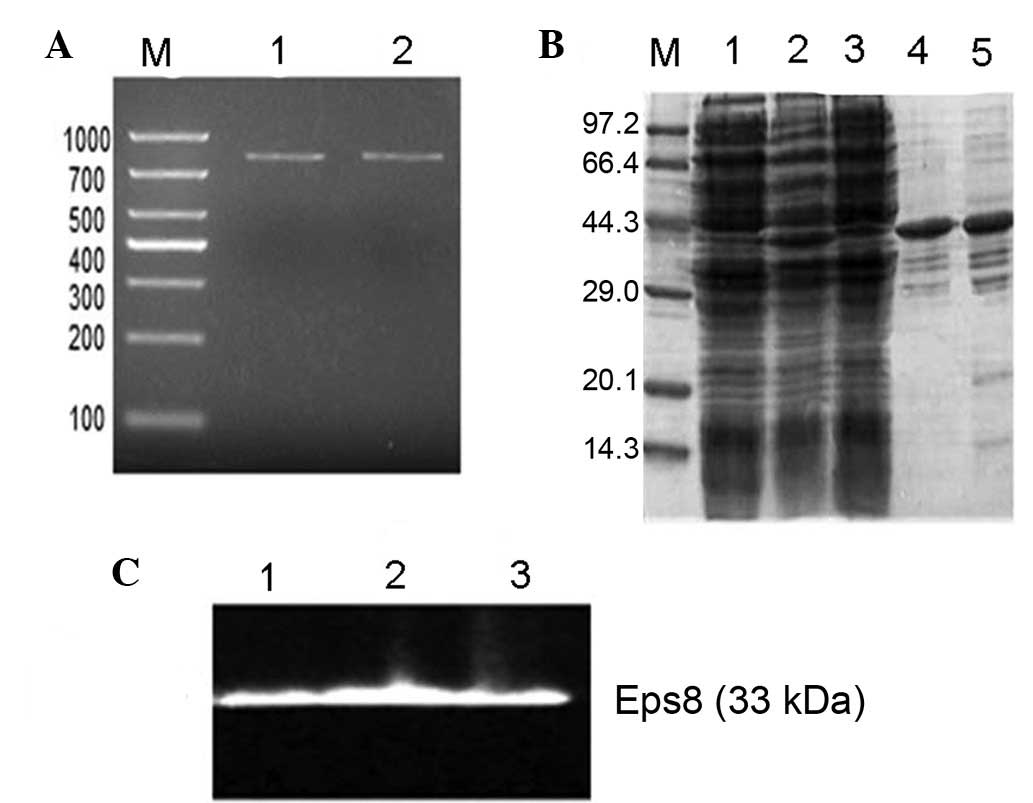
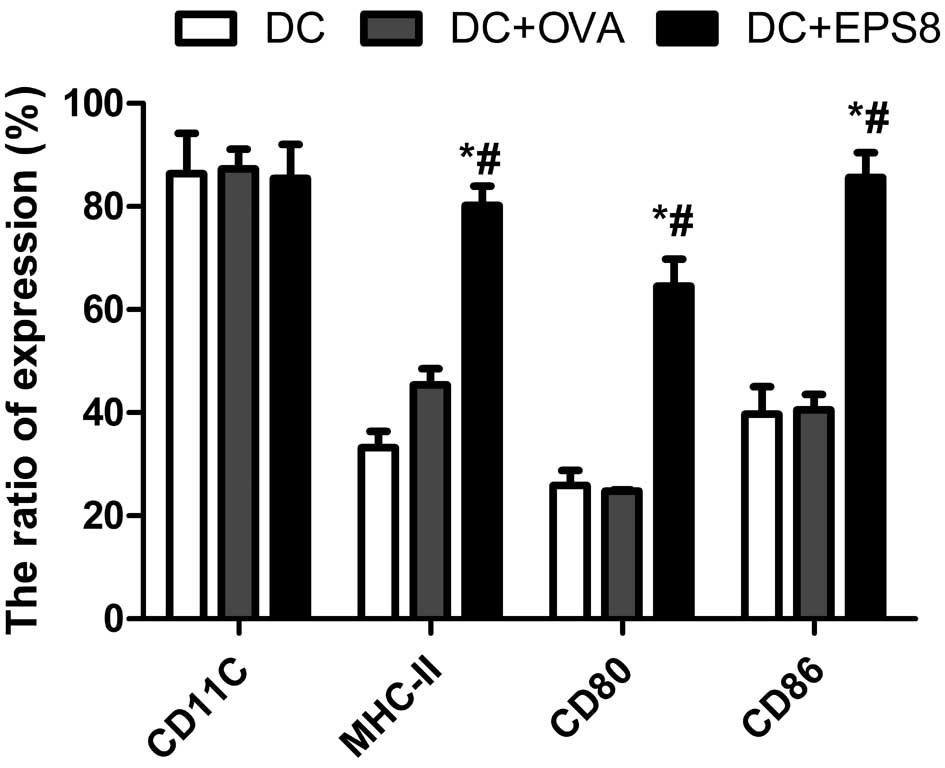
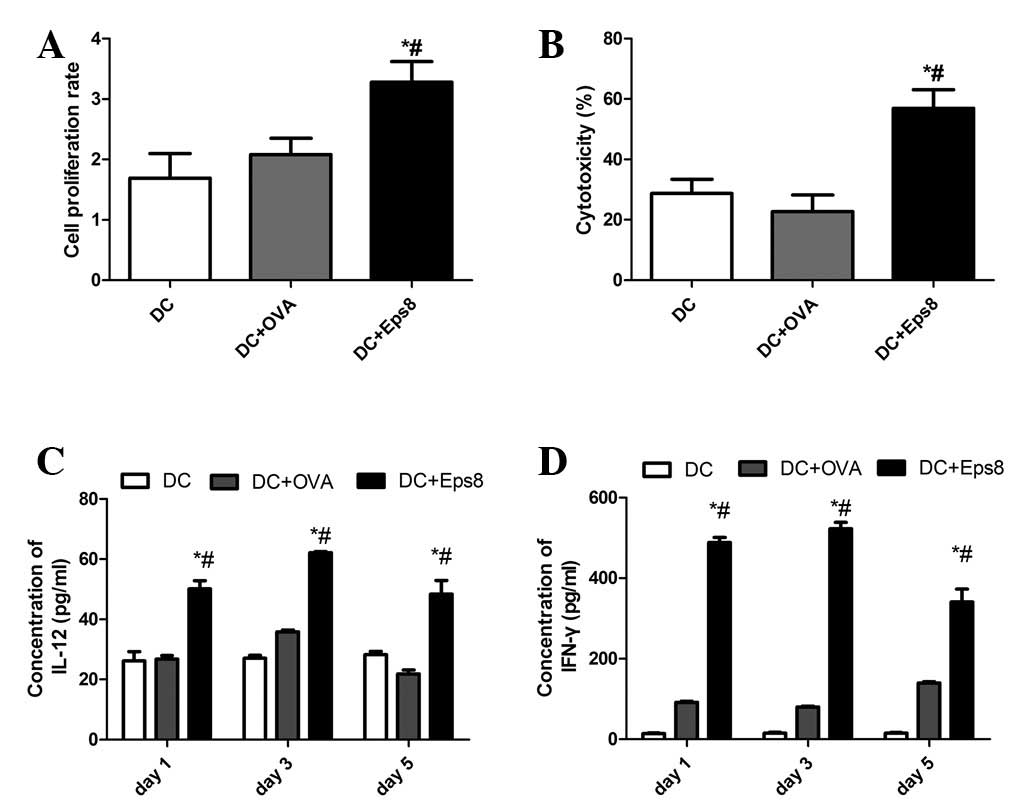
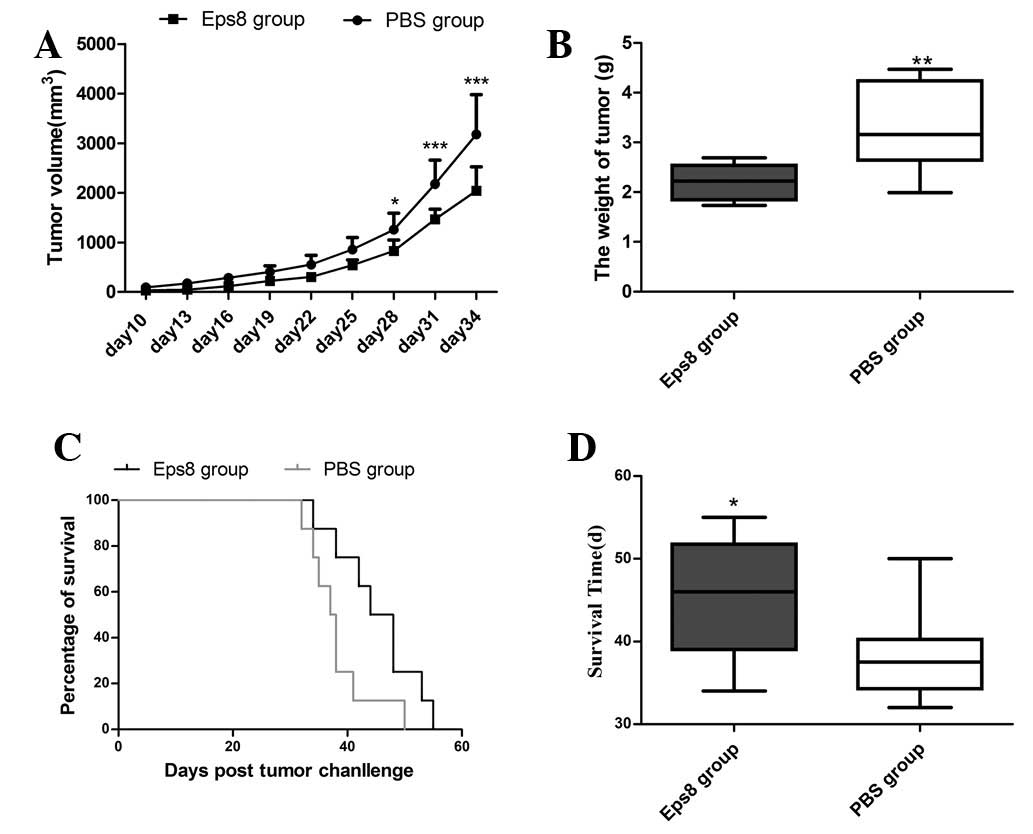
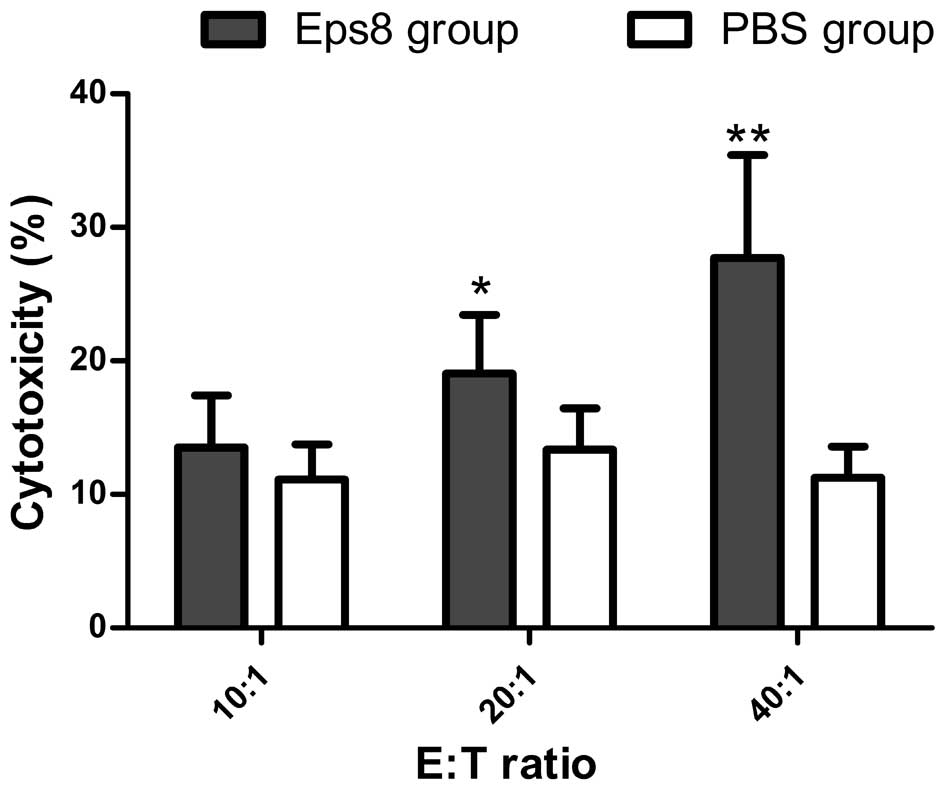
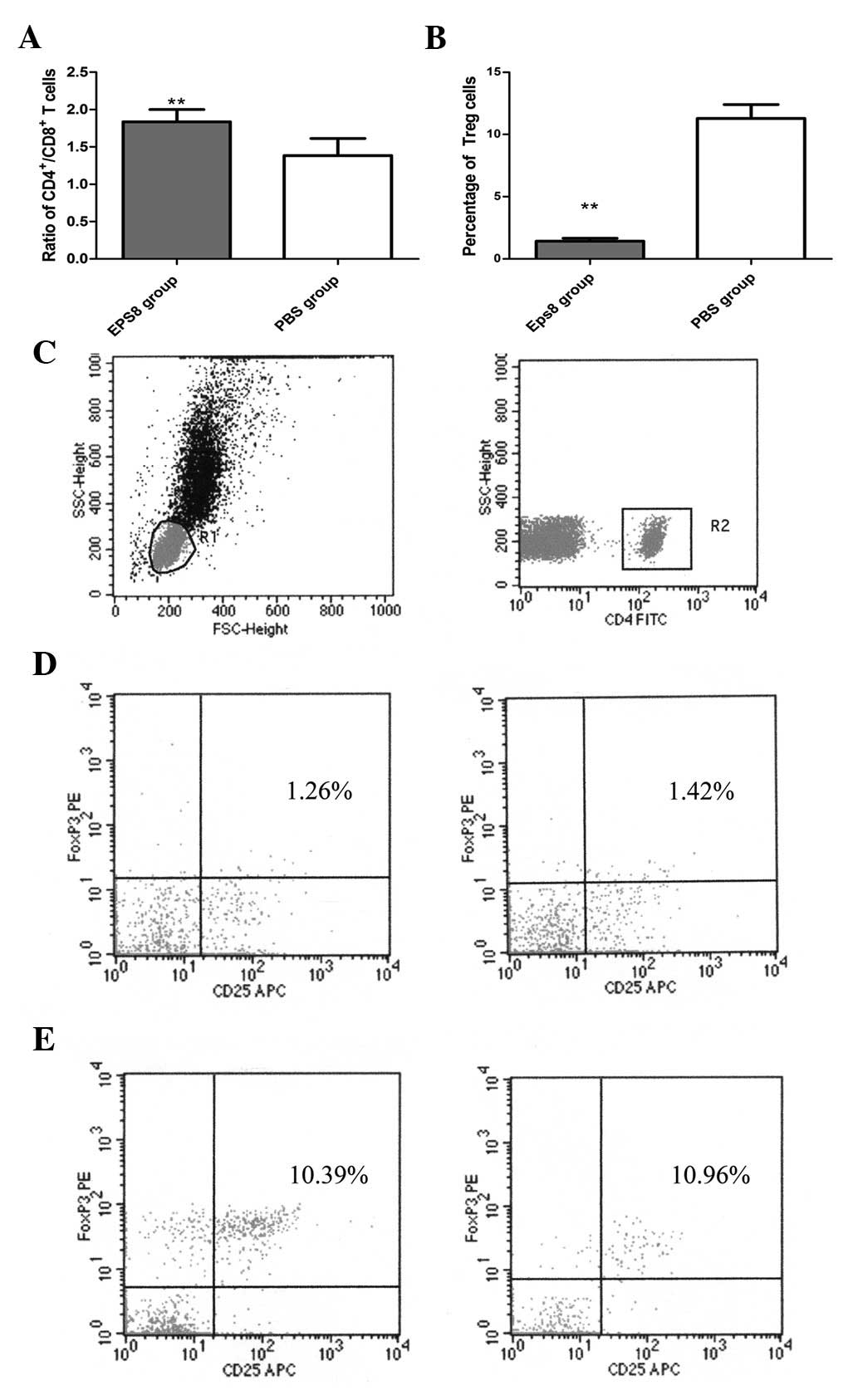
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lanari C, Wargon V, Rojas P and Molinolo
AA: Antiprogestins in breast cancer treatment: are we ready? Endocr
Relat Cancer. 19:R35–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fletcher SW: Breast cancer screening: a
35-year perspective. Epidemiol Rev. 33:165–175. 2011.PubMed/NCBI
|
|
4
|
Esteva FJ: Monoclonal antibodies, small
molecules, and vaccines in the treatment of breast cancer.
Oncologist. 9(Suppl 3): S4–S9. 2004. View Article : Google Scholar
|
|
5
|
Fioretti D, Iurescia S, Fazio VM and
Rinaldi M: DNA vaccines: developing new strategies against cancer.
J Biomed Biotechnol. 2010:1743782010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peoples GE, Gurney JM, Hueman MT, et al:
Clinical trial results of a HER2/neu (E75) vaccine to prevent
recurrence in high-risk breast cancer patients. J Clin Oncol.
23:7536–7545. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohebtash M, Tsang KY, Madan RA, et al: A
pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients
with metastatic breast and ovarian cancer. Clin Cancer Res.
17:7164–7173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Emens LA, Reilly RT and Jaffee EM: Breast
cancer vaccines: maximizing cancer treatment by tapping into host
immunity. Endocr Relat Cancer. 12:1–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Curigliano G, Spitaleri G, Pietri E, et
al: Breast cancer vaccines: a clinical reality or fairy tale? Ann
Oncol. 17:750–762. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Offenhäeuser N, Borgonovo A, Disanza A,
Romano P, Ponzanelli I and Iannolo G: The eps8 family of proteins
links growth factor stimulation to actin reorganization generating
functional redundancy in the Ras/Rac pathway. Mol Biol Cell.
15:91–98. 2004.PubMed/NCBI
|
|
11
|
Fazioli F, Minichiello L, Matoska V,
Castagnino P, Miki T, Wong WT and Di Fiore PP: Eps8, a substrate
for the epidermal growth factor receptor kinase, enhances
EGF-dependent mitogenic signals. EMBO J. 12:3799–3808.
1993.PubMed/NCBI
|
|
12
|
Yap LF, Jenei V, Robinson CM, et al:
Upregulation of Eps8 in oral squamous cell carcinoma promotes cell
migration and invasion through integrin-dependent Rac1 activation.
Oncogene. 28:2524–2534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen YJ, Shen MR, Chen YJ, Maa MC and Leu
TH: Eps8 decreases chemosensitivity and affects survival of
cervical cancer patients. Mol Cancer Ther. 7:1376–1385. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maa MC, Lee JC, Chen YJ, et al: Eps8
facilitates cellular growth and motility of colon cancer cells by
increasing the expression and activity of focal adhesion kinase. J
Biol Chem. 282:19399–19409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Welsch T, Endlich K, Giese T, Büchler MW
and Schmidt J: Eps8 is increased in pancreatic cancer and required
for dynamic actin-based cell protrusions and intercellular
cytoskeletal organization. Cancer Lett. 255:205–218. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Patel V, Miyazaki H, Gutkind JS
and Yeudall WA: Role for EPS8 in squamous carcinogenesis.
Carcinogenesis. 30:165–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu M, Shorts-Cary L, Knox AJ,
Kleinsmidt-DeMasters B, Lillehei K and Wierman ME: Epidermal growth
factor receptor pathway substrate 8 is overexpressed in human
pituitary tumors: role in proliferation and survival.
Endocrinology. 150:2064–2071. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bashir M, Kirmani D, Bhat HF, et al:
P66shc and its downstream Eps8 and Rac1 proteins are upregulated in
esophageal cancers. Cell Commun Signal. 8:13–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matoskova B, Wong WT, Salcini AE, Pelicci
PG and Di Fiore PP: Constitutive phosphorylation of eps8 in tumor
cell lines: relevance to malignant transformation. Mol Cell Biol.
15:3805–3812. 1995.PubMed/NCBI
|
|
20
|
Funato Y, Terabayashi T, Suenaga N, Seiki
M, Takenawa T and Miki H: IRSp53/Eps8 complex is important for
positive regulation of Rac and cancer cell motility/invasiveness.
Cancer Res. 64:5237–5244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Teh MT, Ji Y, et al: EPS8
upregulates FOXM1 expression, enhancing cell growth and motility.
Carcinogenesis. 31:1132–1141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Terme M, Tomasello E, Maruyama K, et al:
IL-4 confers NK stimulatory capacity to murine dendritic cells: a
signaling pathway involving KARAP/DAP12-triggering receptor
expressed on myeloid cell 2 molecules. J Immunol. 172:5957–5966.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matheu MP, Sen D, Cahalan MD and Parker I:
Generation of bone marrow derived murine dendritic cells for use in
2-photon imaging. J Vis Exp. 17:773–776. 2008.PubMed/NCBI
|
|
24
|
Liu J, Schmidt CS, Zhao F, et al:
LIGHT-deficiency impairs CD8+ T cell expansion, but not effector
function. Int Immunol. 5:861–870. 2003.PubMed/NCBI
|
|
25
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park HJ, Ahn KJ, Ahn SD, et al:
Susceptibility of cancer cells to beta-lapachone is enhanced by
ionizing radiation. Int J Radiat Oncol Biol Phys. 61:212–219. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Märten A, Renoth S, von Lilienfeld-Toal M,
et al: Enhanced lytic activity of cytokine-induced killer cells
against multiple myeloma cells after co-culture with
idiotype-pulsed dendritic cells. Haematologica. 86:1029–1037.
2001.
|
|
28
|
Burke P, Schooler K and Wiley HS:
Regulation of epidermal growth factor receptor signaling by
endocytosis and intracellular trafficking. Mol Biol Cell.
12:1897–1910. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Disis ML, Schiffman K, Guthrie K, et al:
Effect of dose on immune response in patients vaccinated with an
her-2/neu intracellular domain protein-based vaccine. J Clin Oncol.
22:1916–1925. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Foy TM, Bannink J, Sutherland RA, et al:
Vaccination with Her-2/neu DNA or protein subunits protects against
growth of a Her-2/neu expressing murine tumor. Vaccine.
19:2598–2606. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar
|
|
32
|
Santegoets SJ, van den Eertwegh AJ, van de
Loosdrecht AA, Scheper RJ and de Gruijl TD: Human dendritic cell
line models for DC differentiation and clinical DC vaccination
studies. J Leukoc Biol. 84:1364–1373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morse MA, Chui S, Hobeika A, Lyerly HK and
Clay T: Recent developments in therapeutic cancer vaccines. Nat
Clin Pract Oncol. 2:108–113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nishikawa H, Kato T, Hirayama M, et al:
Regulatory T Cell-resistant CD8+ T cells induced by
glucocorticoid-induced tumor necrosis factor receptor signaling.
Cancer Res. 68:5948–5954. 2008.PubMed/NCBI
|
|
35
|
Akbari A and Rezaei A: In vitro selective
depletion of CD4(+)CD25(+) regulatory T-cells from PBMC using
anti-tac-SAP. J Immunotoxicol. 9:368–373. 2012.
|
|
36
|
Curiel TJ, Coukos G, Zou L, et al:
Specific recruitment of regulatory T cells in ovarian carcinoma
fosters immune privilege and predicts reduced survival. Nat Med.
10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Banerjee DK, Dhodapkar MV, Matayeva E,
Steinman RM and Dhodapkar KM: Expansion of FOXP3high regulatory T
cells by human dendritic cells (DCs) in vitro and after injection
of cytokine-matured DCs in myeloma patients. Blood. 108:2655–2661.
2006. View Article : Google Scholar : PubMed/NCBI
|