Introduction
Non-small-cell lung cancer (NSCLC) is the most
common type of lung cancer, with a mortality rate of >80%.
However, the value of chemotherapy in the treatment of NSCLC is
controversial. To date, cis-diamminedichloroplatinum (cisplatin,
CDDP) remains one of the most important drugs for combination
chemotherapy (1). In 1995, a
meta-analysis was performed to investigate the impact of
CDDP-containing combination chemotherapy (2,3). The
results indicated that patients administered with CDDP-containing
combination chemotherapy revealed a small but significant 10%
survival benefit at 12 months compared with the best supportive
care in advanced NSCLC. As a result of these observations,
CDDP-containing combination chemotherapy has been commonly used for
the treatment of NSCLC; however, the treatment efficacy remains
unsatisfactory (4).
2′,2′-Difluorodeoxycytidine (gemcitabine, GEM), an active
antineoplastic agent for NSCLC, has been previously hypothesized to
be suitable for use in combination with CDDP, following
observations indicating that these drugs may exhibit complementary
mechanisms (5). The
sequence-dependent effects of CDDP/GEM treatment remain an
important area of clinical and experimental studies, with results
demonstrating that various treatment protocols affect the outcome
of combination chemotherapy. Previous studies have reported that
GEM followed by CDDP represents the most effective treatment in
in vitro and in vivo models (6–10).
The present study aimed to investigate the sequence-dependent
effects of CDDP/GEM treatment on the human non-small-cell lung
carcinoma cell line, A549.
Materials and methods
Chemicals and reagents
GEM and CDDP were purchased from Jiangsu Hansoh
Pharmaceutical Co., Ltd. (Lianyungang, China). GEM and CDDP were
dissolved in 0.9% NaCl and sterile-filtered. Drug concentrations
were set according to their respective IC50 values,
followed by serial dilutions. In the GEM group, the concentrations
used were 0.001, 0.01, 0.1, 1 and 10 mg/l. In the CDDP group, the
concentrations were 0.5, 1, 2, 4 and 8 mg/l, respectively.
Cell culture
A549 cells were grown in monolayers in Dulbecco's
modifed Eagle's medium (Gibco, Invitrogen, NY, USA) supplemented
with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT,
USA), 1% L-glutamine and 1% penicillin-streptomycin and incubated
at 37°C in 5% CO2. The study was approved by the
Institutional Review Board at Liaoning Tumor Hospital (Shenyang,
China).
Drug administration
In this study, the cells were divided into six
groups, including a control group and five treatment groups. The
treatment groups were as follows: GEM, CDDP, GEM followed by CDDP
(GEM - CDDP), CDDP followed by GEM (CDDP - GEM) and simultaneous
administration of GEM and CDDP (GEM + CDDP). To study the
sequence-dependent effects of treatments, cultured cells were
exposed to GEM and CDDP alone and in combination. Cells were
treated with the first drug for 24 h, at concentrations of
IC50, 75% IC50, 50% IC50 and 25%
IC50. The cells were then washed with PBS and treated
with the second drug, also at concentrations of IC50,
75% IC50, 50% IC50 and 25% IC50,
with the same dilution ratios for both drugs.
Inhibition rate
A549 cells at the exponential growth phase were
plated in 96-well plates (5×103 cells in a volume of 100
μl medium/well). Following cell adherence, 100 μl GEM (final
concentrations, 0.001, 0.01, 0.1, 1 and 10 mg/l) and/or CDDP (final
concentrations, 0.5, 1, 2, 4 and 8 mg/l) were added to the medium.
Cells were cultured at 37°C for 48 h. Next, medium in the control
and drug-containing wells was removed and replaced by 200 μl fresh,
drug-free MTT medium (0.5 mg/ml; Sigma, St. Louis, MO, USA).
Following culture for 4 h at 37°C, the medium was replaced by 150
μl dimethyl sulfoxide (DMSO; Sigma). Finally, a microplate reader
(Tecan, Männedorf, Switzerland) was used to detect the optical
density (OD) values in each group at a wavelength of 490 nm. Each
group was performed in triplicate in three independent experiments.
Inhibition rates and IC50 were calculated based on these
values.
Calculation of combination index
(CI)
CI was calculated using the following formula
(11): CI =
(Da/aIC50) +
(Db/bIC50), where a and b represent two drugs
with a similar function. Da and Db represent doses that lead to a
growth inhibition of 50%. aIC50 and
bIC50 represent the drug concentration at
which an inhibition rate of 50% was achieved. CI<1 indicated
synergism, CI>1 indicated antagonism and CI=1 indicated
additivity. The inhibition rate was calculated based on an MTT
assay and the IC50 was calculated using logistic
analysis.
Cell cycle analysis
Cells were exposed to cisplatin and/or gemcitabine
treatment. Following 48 h, cells were washed with ice-cold PBS,
recovered by trypsinization, fixed in 70% cold ethanol and stored
at 4°C. Following centrifugation, 500 μl resuspended cells with a
concentration of 1.0×106/ml were collected. Next,
propidium iodide (PI) solution was added and incubated for 45 min
at room temperature (in the dark). Flow cytometric analysis was
performed with a BD FACScan flow cytometer (Becton Dickinson, San
Jose, CA, USA) using a 480 nm laser as an excitation source and an
absorbance wavelength of 630 nm.
Analysis of apoptosis
Apoptotic cells were identified using the BD
Pharmingen FITC Annexin V Apoptosis Detection kit (San Diego, CA,
USA), according to the manufacturer's instructions. Annexin V is a
35–36 kDa Ca2+-dependent phospholipid-binding protein
that binds phosphatidylserine, a phospholipid continuously exposed
in apoptotic cells. Annexin V conjugates with FITC, a fluorochrome,
functioning as a sensitive probe for the flow cytometry of cells
undergoing apoptosis. Damaged cells were stained by PI. Cells were
plated in 6-well plates and divided into 6 groups. Following drug
treatment, cells were collected and washed twice with cold PBS.
Next, cells were resuspended in binding buffer at a concentration
of 1.0×106 cells/ml. In addition, 100 μl solution was
added to 5-ml culture tubes together with 5 μl FITC Annexin V and 5
μl PI. Cells were incubated for 15 min at room temperature under
dark conditions. Binding buffer (400 μl) was added to the tubes and
analyzed using the BD FACScan flow cytometer.
Statistical analysis
The SPSS 13.0 statistical software package (SPSS,
Inc., Chicago, IL, USA) was used for statistical analysis. The
results of cells treated with different drugs were analyzed by
one-way ANOVA. Pearson correlation analysis was used to analyze the
dose- and time-dependent effects. P<0.05 was considered
statistically significant.
Results
Dose and time-dependent effects of GEM
and CDDP
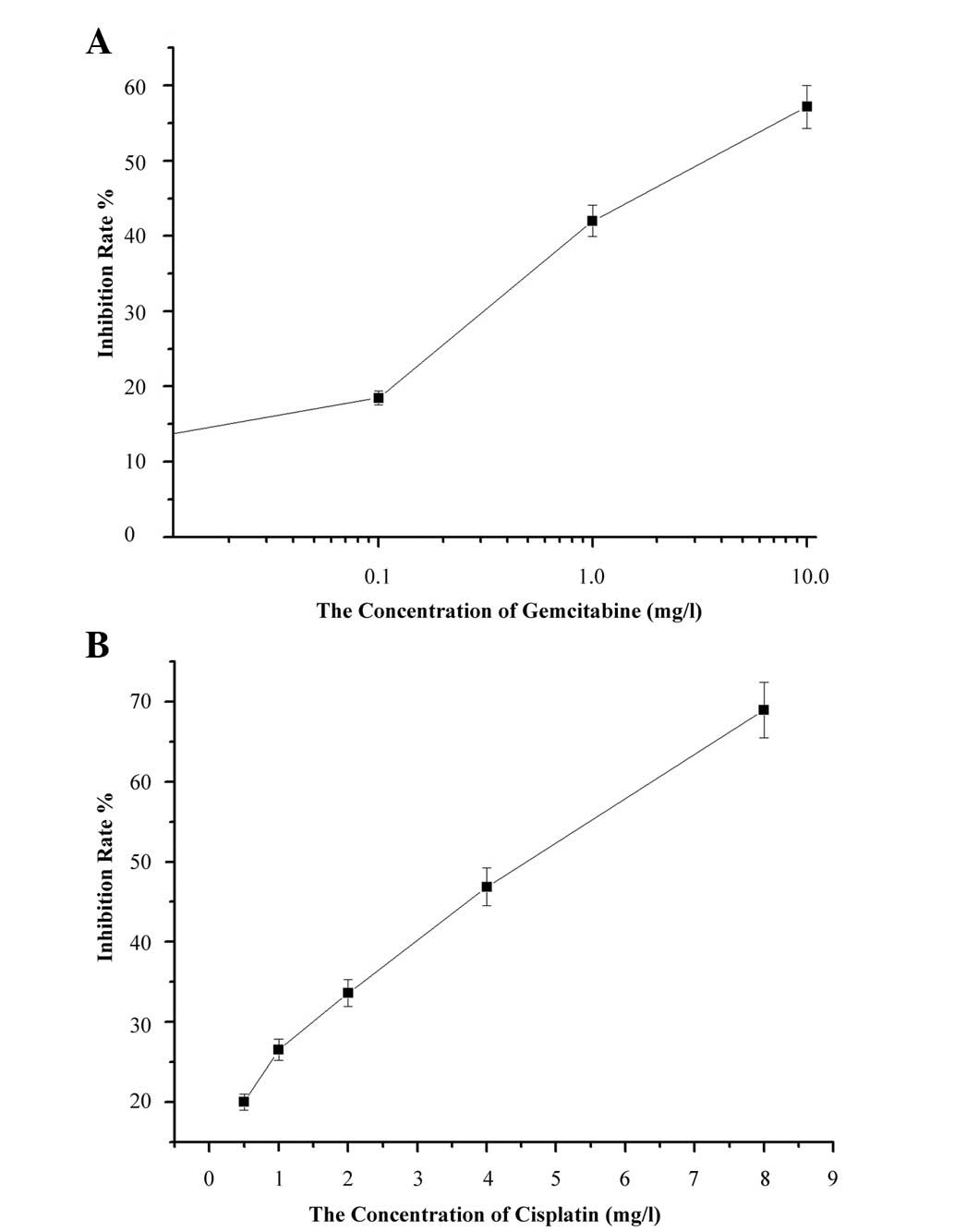
To investigate the dose- and time-dependent effects
of GEM and CDDP on A549 cells, cells were treated with GEM alone
with concentrations of 0.1 to 1 mg/l or CDDP alone with
concentrations of 0.5, 1, 2 and 4 mg/l, respectively. MTT assay was
performed to detect cell viability 24, 48 and 72 h after drug
exposure. Pearson correlation analysis indicated a dose-effect
relationship in the GEM (r=0.827; P<0.05) and CDDP groups
(r=0.983; P<0.01) within 24 h (Fig.
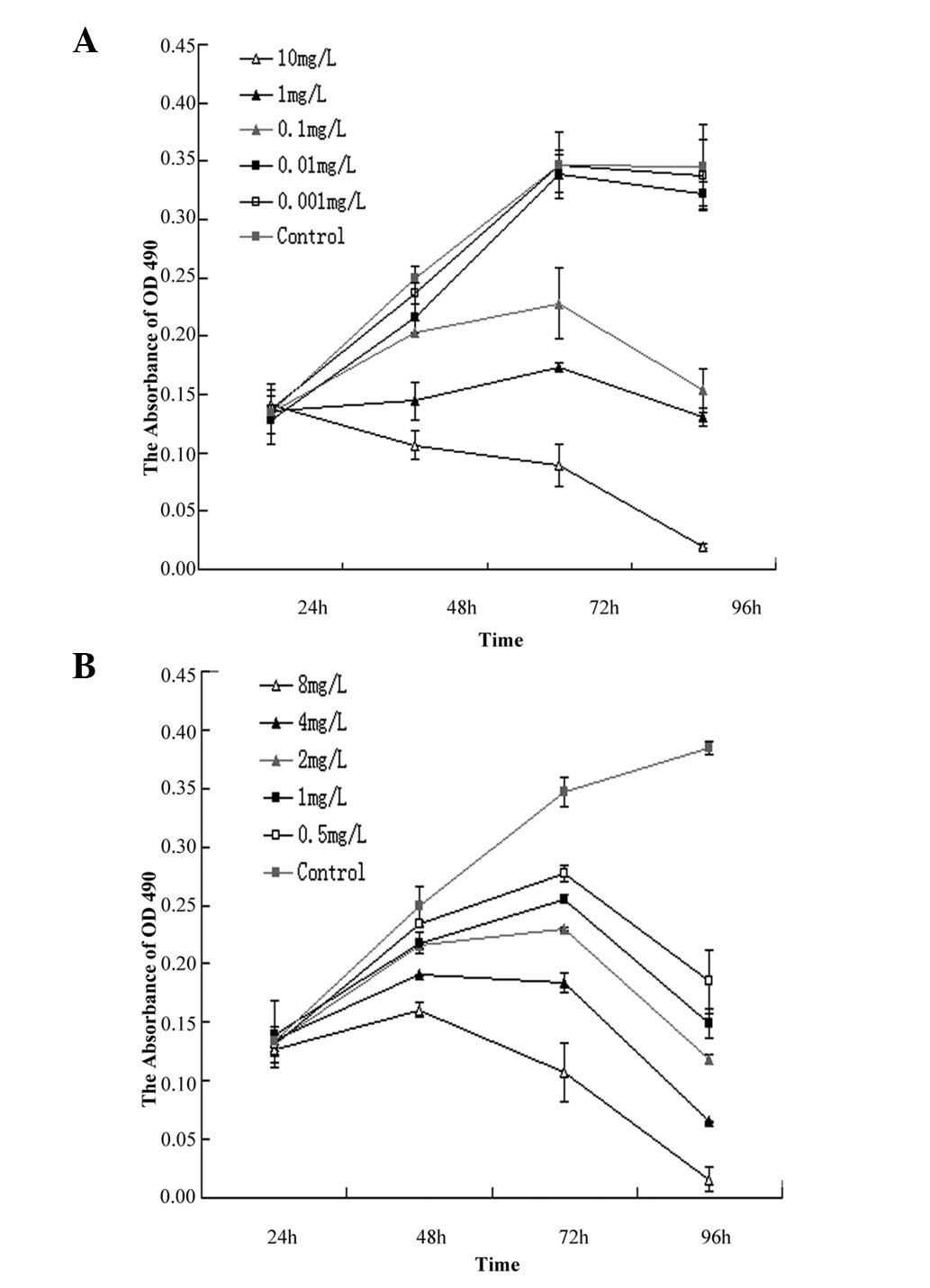
1). A time-dependent was also observed in Fig. 2. In the GEM group, IC50
for 24, 48 and 72 h was 3.79±0.32, 1.11±0.12 and 0.23±0.06 mg/l,
respectively (Fig. 2A). In the
CDDP group, IC50 for 24, 48 and 72 h was 18.58±2.01,
3.78±0.25, and 0.76±0.10 mg/l, respectively (Fig. 2B).
Sequence-dependent effects of GEM and
CDDP on the inhibition of cell growth
To investigate the sequence-dependent effects
of GEM and CDDP on the inhibition of A549 cells, drug
administration with different sequences was performed on cells.
When the inhibition rate reached 50%, the concentration for GEM was
0.39±0.01 mg/l and 1.32±0.03 mg/l for CDDP in the GEM-CDDP group;
the concentration for GEM was 0.65±0.03 mg/l and 2.21±0.04 mg/l for
CDDP in the CDDP-GEM group; the concentration for GEM was 0.58±0.02
mg/l and 1.80±0.03 mg/l for CDDP in the GEM+CDDP group. Inhibition
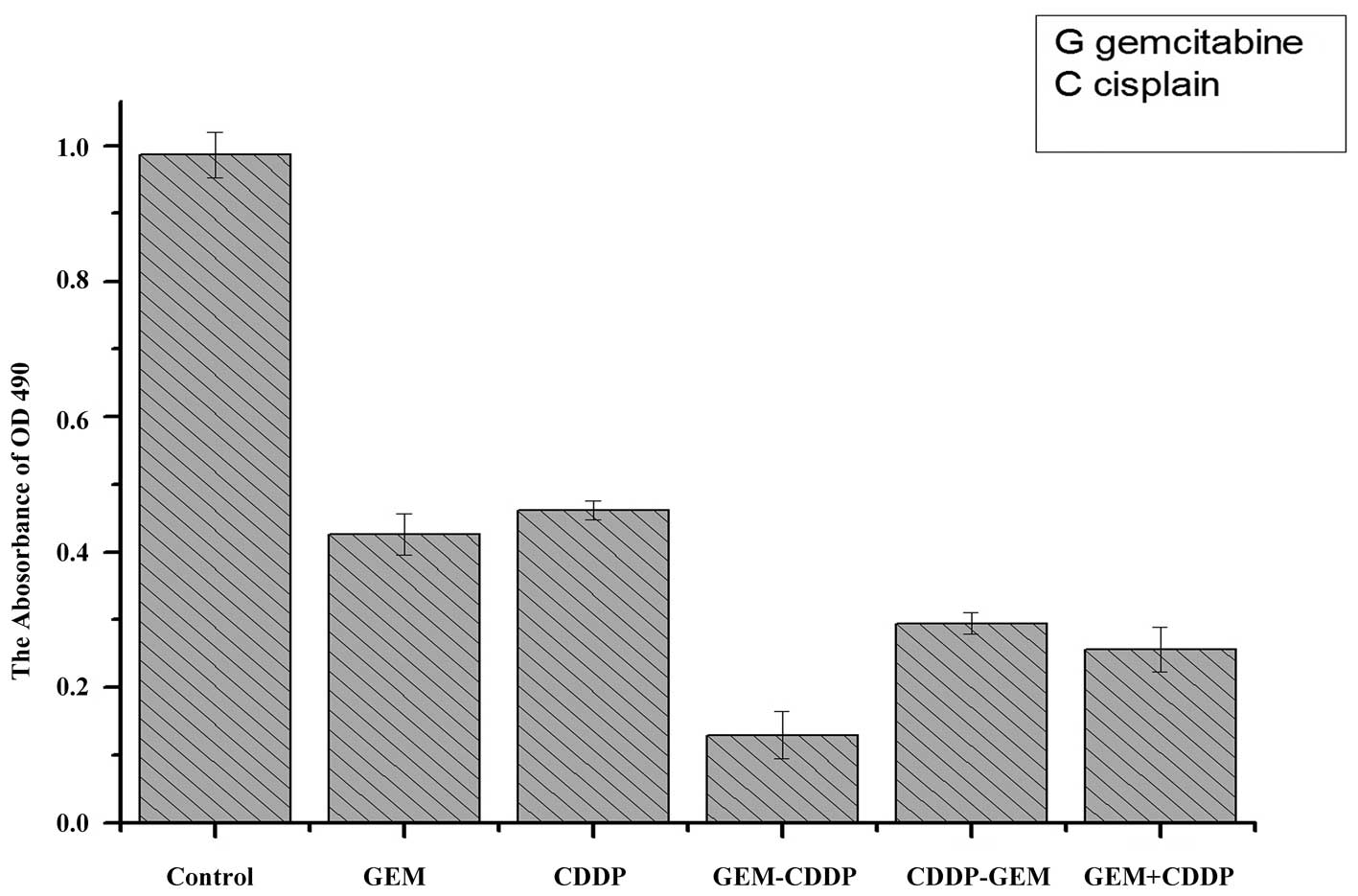
rate and CIs for different groups are presented in Table I. Compared with the control group
(Fig. 3), significant inhibitory
effects were observed in the GEM and CDDP groups. The
sequence-dependent effects of GEM and CDDP were also determined in
this study; the GEM - CDDP group revealed the highest rate of
inhibition compared with the remaining five groups, indicating that
GEM - CDDP chemotherapy exhibited the highest efficiency for
killing A549 cells in vivo.
 | Table IOD values, inhibition rates and CIs of
the control and experimental groups. |
Table I
OD values, inhibition rates and CIs of
the control and experimental groups.
| Group | OD value (490) | Inhibition rate | CI |
|---|
| Control | 0.986±0.033 | - | - |
| GEM | 0.427±0.034 | 0.616667±0.045 | - |
| CDDP | 0.462±0.031 | 0.552941±0.027 | - |
| GEM − CDDP | 0.130±0.013 | 0.87451±0.016 | 0.8007 |
| CDDP − GEM | 0.295±0.035 | 0.72451±0.037 | 1.1735 |
| GEM + CDDP | 0.254±0.008 | 0.74001±0.017 | 0.9986 |
Sequence-dependent effects of GEM and
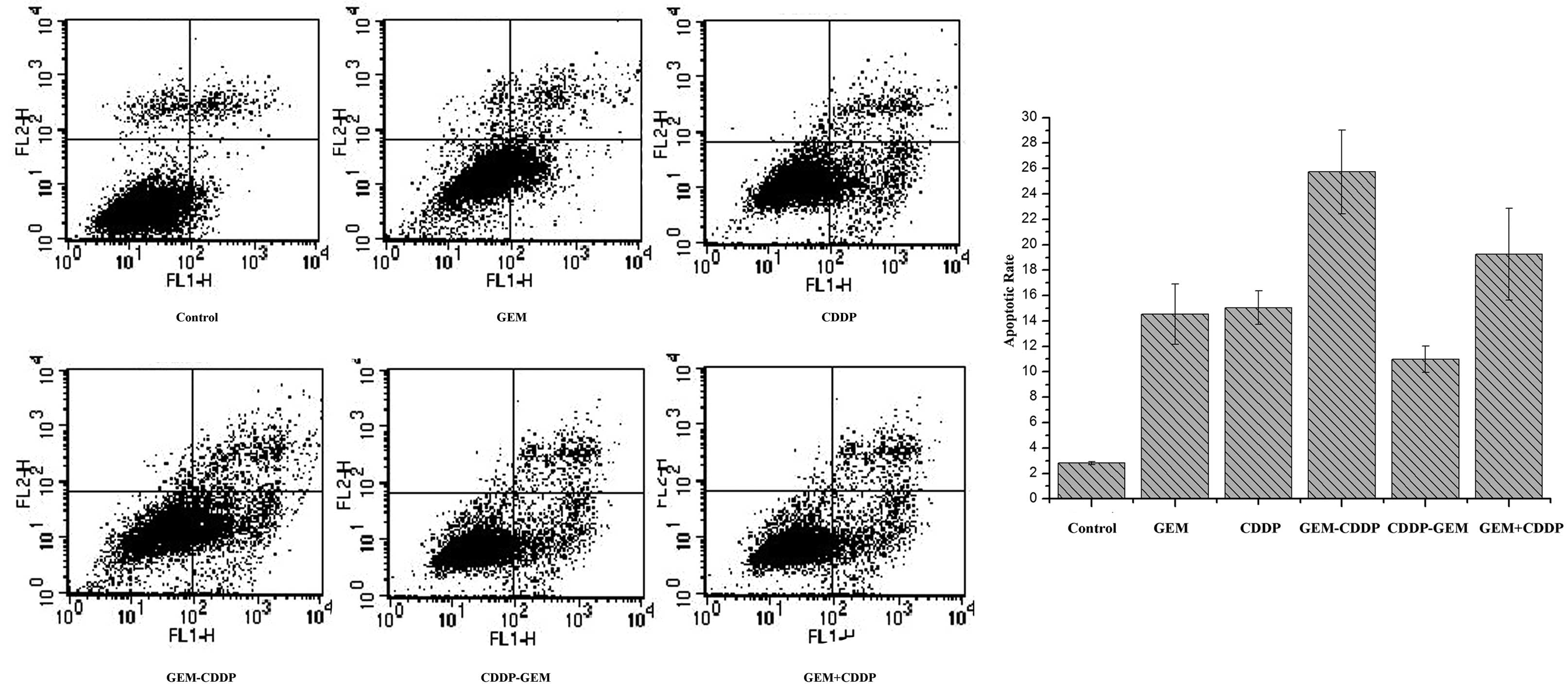
CDDP on apoptosis
The concentration of GEM and CDDP in the combination
is based on their respective IC50 values. As
demonstrated in Fig. 4, the
apoptotic rate in the control group was 2.82±0.12%. In the GEM
group, the apoptotic rate was 14.54±2.36%, while that of the CDDP
group was 15.06±1.31%. In the combination groups, the rates of
apoptosis were 25.72±3.29, 10.99±1.04 and 19.24±3.61% in the GEM -
CDDP, CDDP - GEM and GEM + CDDP groups, respectively. Among these
groups, GEM - CDDP revealed the highest rate of apoptosis compared
with the other groups (P<0.05).
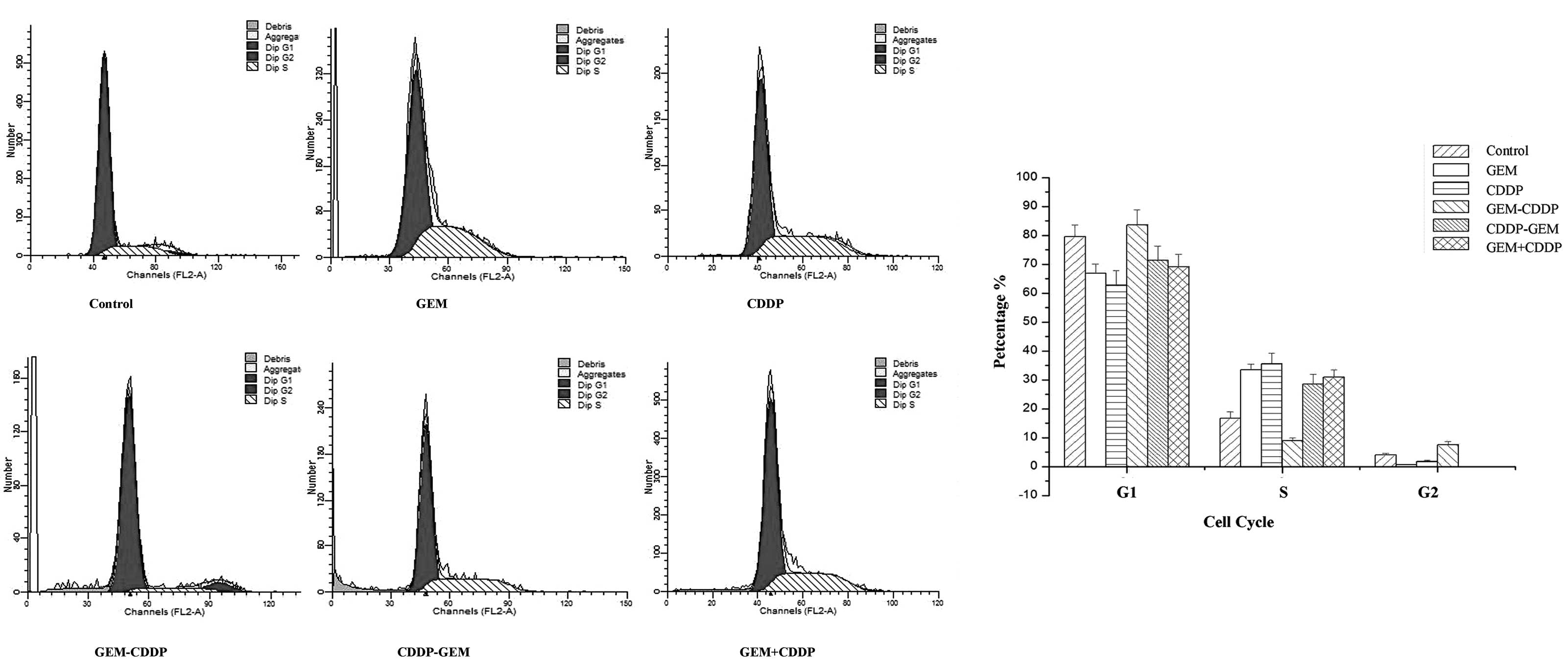
Sequence-dependent effects of GEM and
CDDP on the cell cycle
In this study, the sequence-dependent effects of
various treatment protocols on the cell cycle were determined
(Fig. 5). In groups treated with
GEM or CDDP, an increased percentage of cells were arrested in the
S phase compared with the control group (P<0.05). Although the
amount of cells arrested in the S phase in the CDDP group was
larger than that in the GEM group, no statistical difference was
observed. Consistent results were also detected in the CDDP - GEM,
CDDP and GEM groups, where an increased percentage of cells were
arrested in the S phase compared with the control group
(P<0.05). GEM - CDDP was identified to exhibit the highest rate
of apoptosis, with the majority of cells arrested in the
G1 and G2 phases.
Discussion
CDDP is one of the most widely used anticancer
drugs, due to its broad spectrum of activities. CDDP has revealed
specific clinical activity for the treatment of NSCLC; however,
CDDP induces nephro- and neurotoxicity, ototoxicity, severe nausea,
and vomiting (12) and is often
withheld from elderly people (13). Therefore, the identification of
novel therapeutic approaches with mild toxicities is required to
improve the clinical outcome of NSCLC. GEM has been hypothesized to
be an excellent candidate for combination therapy, as it is
associated with moderate side-effects and dose-dependent toxicity.
The combination of GEM and CDDP represents an attractive candidate
for combination chemotherapy, as the drugs exhibit complementary
mechanisms and non-overlapping side effects (14).
Results of the present study indicate that CDDP and
GEM exert synergistic effects in vitro, consistent with
previous observations (5). In
addition, the effect of sequence of drug administration on the
outcome of GEM/CDDP treatment was investigated, and it was
identified that the administration of GEM followed by CDDP
represented the most efficient treatment protocol, consistent with
a number of in vitro and in vivo studies (15–18).
GEM functions by blocking nucleic acid synthesis and
enzymes involved in the nucleotide biosynthesis pathway. The drug
inhibits DNA synthesis and the DNA repair process by reducing the
levels of deoxynucleoside triphosphate recruited during DNA
synthesis and repair. To date, GEM is considered to be one of the
most efficient chemotherapeutic drugs, as it inhibits cell
proliferation by preventing cells from progressing from the
G1 to the S phase. At present, GEM/CDDP combination
therapy is preferred for the treatment of advanced NSCLC. Despite
previous observations indicating that the administration of
GEM/CPPD following an appropriate protocol leads to the synergistic
interaction of the drugs (17),
high levels of morbidity and a marginal impact on survival time
have rendered this combination unsatisfactory. Based on these
observations, the aim of the current study was to investigate the
sequence-dependent effects of GEM/CDDP treatment in the NSCLC cell
line, A549, which is one of the most drug-resistant human tumor
cells.
Previous studies have reported sequence-dependent
effects of GEM and CDDP in tumor cells. van Moorsel et
al(15) reported synergism
between GEM and CDDP in NSCLC tumor Lewis lung carcinomas in
C57/B16 mice. In addition, Crino et al reported a 54%
efficacy rate in a group treated with GEM followed by CDDP in a
phase II NSCLC trial (16). In the
trial, 48 previously untreated patients with NSCLC were analyzed.
GEM was administered weekly at a dose of 1 g/ml and CDDP was
administered at a dose of 100 mg/ml on day 2 of each 28-day cycle.
The results indicated that GEM administration followed by CDDP
induced a high response rate in stage IIIB and IV NSCLC (16). Finally, antagonism was reported in
a study performed with CDDP administration followed by GEM
(concentration, 60% of the IC50) for 24 h (18). Results of the present study are
consistent with these observations, providing further evidence for
the sequence- and dose-dependent effects of GEM and CDDP therapy on
tumor cells.
A number of variables, including the schedule, dose
and type of study, are considered to be important for the efficacy
of administration of GEM and CDDP combination. However, the
mechanisms by which these drugs mediate their effects remain
unclear.
In the present study, synergism was observed in the
group treated with GEM followed by CDDP at12 h. Antagonism was
observed in the group treated with CDDP followed by GEM at 12 h.
Additivity was observed in the group treated with GEM and CDDP
simultaneously. Results of previous studies, together with the
present study, indicate the presence of sequence-dependent effects
of GEM and CDDP treatment, and suggest that these are dependent on
dosage, schedule and cell lines to a significant extent. However,
at present, no mechanisms accounting for the synergism between GEM
and CDDP have been hypothesized. In the current study, analysis of
the cell cycle was performed to determine whether the cell cycle is
involved in the sequence-dependent effects of GEM and CDDP
treatment. Cells treated with GEM or CDDP alone induced cell arrest
in the S phase, with increased rates of cell apoptosis. Cells
treated with CDDP - GEM or CDDP + GEM exhibited an increased
proportion of cells arrested in the S phase, while in the GEM -
CDDP group, cells were arrested in the G1 and
G2 phases. The interaction between CDDP and GEM enables
CDDP to affect the incorporation of GEM into DNA and RNA in a cell
line-dependent manner (19). In
addition, a marked effect on DNA synthesis was noted following
administration of CDDP. Thus, CDDP may affect the interaction of
GEM with DNA. GEM increases the cellular uptake of CDDP and
subsequent DNA-platination (20,21).
In a previous study, nucleotide excision repair was observed to
occur when CDDP was administered and followed by GEM (6). In addition, levels of ribonucleotide
reductase, the target of GEM, increased significantly during the
inhibition of DNA synthesis. As CDDP is known to inhibit
ribonucleotide reductase, the inhibitory effect of CDDP on
ribonucleotide reductase may be reduced by GEM metabolism. In
studies performed with GEM followed by CDDP, the accumulation of
single- and double-strand breaks was significant and intracellular
CDDP was hypothesized to generate active products that form DNA
intra- and interstrand cross-links (22,23),
increasing the probability of the induction of cell apoptosis.
Emerging drugs, particularly molecular targeted
therapies, represent novel options for drug combination therapies
(24). Specifically, the epidermal
growth factor receptor tyrosine kinase inhibitor, gefitinib
(25), and histone deacetylation
inhibitors (26,27) have been combined with traditional
chemotherapy drugs, demonstrating promising efficacies and novel
insights into NSCLC therapies.
In conclusion, results of the current study indicate
that drug administration sequence is an important variable in the
efficacy of drug combination chemotherapy. These observations are
likely to provide insight into the synergism of GEM followed by
CDDP.
References
|
1
|
Kroep JR, Peters GJ, van Moorsel CJ, et
al: Gemcitabine-cisplatin: A schedule finding study. Ann Oncol.
10:1503–1510. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
No authors listed. Chemotherapy in
non-small cell lung cancer: a meta-analysis using updated data on
individual patients from 52 randomised clinical trials. Non-Small
Cell Lung Cancer Collaborative Group. BMJ. 311:899–909. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
No authors listed. Clinical practice
guidelines for the treatment of unresectable non-small-cell lung
cancer. Adopted on May 16, 1997 by the American Society of Clinical
Oncology. J Clin Oncol. 15:2996–3018. 1997.PubMed/NCBI
|
|
4
|
Berghmans T, Paesmans M, Lalami Y, et al:
Activity of chemotherapy and immunotherapy on malignant
mesothelioma: a systematic review of the literature with
meta-analysis. Lung Cancer. 38:111–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Braakhuis BJ, van Dongen GA, Vermorken JB
and Snow GB: Preclinical in vivo activity of
2′,2′-difluorodeoxycytidine (Gemcitabine) against human head and
neck cancer. Cancer Res. 51:211–214. 1991.
|
|
6
|
van Moorsel CJ, Veerman G, Bergman AM, et
al: Combination chemotherapy studies with gemcitabine. Semin Oncol.
24(2 Suppl 7): S7-17–S7-23. 1997.
|
|
7
|
Peters GJ, Ruiz van Haperen VW, Bergman
AM, et al: Preclinical combination therapy with gemcitabine and
mechanisms of resistance. Semin Oncol. 23(5 Suppl 10): 16–24.
1996.PubMed/NCBI
|
|
8
|
Braakhuis BJ, Ruiz van Haperen VW, Welters
MJ and Peters GJ: Schedule-dependent therapeutic efficacy of the
combination of gemcitabine and cisplatin in head and neck cancer
xenografts. Eur J Cancer. 31A:2335–2340. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bergman AM, Ruiz van Haperen VW, Veerman
G, et al: Synergistic interaction between cisplatin and gemcitabine
in vitro. Clin Cancer Res. 2:521–530. 1996.PubMed/NCBI
|
|
10
|
Tsai CM, Chang KT, Chen JY, et al:
Cytotoxic effects of gemcitabine-containing regimens against human
non-small cell lung cancer cell lines which express different
levels of p185neu. Cancer Res. 56:794–801. 1996.
|
|
11
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McKeage MJ: Comparative adverse effect
profiles of platinum drugs. Drug Saf. 13:228–244. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bunn PA Jr: Chemotherapy for advanced
non-small-cell lung cancer: who, what, when, why? J Clin Oncol.
20:23S–33S. 2002.PubMed/NCBI
|
|
14
|
Voigt W, Bulankin A, Müller T, et al:
Schedule-dependent antagonism of gemcitabine and cisplatin in human
anaplastic thyroid cancer cell lines. Clin Cancer Res. 6:2087–2093.
2000.PubMed/NCBI
|
|
15
|
van Moorsel CJ, Pinedo HM, Veerman G, et
al: Scheduling of gemcitabine and cisplatin in Lewis lung tumour
bearing mice. Eur J Cancer. 35:808–814. 1999.PubMed/NCBI
|
|
16
|
Crinò L, Scagliotti G, Marangolo M, et al:
Cisplatin-gemcitabine combination in advanced non-small-cell
lungcancer: a phase II study. J Clin Oncol. 15:297–303. 1997.
|
|
17
|
Zanellato I, Boidi CD, Lingua G, et al: In
vitro anti-mesothelioma activity of cisplatin-gemcitabine
combinations: evidence for sequence-dependent effects. Cancer
Chemother Pharmacol. 67:265–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Voigt W, Bulankin A, Müller T, et al:
Schedule-dependent antagonism of gemcitabine and cisplatin in human
anaplastic thyroid cancer cell lines. Clin Cancer Res. 6:2087–2093.
2000.PubMed/NCBI
|
|
19
|
Jackson RC: Amphibolic drug combinations:
the design of selective antimetabolite protocols based upon the
kinetic properties of multienzyme systems. Cancer Res.
53:3998–4003. 1993.PubMed/NCBI
|
|
20
|
Eastman A: The formation, isolation and
characterization of DNA adducts produced by anticancer platinum
complexes. Pharmacol Ther. 34:155–166. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bunch RT and Eastman A:
7-Hydroxystaurosporine (UCN-01) causes redistribution of
proliferating cell nuclear antigen and abrogates cisplatin-induced
S-phase arrest in Chinese hamster ovary cells. Cell Growth Differ.
8:779–788. 1997.
|
|
22
|
Karagiannis TC and El-Osta A:
Double-strand breaks: signaling pathways and repair mechanisms.
Cell Mol Life Sci. 61:2137–2147. 2004.PubMed/NCBI
|
|
23
|
Bergstralh DT and Sekelsky J: Interstrand
crosslink repair: can XPF-ERCC1 be let off the hook? Trends Genet.
24:70–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pérol M, Chouaid C, Pérol D, et al:
Randomized, phase III study of gemcitabine or erlotinib maintenance
therapy versus observation, with predefined second-line treatment,
after cisplatin-gemcitabine induction chemotherapy in advanced
non-small-cell lung cancer. J Clin Oncol. 30:3516–3524. 2012.
|
|
25
|
Hida T, Ogawa S, Park JC, et al: Gefitinib
for the treatment of non-small-cell lung cancer. Expert Rev
Anticancer Ther. 9:17–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ozaki K, Kishikawa F, Tanaka M, et al:
Histone deacetylase inhibitors enhance the chemosensitivity of
tumor cells with cross-resistance to a wide range of DNA-damaging
drugs. Cancer Sci. 99:376–384. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Donadelli M, Costanzo C, Beghelli S, et
al: Synergistic inhibition of pancreatic adenocarcinoma cell growth
by trichostatin A and gemcitabine. Biochim Biophys Acta.
1773:1095–1106. 2007. View Article : Google Scholar : PubMed/NCBI
|