regulatory mechanisms involved in arterial and bone
pathologies.
Notch1 signaling plays a crucial role in cell fate
determination and various developmental processes, and translates
cell-cell interactions into specific transcriptional programs
(19–23). In the transcriptional process,
cell-cell interactions cause cleavage of the Notch intracellular
domain (NICD), which migrates into the nucleus and associates with
RBP-Jκ, further activating the transcription of target genes
(22,23). Msx2, the target gene activated by
the Notch1-RBP-Jκ signaling pathway, is considered to represent a
key regulator of vascular calcification and has been identified as
a homeodomain transcription factor responsible for osteoblast
differentiation and mineralization (9,22,24,25).
However, to date, a limited number of studies have
focused on the effect of alendronate on the expression of the
Notch1-RBP-Jκ signaling pathway. In the present study, an in
vitro rat model of vascular calcification was used to determine
the effect of alendronate on expression of the Notch1-RBP-Jκ
signaling pathway, and the possible mechanisms behind the
inhibition of artery calcification by alendronate were
explored.
Materials and methods
Cell culture
Primary vascular smooth muscle cells (VSMCs) were
isolated from the thoracic aortas of Sprague-Dawley rats (4 weeks
old; male; Animal Center, Tongji Medical College, Wuhan, China),
maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10%
FBS, 100 U/ml penicillin and 100 mg/ml streptomycin and incubated
in 75-cm2 tissue culture flasks at a density of
1×104 cells/ml. VSMCs at passages 5 and 6 were used for
subsequent experiments. The study was approved by the ethics
committee of the Institutional Animal Care and Use Committee at
Tongji Medical College, Huazhong University of Science and
Technology, Wuhan, China.
Osteoblastic differentiation was induced by
culturing cells in osteogenic medium containing 50 mg/ml
ascorbate-2-phosphate and 10 mmol/l β-glycerol phosphate. DMEM was
used as a control. N-[N-(3,5-difluorophenacetyl-L-alanyl)]-S-phen-
yl-glycinet-butyl ester (DAPT; 10 mmol/l), a potent γ-secretase
inhibitor, was used as a positive control for inhibition of the
Notch1-RBP-Jκ-dependent signaling pathway (26). VSMCs were also cultured in the
presence of various concentrations of alendronate (10−8,
10−6, 10−4 and 10−3 mmol/l). After
14 days, cultured VSMCs were subjected to the following
experimental conditions in 7 groups: the control (VSMCs cultured
with DMEM); osteogenic (OS; VSMCs cultured with osteogenic medium);
and DAPT groups (VSMCs cultured with OS and DAPT); and the
alendronate groups (VSMCs cultured with OS and alendronate), which
were divided into four subgroups based on varying concentrations of
alendronate (10−8, 10−6, 10−4 and
10−3 mmol/l). The medium was replaced with fresh medium
every 2 days. For time-course experiments, the first day of culture
in calcification medium was defined as day 0.
von Kossa staining
Cells in culture flasks were washed 3 times with
PBS, followed by a fixation with 4% paraformaldehyde for 15 min.
Next, cells were washed 3 times with distilled water, incubated
with 5% silver nitrate solution and exposed to bright sunlight for
30 min, then washed with distilled water for 5 min and treated with
5% sodium thiosulfate for 2 min. Calcium particles were observed by
microscope in visual fields (magnification, ×40).
Measurement of calcium content
Cultures were decalcified with 0.6 mol/l HCl for 24
h. The calcium content of the HCl supernatant was determined by the
o-cresolphthalein complexone method (calcium kit; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). Following
decalcification, the cells were washed with PBS and solubilized
with 0.1 mol/l NaOH-0.1% SDS. The cells were dissolved in
HNO3 and then dried in an oven and redissolved with
blank solution (27 nmol/l KCl and 27 μmol/l LaCl3 in
deionized water). Calcium content was measured using an atomic
absorption spectrophotometer (SpectrAA-240 FS; Agilent
Technologies, Santa Clara, CA, USA) at 422.7 nm.
Alkaline phosphatase (ALP) assay
ALP activity of various cells was measured using a
Lab Assay ALP kit (Nanjing Jiancheng Bioengineering Institute),
according to the manufacturer’s instructions.
Real-time quantitative PCR
Total RNA was isolated from the VSMCs using the
TRIzol chloroform method, according to the manufacturer’s
instructions (Invitrogen Life Technologies, Carlsbad, CA, USA), and
reverse transcribed into cDNA with a Toyoba reverse transcription
kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). Real-time
quantitative PCR was performed using the ABI PRISM 7900 sequence
detector system (Applied Biosystems, Foster City, CA, USA),
according to the manufacturer’s instructions. β-actin was used as
an endogenous control. The PCR mixture contained SYBR
Green/Fluorescein qPCR Master Mix (2X; Thermo Fisher Scientific
Inc.), cDNA and the primers. Relative gene expression levels (the
amount of target, normalized against an endogenous control gene)
were calculated using the comparative Ct method formula,
2−ΔΔCt. The following primer sequences were used for
real-time quantitative PCR: rat Notch1 forward,
5′-GAGGCTTGAGATGCTCCCAG-3′ and reverse,
5′-ATTCTTACATGGTGTGCTGAGG-3′; rat RBP-Jκ forward,
5′-GAGCCATTCTCAGAGCCAAC-3′ and reverse, 5′-TCCCCAAGAAACCACAAAAG-3′;
rat msx2 forward, 5′-AAGGCAAAAAGACTGCAGGA-3′ and reverse,
5′-GGATGGGAAGCACAGGTCTA-3′; and β-actin forward,
5′-CACGATGGAGGGGCCGGACTCATC-3′ and reverse,
5′-TAAAGACCTCTATGCCAACACAGT-3′
Western blot analysis
Total protein was extracted from cultured VSMCs in
radio immunoprecipitation assay buffer containing 50 mmol/l Tris,
150 mmol/l NaCl, 0.1% SDS, 0.5% sodium deoxycholate and 1% Triton
X-100 in the presence of aprotinin, PMSF, okadaic acid and
leupeptin. Total protein (50 μg/sample) was loaded onto 12%
SDS-polyacrylamide gels and separated at 100 V, followed by
transfer to PVDF membranes at 200 mA and 4°C for 70 and 110 min for
Notch1 and Msx2, and RBP-Jκ signals, respectively. Membranes were
blocked in 5% non-fat milk in 0.1 mol/l PBS (pH 7.4) at room
temperature for 2 h and then incubated with the following primary
antibodies: goat anti-Notch1 polyclonal (1:400), rabbit anti-RBP-Jκ
polyclonal (1:300) or goat anti-Msx2 polyclonal (1:400; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Following washing,
membranes were incubated in HRP-conjugated rabbit anti-goat or goat
anti-rabbit secondary antibodies (1:40,000; Wuhan Boster Biological
Technology Ltd., Wuhan, Hubei, China) for 2 h at room temperature,
followed by washing and 5 min incubation with enhanced
chemiluminescence reagents. The membranes were stripped and equal
protein loading was determined by GAPDH expression using a mouse
monoclonal antibody (1:75,000; GoodHere Biological Technology,
Ltd., Hangzhou, China).
Statistical analysis
Data are presented as the mean ± SEM. Significant
differences were estimated by ANOVA followed by
Student-Newman-Keuls multiple comparison tests. P≤0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS software (version
17.0; SPSS Inc., Chicago, IL, USA).
Results
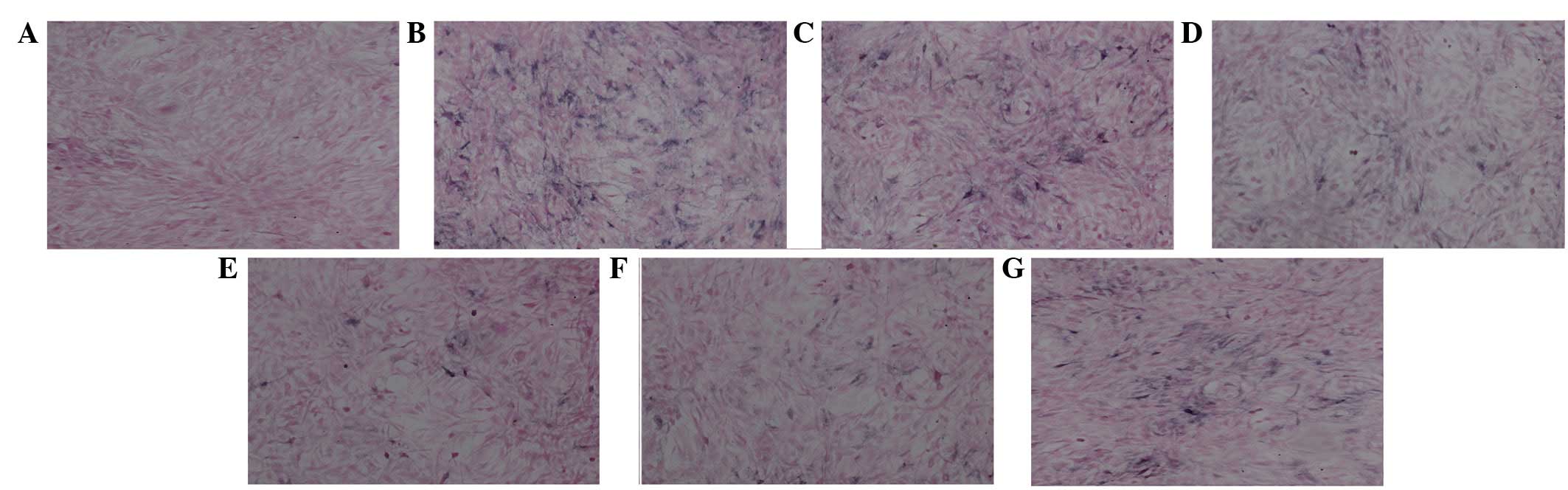
von Kossa staining for VSMC
calcification
To determine the calcification of VSMCs, von Kossa
staining was performed and the results showed that no calcification
was detected in the control group (Fig. 1A), while a marked positive staining
(black) was detected in VSMCs cultured in osteogenic medium
(Fig. 1B), indicating increased
calcification in VSMCs. Increased calcification was eradicated
significantly by DAPT treatment (Fig.
1G). The calcification of VSMCs was markedly reduced in the
presence of alendronate in a dose-dependent manner (Fig. 1C-F) compared with those in
osteogenic medium only (Fig. 1B),
indicating that alendronate and DAPT repressed the calcification of
VSMCs induced by osteogenic media.
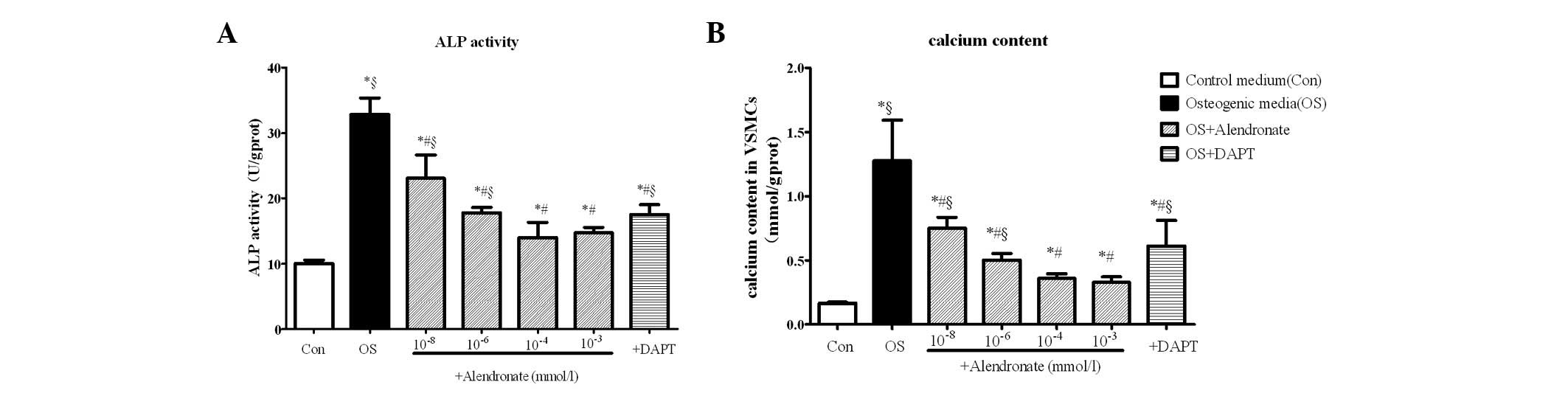
ALP activity and calcium content of
VSMCs
To further determine the calcification of VSMCs, ALP
activity and calcium content were analyzed. The results showed that
ALP activity (Fig. 2A) and calcium
content (Fig. 2B) were
significantly increased in VSMCs treated with osteogenic media
(P<0.05 vs. control). Again, the osteogenic conversion of VSMCs
was repressed by DAPT and reduced in the presence of alendronate in
a dose-dependent manner (P<0.01 vs. the osteogenic group). ALP
activity and calcium content reached minimum levels at
10−4 mmol/l alendronate (P<0.05 vs. the osteogenic
group; Fig. 2).
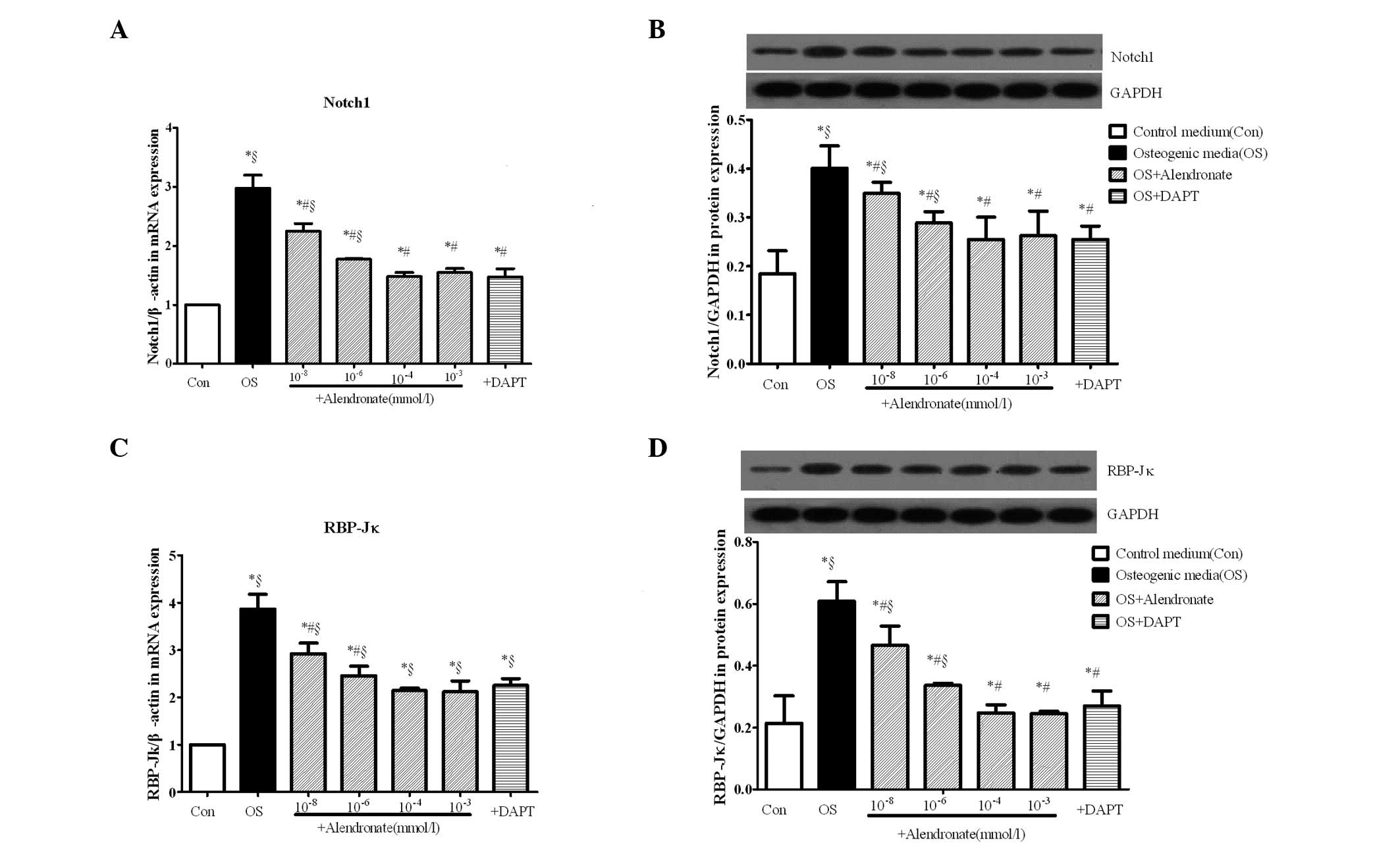
Alendronate downregulates the
Notch1-RBP-Jκ-dependent signaling pathway
mRNA and protein levels of Notch1 (Fig. 3A and B) and RBP-Jκ (Fig. 3C and D) in VSMCs were measured by
real-time RT-PCR and western blot analysis. As shown in Fig. 3, Notch1 and RBP-Jκ were
significantly increased at the mRNA and protein levels in the
osteogenic group compared with controls (P<0.05), indicating
that the Notch1-RBP-Jκ signaling pathway was activated in the
osteogenic conversion of VSMCs.
To determine the effects of alendronate on
expression of the Notch1-RBP-Jκ signaling pathway in vitro,
four concentrations (10−8, 10−6,
10−4 and 10−3 mmol/l) of alendronate were
added to osteogenic VSMCs and DAPT was added as a positive control
for inhibition of the Notch1-RBP-Jκ signaling pathway. The results
showed that alendronate reduced the expression of Notch1 and RBP-Jκ
compared with the osteogenic media group in a dose-dependent
manner, and the expression of Notch1 and RBP-Jκ reached minimum
levels at a dose of 10−4 mmol/l (P<0.05 vs. the
osteogenic and 10−4 mmol/l alendronate groups).
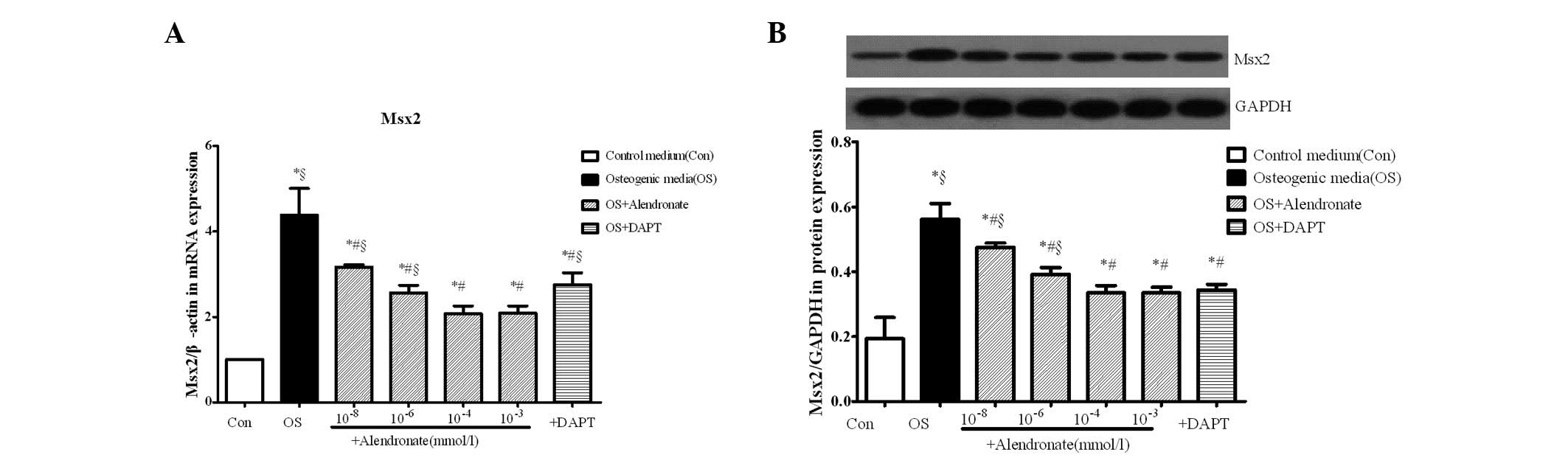
Alendronate downregulates the expression
of Msx2
The expression of Msx2 was measured by real-time
RT-PCR and western blot analysis. The mRNA and protein levels of
Msx2 were significantly increased in the osteogenic group compared
with the control group (P<0.05), indicating that VSMCs were
transformed to osteoblast-like cells. Results in the alendronate
group showed that the transformation regulated by Msx2 was
inhibited in an alendronate dose-dependent manner compared with the
osteogenic media group (P<0.05). The expression of Msx2 in the
DAPT group, as the negative control, was inhibited, which indicated
that inhibition of the Notch1-RBP-Jκ signaling pathway decreased
the expression of Msx2 (P<0.05 vs. the osteogenic group;
Fig. 4).
Discussion
Osteoporosis and vascular calcification share a
number of common risk factors and pathogenetic mechanisms,
involving proteins, hormones, elements, lipids and vitamins
(27). Previous studies have
indicated that bone morphogenetic proteins, the RANKL/RANK/OPG and
Wnt signaling pathways, matrix Gla protein, vitamins K and D,
osteopontin and the renin-angiotensin-aldosterone system are
involved in this process (2,23).
In addition, the Notch signaling pathway plays a key role in the
development and homeostasis of the cardiovascular system and bone
(19,23,29–32).
VSMC fate decisions, including cell growth,
migration and apoptosis, are fundamental features in the
pathogenesis of vascular disease. The signaling pathways that
regulate VSMC growth, death, differentiation and migration are the
focus of regulation and control targets, and recent studies have
attempted to clarify the pathway for osteoblastic transformation of
VSMCs, including the Wnt signaling pathway (28,33–35).
The Notch signaling pathway is also important and the Notch1
signaling pathway has been reported to induce the osteogenic
differentiation and mineralization of VSMCs (22). The Notch1 signaling pathway
promotes the osteogenic differentiation and mineralization of VSMCs
by direct activation of Msx2 gene transcription via RBP-Jκ
(22,36,37).
RBP-Jκ, a major mediator of Notch1 signaling, binds NICDs and forms
a complex that further activates the transcription of target genes
from their cognate DNA binding sequence in the nucleus (21,22,37).
Msx2, the downstream target gene of the
Notch1-RBP-Jκ signaling pathway, is a key regulatory osteogenic
factor of vascular calcification. Decreased levels of Msx2 imply
that the osteogenic differentiation of VSMCs is inhibited (22,38).
In the present study, the expression of Notch1 and RBP-Jκ was
increased in osteogenic VSMCs, consistent with the increased
intracellular calcium deposition (P<0.05). The osteogenic
effects on VSMCs, including increased Msx2, enhanced ALP activity
and the deposition of calcium in VSMCs, were eradicated by DAPT, an
inhibitor of the Notch1 signaling pathway (26), indicating that the Notch1-RBP-Jκ
signaling pathway contributes to the calcification of VSMCs
(P<0.05).
In clinical medicine, alendronate has been
successfully developed as a treatment for osteoporosis and other
pathological conditions associated with bone loss (6,13,29).
Clinical studies have shown that alendronate may also be used for
treating vascular calcification (3,4,25).
In addition, alendronate inhibits the calcification of arteries and
valves without affecting serum levels of calcium or phosphate
(12). Results of the present
study demonstrated that alendronate represses VSMC calcification
via downregulation of the Notch1-RBP-Jκ signaling pathway in
vitro in a dose-dependent manner. The expression of Msx2, the
downstream target of the Notch1-RBP-Jκ signaling pathway, was
repressed in a dose-dependent manner. These observations indicate
that osteogenic conversion of VSMCs may be repressed by alendronate
through downregulation of the Notch1-RBp-Jκ signaling pathway.
In summary, results of this study indicate that
VSMCs differentiate into osteoblast-like cells by upregulating the
Notch1-RBP-Jκ signaling pathway, and osteogenic transformation
leads to vascular calcification. Alendronate was found to inhibit
osteogenic transformation regulated by this pathway to protect
arteries. Alendronate, a drug that has been found to protect the
arteries and bones, is likely to be important for the prevention of
CVD and osteoporosis to preserve the quality of life of elderly
individuals.
References
|
1
|
United Nations. World Population Ageing.
New York, USA: 2009
|
|
2
|
World Health Report. World Health
Organization; Geneva: 2002
|
|
3
|
Santos LL, Cavalcanti TB and Bandeira FA:
Vascular effects of bisphosphonates - a systematic review. Clin Med
Insights Endocrinol Diabetes. 5:47–54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lampropoulos CE, Papaioannou I and D’Cruz
DP: Osteoporosis - a risk factor for cardiovascular disease? Nat
Rev Rheumatol. 8:587–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perrini S, Natalicchio A, Laviola L, et
al: Abnormalities of insulin-like growth factor-I signaling and
impaired cell proliferation in osteoblasts from subjects with
osteoporosis. Endocrinology. 149:1302–1313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mithal A: Osteoporosis in Asia: a call to
action. Osteoporos Int. 23:S739–S740. 2012.
|
|
7
|
Yesil Y, Ulger Z, Halil M, et al:
Coexistence of osteoporosis (OP) and coronary artery disease (CAD)
in the elderly: It is not just a by chance event. Arch Gerontol
Geriatr. 54:473–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rhee EJ, Lee WY, Kim SY, et al:
Relationship of serum osteoprotegerin levels with coronary artery
disease severity, left ventricular hypertrophy and C-reactive
protein. Clin Sci (Lond). 108:237–243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vattikuti R and Towler DA: Osteogenic
regulation of vascular calcification: an early perspective. Am J
Physiol Endocrinol Metab. 286:E686–E696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giachelli CM: Vascular calcification
mechanisms. J Am Soc Nephrol. 15:2959–2964. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hofbauer LC, Brueck CC, Shanahan CM,
Schoppet M and Dobnig H: Vascular calcification and osteoporosis -
from clinical observation towards molecular understanding.
Osteoporos Int. 18:251–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Price PA, Faus SA and Williamson MK:
Bisphosphonates alendronate and ibandronate inhibit artery
calcification at doses comparable to those that inhibit bone
resorption. Arterioscler Thromb Vasc Biol. 21:817–824. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Skolnick AH, Osranek M, Formica P and
Kronzon I: Osteoporosis treatment and progression of aortic
stenosis. Am J Cardiol. 104:122–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ylitalo R, Kalliovalkama J, Wu XM, et al:
Accumulation of bisphosphonates in human artery and their effects
on human and rat arterial function in vitro. Pharmacol Toxicol.
83:125–131. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
London GM: Bone-vascular cross-talk. J
Nephrol. 25:619–625. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boanini E, Torricelli P, Gazzano M, Fini M
and Bigi A: The effect of alendronate doped calcium phosphates on
bone cells activity. Bone. 51:944–952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keam SJ and Plosker GL: Prevention and
treatment of osteoporosis in postmenopausal women. Dis Manag Health
Outcome. 12:19–37. 2004. View Article : Google Scholar
|
|
18
|
Iwamoto J, Takeda T, Sato Y and Uzawa M:
Effects of alendronate on metacarpal and lumbar bone mineral
density, bone resorption, and chronic back pain in postmenopausal
women with osteoporosis. Clin Rheumatol. 23:383–389. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Manderfield LJ, High FA, Engleka KA, et
al: Notch activation of Jagged1 contributes to the assembly of the
arterial wall. Circulation. 125:314–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurpinski K, Lam H, Chu JL, et al:
Transforming growth factor-beta and notch signaling mediate stem
cell differentiation into smooth muscle cells. Stem Cells.
28:734–742. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morimoto M, Liu ZY, Cheng HT, Winters N,
Bader D and Kopan R: Canonical Notch signaling in the developing
lung is required for determination of arterial smooth muscle cells
and selection of Clara versus ciliated cell fate. J Cell Sci.
123:213–224. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimizu T, Tanaka T, Iso T, et al: Notch
signaling induces osteogenic differentiation and mineralization of
vascular smooth muscle cells: role of Msx2 gene induction via
Notch-RBP-Jk signaling. Arterioscler Thromb Vasc Biol.
29:1104–1111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morrow D, Guha S, Sweeney C, et al: Notch
and vascular smooth muscle cell phenotype. Circ Res. 103:1370–1382.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iyemere VP, Proudfoot D, Weissberg PL and
Shanahan CM: Vascular smooth muscle cell phenotypic plasticity and
the regulation of vascular calcification. J Intern Med.
260:192–210. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sage AP, Tintut Y and Demer LL: Regulatory
mechanisms in vascular calcification. Nat Rev Cardiol. 7:528–536.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang M, Wu L, Wang L and Xin X:
Down-regulation of Notch1 by gamma-secretase inhibition contributes
to cell growth inhibition and apoptosis in ovarian cancer cells
A2780. Biochem Biophys Res Commun. 393:144–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Price PA, June HH, Buckley JR and
Williamson MK: Osteoprotegerin inhibits artery calcification
induced by warfarin and by vitamin D. Arterioscler Thromb Vasc
Biol. 21:1610–1616. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xin H, Xin F, Zhou S and Guan S: The
Wnt5a/Ror2 pathway is associated with determination of the
differentiation fate of bone marrow mesenchymal stem cells in
vascular calcification. Int J Mol Med. 31:583–588. 2013.PubMed/NCBI
|
|
29
|
Zuo C, Huang Y, Bajis R, et al:
Osteoblastogenesis regulation signals in bone remodeling.
Osteoporos Int. 23:1653–1663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zanotti S and Canalis E: Notch signaling
and bone. IBMS BoneKEy. 8:318–327. 2011. View Article : Google Scholar
|
|
31
|
Doi H, Iso T, Shiba Y, et al: Notch
signaling regulates the differentiation of bone marrow-derived
cells into smooth muscle-like cells during arterial lesion
formation. Biochem Biophys Res Commun. 381:654–659. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Engin FZ, Yao ZQ, Yang T, et al: Dimorphic
effects of Notch signaling in bone homeostasis. Nat Med.
14:299–305. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maeda K, Kobayashi Y, Udagawa N, et al:
Wnt5a-Ror2 signaling between osteoblast-lineage cells and
osteoclast precursors enhances osteoclastogenesis. Nat Med.
18:405–412. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Al-Aly Z: Arterial calcification: a tumor
necrosis factor-alpha mediated vascular Wnt-opothy. Transl Res.
151:233–239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nie B, Zhou S, Fang X, Li W, Wang B and
Guan S: Implication of receptor activator of NF-kappaB ligand in
Wnt/beta-catenin pathway promoting osteoblast-like cell
differentiation. J Huazhong Univ Sci Technolog Med Sci. 32:818–822.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iso T, Hamamori Y and Kedes L: Notch
signaling in vascular development. Arterioscler Thromb Vasc Biol.
23:543–553. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Morrow D, Scheller A, Birney YA, et al:
Notch-mediated CBF-1/RBP-J{kappa}-dependent regulation of human
vascular smooth muscle cell phenotype in vitro. Am J Physiol Cell
Physiol. 289:C1188–C1196. 2005.
|
|
38
|
Shimizu T, Tanaka T, Iso T and Kurabayashi
M: Notch signaling directly targets Msx2: possible role of notch
signaling in osteogenic conversion of vascular smooth muscle cells
and vascular calcification. Circulation. 118:S3682008.
|