Introduction
The prevention and reduction of invasion and
metastasis in malignant tumors is extremely important and these
processes represent the main cause of clinical mortality. An
important method of metastasis occurs via the lymphatic system.
Tumor metastasis is a complex process that involves genetic
abnormalities in tumor cells, changes in the signal regulation
system, invasiveness, adhesion and angiogenesis (1). To improve understanding of the effect
of regulating the function and expression of metastasis-associated
molecules on the molecular mechanism of tumor metastasis, drug
targets and anti-metastatic drugs must be identified and screened
for in order to inhibit tumor metastasis and reduce the mortality
rate of cancer patients.
Hepatocyte growth factor (HGF), also known as
mesenchymal factor, is a cytokine produced in stromal cells,
including fibroblasts and macrophages. HGF targets adjacent cells
in a paracrine manner, binding to the cell surface receptor and
activating tyrosine kinases, and is important for the regulation of
cell growth and migration (2).
The HGF receptor is also known as cMet, as it is the
product of the proto-oncogene, cMet. cMet possesses tyrosine
kinase activity and is a transmembrane receptor protein. HGF
recognizes and binds to cMet, which leads to receptor dimerization
and the phosphorylation of tyrosine residues in intracellular
domains. Intracellular signaling molecules containing SH2 domains
recognize the phosphorylation of these tyrosine residues,
activating various intracellular signal transduction pathways.
There are at least three known signal transduction pathways
activated by cMet, including phospholipase
Cγ/diacylglycerol/protein kinase C (PLCγ/DAG/PKC),
phosphoinositol-3-kinase/phosphoinositol-dependent protein
kinase/protein kinase B (PI3K/PDK/PKB or PI3K/AKT) and
mitogen-activated protein kinase (MAPK). Abnormal PI3K/AKT and
PLCγ/DAG/PKC signaling pathways have been previously reported to be
markedly associated with tumorigenesis, particularly invasion and
metastasis (3).
cMet is activated by molecules in the extracellular
matrix, including laminin (LN) and the cell surface adhesion
molecule fibronectin (FN) (4–7).
During invasion and metastasis, LN may activate cMet and promote
the expression and activation of proteolytic enzymes, including
collagenase IV, matrix metalloproteinases and urokinase-type
plasminogen activator. FN, on the surface of the cells of the
target organ tissue, may also activate cMet, thereby promoting the
expression of vascular endothelial growth factor, which may
specifically promote endothelial cell division and increase
vascular permeability, followed by the stimulation of tumor
angiogenesis, alteration of the tumor stroma and promotion of the
growth of secondary tumors (8). In
the present study, the molecular mechanisms of cMet-mediated cell
signal transduction in the regulation of tumor metastasis were
analyzed in two mouse ascites hepatoma cell lines associated with
different lymphatic metastatic potentials, Hca-F (high metastatic
potential) and Hca-P (low metastatic potential). The
phosphorylation of tyrosine residues of cMet and the activation of
the intracellular PI3K/AKT and PLCγ/DAG/PKC signaling pathways
following stimulation with different ligands were analyzed and
compared between the two cell lines.
Materials and methods
Materials
Primary antibodies anti-p-Met (Tyr 1313) IgG,
anti-p-Met (Tyr 1349) IgG, anti-p-Met (Tyr 1365) IgG, anti-p-PLCγ1
(Tyr 783) IgG, anti-PLCγ1 IgG, anti-p-Akt1/2/3 (Thr 308) IgG,
anti-p-Akt1/2/3 (Ser 473) IgG and anti-β-actin IgG, biotinylated
anti-mouse, -rabbit and -goat secondary antibodies and alkaline
phosphatase-labeled streptavidin were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). BCIP/NBT chromogenic
agents were obtained from Wuhan Boster Biological Technology, Ltd.
(Wuhan, China).
Reagents
Recombinant human HGF was obtained from Peprotech
(Rocky Hill, NJ, USA). LN and FN were obtained from Sigma-Aldrich
(St. Louis, MO, USA). Inhibitors were obtained from Calbiochem (San
Diego, CA, USA; U73122) or Sigma-Aldrich (LY294002).
Cell culture
For all experiments, Hca-P and Hca-F cell lines were
grown in multi-well plates in RPMI-1640 medium supplemented with
10% heat-inactivated FBS, 100 U/ml penicillin and 100 mg/ml
streptomycin under a 5% CO2 atmosphere at 37°C.
Analysis of tyrosine phosphorylation of
cMet in the cell lines
Culture medium was removed and the cells were washed
twice in serum-free RPMI-1640 medium and incubated overnight in
serum-free medium. Cells were collected, divided into the control
and treatment groups and added to serum-free medium containing 20
ng/ml HGF, 2 ng/ml FN or 2 ng/ml LN for 10 min at room temperature.
Centrifugal supernatants were discarded and the cells were lysed in
200 μl RIPA buffer (1% Triton X-100, 150 mM NaCl, 25 mM Tris at pH
7.5, 0.5% sodium deoxycholate, 0.1% SDS, 5 mM pyrophosphate and 50
mM NaF) containing 1 mM Na3VO4, 1 mM DTT, 1%
protease inhibitor cocktail and 1% phosphatase inhibitor cocktail.
Lysates were electrophoresed on a 10% SDS-PAGE gel. Next, proteins
were transferred to PVDF membranes at 100 mA for 2 h. Non-specific
binding on the PVDF membranes was blocked with 3% (w/v) BSA. Target
protein bands on the PVDF membranes were revealed by immunoblotting
with anti-p-Met (Tyr 1313) IgG, anti-p-Met (Tyr 1349) IgG or
anti-p-Met (Tyr 1365) IgG primary antibodies, biotinylated goat
anti-rabbit IgG, horse anti-mouse IgG or rabbit anti-goat IgG
secondary antibodies, alkaline phosphatase-labeled streptavidin and
BCIP/NBT chromogenic agent.
Analysis of signal transduction pathway
activity in the cell lines
Total protein from treated cells was analyzed by
western blot analysis as described. Target protein bands in PVDF
membranes were revealed by immunoblotting with anti-P-Akt1/2/3 (Thr
308) IgG, anti-P-Akt 1/2/3 (Ser 473) IgG, anti-PLCγ1 IgG,
anti-p-PLCγ1 (Tyr 783) IgG, biotinylated goat anti-rabbit IgG,
horse anti-mouse IgG or rabbit anti-goat IgG secondary antibodies,
alkaline phosphatase-labeled streptavidin and BCIP/NBT chromogenic
agents.
In vitro migration assay
Migration assays were performed using a Boyden
chamber (Costar, Cambridge, MA, USA) with 8-μm pore polycarbonate
filters (BD Biosciences, Franklin Lakes, NJ, USA). Following
overnight incubation in serum-free medium, cells (1×105)
were resuspended in 300 μl medium with 20 ng/ml HGF, 2 ng/ml FN or
2 ng/ml LN and placed in the top chamber and 250 μl 10% FBS medium
was placed in the bottom chamber. After incubation for 6 h at 37°C
in 5% CO2, cells on the top membrane surface were
mechanically removed. Cells that migrated to the bottom side of the
membrane were fixed and stained with 0.1% DAPI (Sigma-Aldrich).
Images were captured and stained cells were counted under a
microscope in five randomly selected fields. PLCγ/DAG/PKC and
PI3K/AKT pathways were blocked with 10.5 μM U73122 and 15 μM
LY294002 for 4 h, respectively. Cells were then harvested and used
for migration assays.
Statistical analysis
All results were repeated in at least three
independent experiments and consistently yielded similar results.
Data were analyzed by the Gel-Pro analyzer (Media Cybernetics, USA)
and GraphPad Prism 5 (Beijing, China). P<0.05 was considered to
indicate a statistically significant difference. Results are
presented as the mean ± SEM.
Results
Effect of HGF on tyrosine phosphorylation
of cMet and signaling pathway activities
cMet is activated through the ligand binding to the
receptor, receptor dimerization and phosphorylation of
intracellular tyrosine residues. Different phosphorylated tyrosine
residues activate different downstream intracellular signaling
pathways, which then play various roles in the regulation of a
number of biological functions, including proliferation, invasion
and metastasis (9). As
demonstrated in Fig. 1A,
HGF-stimulated phosphorylation of cMet at Tyr 1313 and 1365 in
highly metastatic Hca-F cells was higher than that in low
metastatic Hca-P cells; however, phosphorylation at Tyr 1349 was
lower than that in the Hca-P cells.
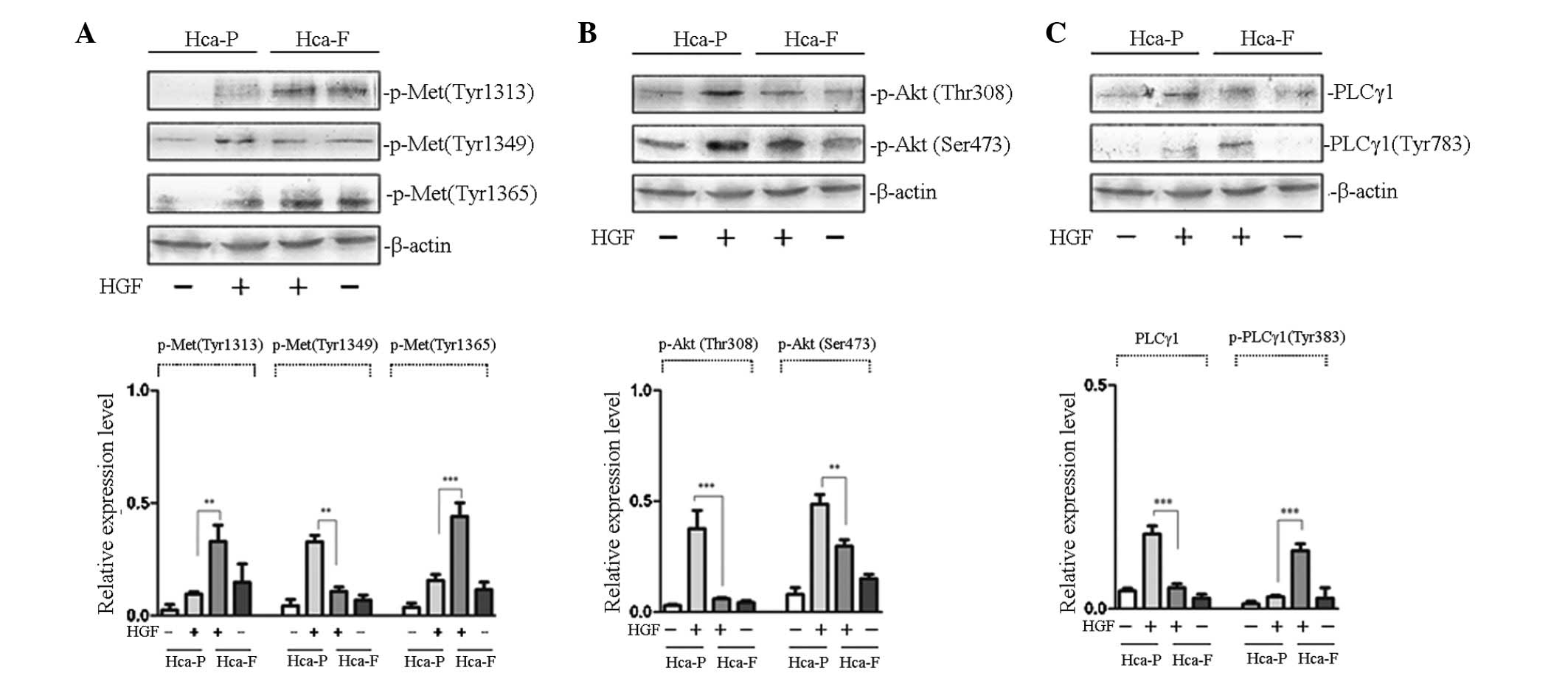 | Figure 1Effect of HGF on the phosphorylation
of cMet and signaling pathway activity in Hca-P and Hca-F cells.
(A) Effect of HGF on the phosphorylation of cMet. (B) Effect of HGF
on the activity of PI3K/AKT signaling pathway. (C) Effect of HGF on
the activity of PLCγ/DAG/PKC signaling pathway. Cells were
stimulated with HGF and subjected to western blot analysis with
anti-p-Met (Tyr 1313), anti-p-Met (Tyr 1365), anti-p-Akt 1/2/3 (Ser
473), anti-p-Akt1/2/3 (Thr 308), anti-PLCγ1 and anti-p-PLCγ1 (Tyr
783) antibodies. The phosphorylation of cMet at Tyr 1313 and 1365
in Hca-F cells was higher than that in Hca-P cells, while
phosphorlyation of Tyr 1349 was lower than that in Hca-P cells. The
activity of the PLCγ1/DAG/PKC signaling pathway was greater in
Hca-F cells than that in Hca-P cells; however, the activity of
PI3K/AKT was lower. HGF, hepatocyte growth factor; cMet, HGF
receptor; Hca-F, highly metastatic cell line; Hca-P, low metastatic
cell line; PI3K, phosphoinositol-3-kinase; Akt, protein kinase B;
PLCγ1, phospholipase γ1; DAG, diacylglycerol; PKC, protein kinase
C. |
Growth factor receptors activate PI3K/AKT signal
transduction, an important pathway involved in the regulation of
tumor metastasis. AKT functions as a protein kinase in PI3K/AKT
signal transduction and the phosphorylation of AKT at Thr 308 and
Ser 473 is activated by its upstream kinase, PDK (10). Therefore, the detection of
intracellular phosphorylated AKT levels may be used to determine
the activity of PI3K/AKT signal transduction. To study the effect
of AKT phosphorylation in the two cell lines treated with HGF, the
phosphorylation of AKT at Thr 308 and Ser 473 was observed. As
revealed in Fig. 1B,
HGF-stimulated phosphorylation of AKT at Thr 308 and Ser 473 in low
metastatic Hca-P cells was higher than that in highly metastatic
Hca-F cells. These results indicated that PI3K/AKT signaling
pathway activity was greater in low metastatic Hca-P than in highly
metastatic Hca-F cells.
PLCγ/DAG/PKC signal transduction is another
important pathway involved in the regulation of tumor metastasis.
When the growth factor receptor is activated, the SH2 domain of
PLCγ1 identifies a specific phosphorylated tyrosine residue and
binds to the receptor. Receptor tyrosine kinase catalyzes the
phosphorylation of PLCγ1 at Tyr 783, thereby activating
PLCγ/DAG/PKC signal transduction (11). To study the effect of PLCγ1
phosphorylation in the two cell lines treated with HGF, the
phosphorylation of PLCγ1 at Tyr 783 was observed. As demonstrated
in Fig. 1C, HGF-stimulated
phosphorylation of PLCγ1 at Tyr 783 in highly metastatic Hca-F
cells was higher than that in low metastatic Hca-P cells. These
results indicated that PLCγ/DAG/PKC signaling pathway activity was
greater in Hca-F than in Hca-P cells.
Effect of FN on the tyrosine
phosphorylation of cMet and signaling pathway activities
To investigate the effect of FN on the
phosphorylation of cMet, phosphorylation of cMet at Tyr 1313, 1349
and 1365 in Hca-F and Hca-P cells was analyzed. Fig. 2A reveals that, following FN
treatment, the phosphorylation of cMet at Tyr 1313 in highly
metastatic Hca-F cells was significantly higher compared with low
metastatic Hca-P cells; however, the phosphorylation of cMet at Tyr
1349 and 1365 was not found to be significantly different between
the two cell lines.
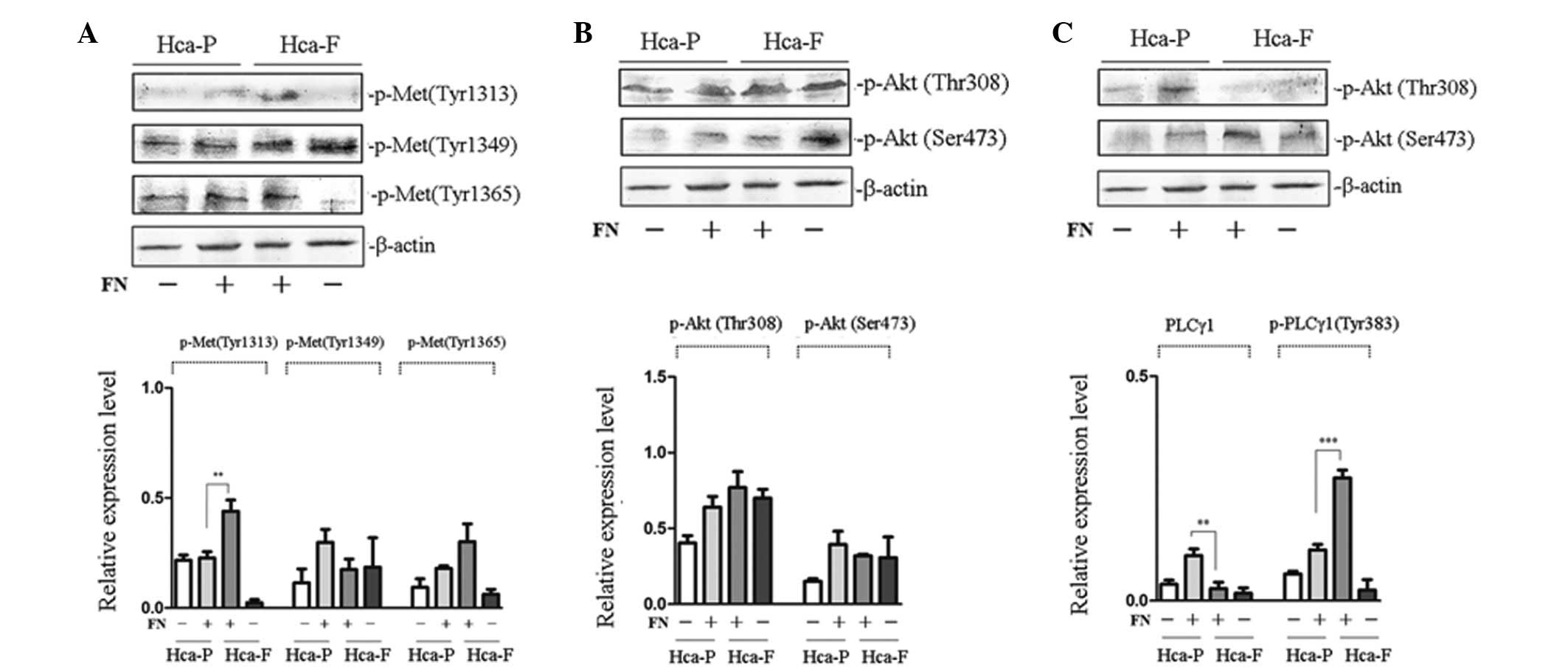 | Figure 2Effect of FN on the phosphorylation of
cMet and signaling pathway activity in Hca-P and Hca-F cells. (A)
Effect of FN on the phosphorylation of cMet. (B) Effect of FN on
the activity of PI3K/AKT signaling pathway. (C) Effect of FN on the
activity of PLCγ/DAG/PKC signaling pathway. Cells were stimulated
with FN and subjected to western blot analysis with anti-p-Met (Tyr
1313), anti-p-Met (Tyr 1365), anti-p-Akt 1/2/3 (Ser 473),
anti-p-Akt1/2/3 (Thr 308), anti-PLCγ1 (1249) and anti-p-PLCγ1 (Tyr
783) antibodies. The phosphorylation of cMet at Tyr-1313 in Hca-F
cells was higher than that in Hca-P cells and the activity of the
PLCγ1/DAG/PKC signaling pathway was greater in Hca-F cells than
that in Hca-P cells. FN, fibronectin; cMet, HGF receptor; Hca-F,
highly metastatic cell line; Hca-P, low metastatic cell line; Akt,
protein kinase B; PLCγ1, phospholipase γ1; DAG, diacylglycerol;
PKC, protein kinase C. |
To study the effect of FN on the activity of the
PI3K/AKT and PLCγ/DAG/PKC signaling pathways of the two cell lines,
the phosphorylation of Akt at Thr 308 and Ser 473 and PLCγ at Tyr
783 was observed. No significant differences in the phosphorylation
of Akt at Thr 308 and Ser 473 were found between Hca-P and Hca-F
cells (Fig. 2B). The
phosphorylation of PLCγ1 at Tyr 783 in highly metastatic Hca-F
cells was higher than that in low metastatic Hca-P cells (Fig. 2C). These results indicated that
there was no significant difference in PI3K/AKT signaling pathway
activity between the two cell lines, whereas PLCγ/DAG/PKC activity
was greater in Hca-F cells than that in Hca-P cells.
Effect of LN on the tyrosine
phosphorylation of cMet and on signaling pathway activities
To investigate the effect of FN on the
phosphorylation of cMet, phosphorylation at Tyr 1313, 1349 and 1365
was analyzed in Hca-F and Hca-P cells. Following LN treatment, the
phosphorylation of cMet at Tyr 1365 in highly metastatic Hca-F
cells was observed to be significantly greater than that in low
metastatic Hca-P cells. In addition, analysis of the
phosphorylation of cMet at Tyr 1313 and 1349 revealed no
significant differences between the two cell lines (Fig. 3A).
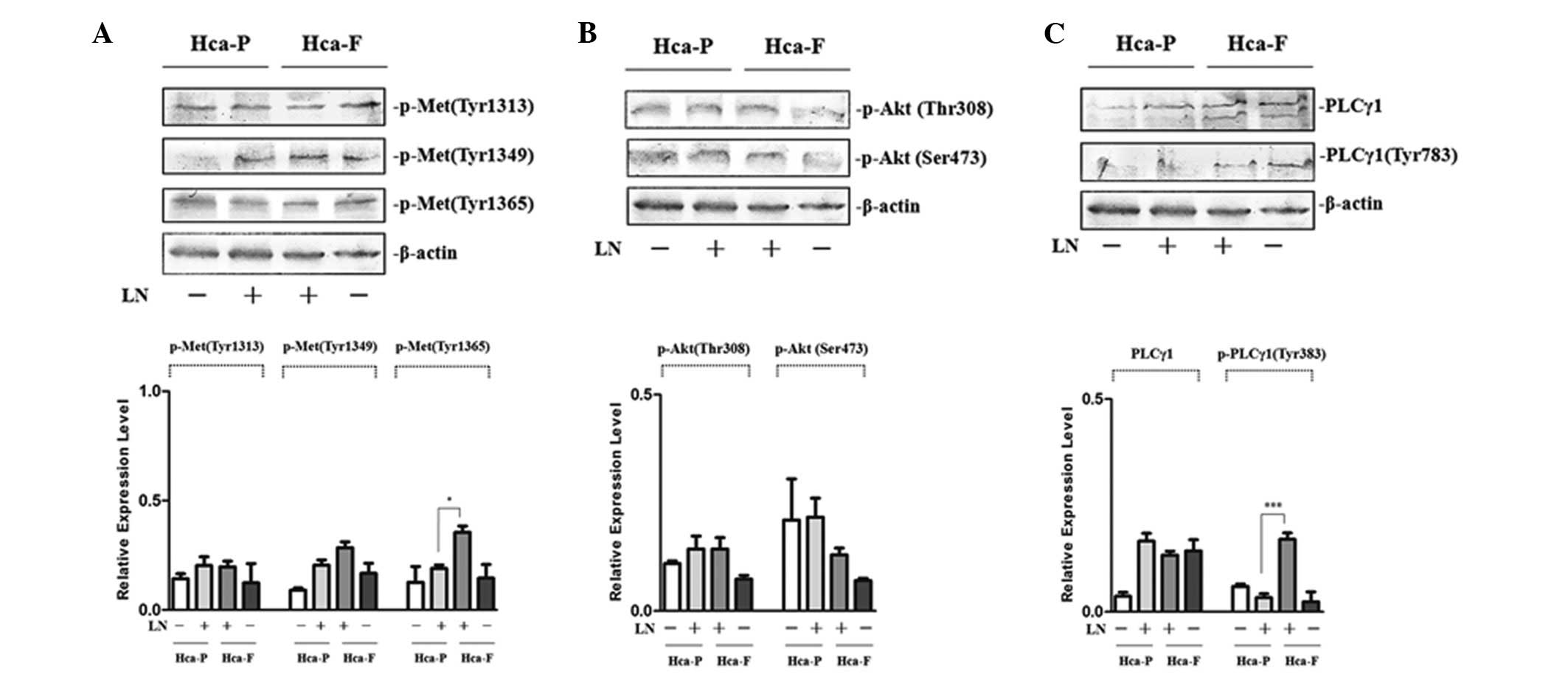 | Figure 3Effect of LN on the phosphorylation of
cMet and signaling pathway activity in Hca-P and Hca-F cells. (A)
Effect of LN on the phosphorylation of cMet. (B) Effect of LN on
the activity of PI3K/AKT signaling pathway. (C) Effect of LN on the
activity of PLCγ/DAG/PKC signaling pathway. Cells were stimulated
with LN and subjected to western blot analysis with anti-p-Met (Tyr
1313), anti-p-Met (Tyr 1365), anti-p-Akt 1/2/3 (Ser 473),
anti-p-Akt1/2/3 (Thr 308), anti-PLCγ1 (1249) and anti-p-PLCγ1 (Tyr
783) antibodies. The phosphorylation of cMet at Tyr 1365 in Hca-F
cells was higher than that in Hca-P cells and the activity of
PLCγ1/DAG/PKC signaling in Hca-F cells was greater than that in
Hca-P cells. LN, laminin; cMet, HGF receptor; Hca-F, highly
metastatic cell line; Hca-P, low metastatic cell line; Akt, protein
kinase B; PLCγ1, phospholipase γ1; DAG, diacylglycerol; PKC,
protein kinase C. |
To study the effect of LN on the PI3K/AKT and
PLCγ/DAG/PKC activity of the two cell lines, phosphorylation of Akt
at Thr 308 and Ser 473 and PLCγ at Tyr 783 was observed. Following
LN treatment, the phosphorylation of Akt at Thr 308 and Ser 473 was
not identified to be significantly different between Hca-P and
Hca-F cells (Fig. 3B); however,
the phosphorylation of PLCγ1 at Tyr 783 in highly metastatic Hca-F
cells was higher than that in low metastatic Hca-P cells (Fig. 3C). These results indicated that
PI3K/AKT activity is not significantly different between the two
cell lines and that PLCγ/DAG/PKC activity was greater in Hca-F
cells compared with Hca-P cells.
Effect of HGF, FN and LN on cell motility
and migration in vitro
PI3K/AKT and PLCγ/DAG/PKC signaling are major
pathways by which cMet modulates cell motility and migration
(12). The effect of HGF, FN and
LN on cell motility and migration in vitro were
investigated. As demonstrated in Fig.
4, HGF, FN and LN promoted Hca-P and Hca-F cell motility and
migration.
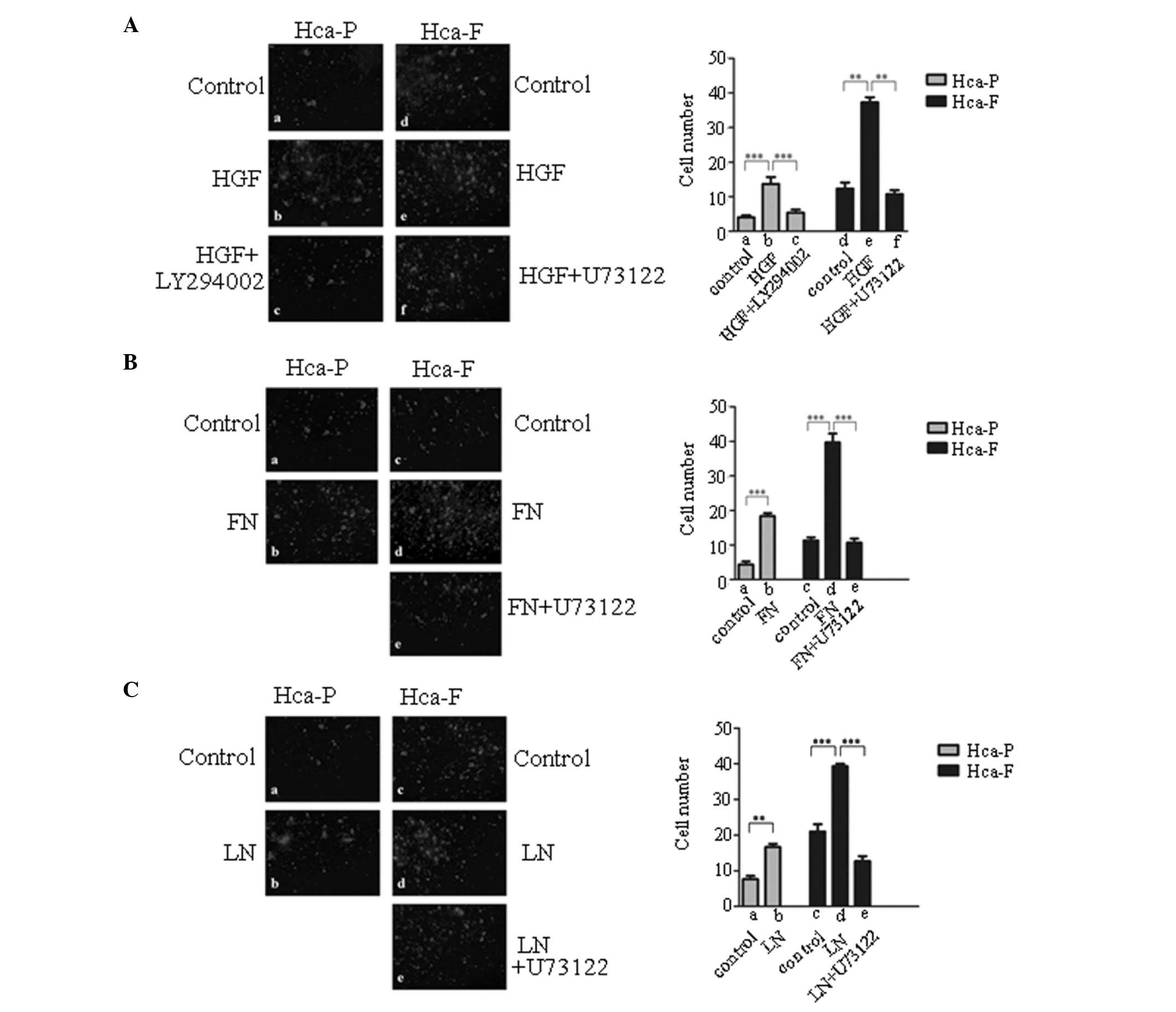 | Figure 4Effect of HGF, FN and LN on the
migration of Hca-P and Hca-F cells. (A) Effect of HGF on the
migration of Hca-P and Hca-F cells. (B) Effect of FN on the
migration of Hca-P and Hca-F cells. (C) Effect of LN on on the
migration of Hca-P and Hca-F cells. Cells were treated with HGF, FN
or LN, PI3K/Akt and PLCγ1/DAG/PKC signaling was blocked with
LY294002 or U73122, respectively, and cells were then subjected to
a migration assay. Membranes were fixed, stained and counted using
fluorescence microscopy (magnification, ×100). HGF, hepatocyte
growth factor; FN, fibronectin; LN, laminin; Hca-F, highly
metastatic cell line; Hca-P, low metastatic cell line; PI3K,
phosphoinositol-3-kinase; Akt, protein kinase B; PLCγ1,
phospholipase γ1; DAG, diacylglycerol; PKC, protein kinase C. |
Effect of blocking PI3K/AKT and
PLCγ/DAG/PKC signaling pathways on cell motility and migration
To determine whether cell motility and migration are
mainly regulated by the PI3K/AKT or PLCγ/DAG/PKC signaling
pathways, the activity of PI3K in the cells was blocked with
LY294002, an inhibitor of PI3K, and the activity of the
PLCγ/DAG/PKC signaling pathway in the cells was blocked with
U73122. Blocking PI3K/AKT signaling with LY294002 reversed the
positive effect of HGF on HGF-stimulated motility and migration in
Hca-P cells in vitro (Fig.
4Aa-c). In Hca-F cells, blocking the PLCγ/DAG/PKC signaling
pathway with U73122 reversed the positive effects of HGF (Fig. 4Ad-f), FN (Fig. 4Bc-e) or LN (Fig. 4Cc-e) on HGF-, FN- or LN-stimulated
motility and migration in vitro, respectively. These results
indicated that HGF, FN and LN modulated cell motility and migration
via the PLCγ/DAG/PKC signaling pathway in Hca-F cells, and that HGF
modulated cell motility and migration via the PI3K/AKT signaling
pathway in Hca-P cells.
Discussion
HGF has a number of important biological functions,
including the promotion of cell proliferation, inhibition of cell
adhesion and increasing the expression of urokinase-type
plasminogen activator receptor, which promotes the degradation of
the extracellular matrix. In addition, HGF increases cytoskeletal
protein phosphorylation and the destruction of the cytoskeleton,
which makes it easier for cells to be deformed and migrate.
Therefore, HGF is also known to promote mitogen, promoting
separation factor, cell morphogen and migration factor in cell
motility and migration. Not only is HGF important for the
regulation of embryonic development, organ formation and cell
growth, the growth factor also has a marked association with
tumorigenesis and tumor progression, invasion, proliferation and
metastasis (9).
HGF mediates these effects via its cognate receptor,
cMet. cMet is a single transmembrane receptor protein that
possesses tyrosine kinase activity. Upon ligand binding, cMet
undergoes dimerization and phosphorylates key tyrosine residues.
The intracellular SH2 domains of signaling molecules recognize the
phosphorylation of specific tyrosine residues, leading to the
activation of signal transduction. The key event in c-Met-mediated
HGF signal transmembrane transduction is the phosphorylation of
tyrosine residues. However, at present, it is unknown whether all
the tyrosine residues are phosphorylated following ligand binding
or only specific tyrosine residues. If the latter hypothesis is
correct, factors affecting the phosphorylation of cMet at specific
tyrosine residues require determination, as well as the effect of
different ligands on the phosphorylation of specific residues. In
addition, the correlation between the phosphorylation of tyrosine
residues and the activation of intracellular signal transduction
pathways, and the molecular mechanisms involved in the regulation
of tumor metastasis require investigation. Clarification of these
issues is likely to contribute to the understanding of the
molecular mechanisms of transmembrane growth factor
receptor-mediated signal transduction and tumor metastasis
(9).
To study the molecular mechanisms of cMet-mediated
cell signal transduction in the regulation of lymph node metastasis
of liver cancer cells, the effect of HGF, FN and LN on the
phosphorylation of c-Met at different tyrosine residues was
compared in two cell lines. Our results showed that, following HGF
treatment, the phosphorylation of cMet at Tyr 1313 and 1365 in
highly metastatic Hca-F cells was higher, whereas the
phosphorylation of cMet at Tyr 1349 was lower than that in low
metastatic Hca-P cells. In addition, the activity of the
PLCγ/DAG/PKC signaling pathway was greater in Hca-F cells compared
with Hca-P cells; however, the activity of the PI3K/AKT signaling
pathway was lower.
FN is an important cell adhesion molecule involved
in cell adhesion during tumor metastasis and has been reported to
directly or indirectly affect cMet activation, thus regulating
tumor metastasis (13). Results of
the current study demonstrated that, following FN treatment, the
phosphorylation of cMet at Tyr 1313 and the activity of the
PLCγ/DAG/PKC signaling pathway in highly metastatic Hca-F cells
were higher than that in low metastatic Hca-P cells.
LN is also an important cell adhesion molecule
involved in cell adhesion and the regulation of c-Met activation
and phosphorylation of tyrosine residues (13). The results of this study indicated
that, following LN treatment, the phosphorylation of cMet at Tyr
1365 and activity of the PLCγ/DAG/PKC signaling pathway in Hca-F
cells were higher than that in Hca-P cells.
In conclusion, under stimulation with various
ligands, phosphorylated tyrosine residues of c-Met and the
activated intracellular signal transduction pathways were found to
vary in the same cell line. We hypothesized that the molecules
involved in tumor metastasis were different at various stages and
their expression required the regulation of different intracellular
signal transduction pathways. Therefore, at different stages in the
process of tumor cell metastasis, different ligands bind to cMet,
leading to the phosphorylation of different tyrosine residues and
activation of different intracellular signal transduction pathways,
which accommodates the various processes involved in the regulation
of tumor cell metastasis.
Under stimulation by the same ligand, the
phosphorylated tyrosine residues of c-Met and the activation of
intracellular signal transduction pathways were different between
Hca-P and Hca-F cells. We hypothesized that these observations may
be associated with differences in membrane structure and the
microenvironment in which c-Met resides between the two cell lines.
On the cell surface, growth factor receptors often combine with
specific lipids (i.e., glycosphingolipids), membrane proteins and
intracellular signaling molecules to form a specific membrane
structure or microdomain, including sugar synapses (glycosynapse)
or lipid rafts. Growth factor receptor-mediated signal
transmembrane transduction is not only the result of ligand
interaction with the receptor, but also the result of complex
interactions of the receptor with other membrane components,
including membrane lipids and proteins. On the cell surface, cMet
is often enriched in specific membrane microdomains with four
transmembrane proteins: CD82, CD9, integrins and tyrosine protein
kinases (14,15). In addition, specific molecules,
including gangliosides, GM2/GM3, CD82 and CD9, inhibit the
activation of c-Met (16,17). Our previous studies also found that
the expression of gangliosides in the two cell lines used in the
present study was significantly different (18), which may explain why different
tyrosine residues were phosphorylated following c-Met activation
using the same ligand, which then activated different intracellular
signal transduction pathways in the two cell lines.
After cMet is activated, different tyrosine residues
are phosphorylated, which leads to the activation of the PI3K/AKT
and PLCγ/DAG/PKC signaling pathways to various extents in the two
cells lines. These observations may result in the different lymph
node metastatic potentials of the two cell lines.
In the current study, analysis of the relationship
between the corresponding changes in the phosphorylation of cMet at
different tyrosine residues and the activity of various signaling
pathways in Hca-P and Hca-F cells suggested that the
phosphorylation of cMet at Tyr 1313 and 1365 was associated with
PLCγ/DAG/PKC signaling pathway activity and the phosphorylation of
cMet at Tyr 1349 may play a role in the activity of the PI3K/AKT
signaling pathway. Furthermore, HGF, FN and LN were demonstrated to
modulate cell motility and migration via the PLCγ/DAG/PKC signaling
pathway.
The mechanisms of cMet phosphorylation and the
regulation of tumor metastasis appear to be complex and require
further study for a more complete understanding of these
processes.
Acknowledgements
The current study was supported by grants from the
National Program on Key Basic Research Project (973 program; no.
2012CB822103) and Liaoning Province Education Bureau (no.
2009T022).
Abbreviations:
|
PI3K
|
phosphoinositol-3-kinase
|
|
AKT
|
protein kinase B
|
|
PLCγ1
|
phospholipase γ1
|
|
DAG
|
diacylglycerol
|
|
PKC
|
protein kinase C
|
References
|
1
|
Edakuni G, Sasatomi E, Satoh T, et al:
Expression of the hepatocyte growth factor/c-Met pathway is
increased at the cancer front in breast carcinoma. Pathol Int.
51:172–178. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamaguchi R, Yano H, Iemura A, et al:
Expression of vascular endothelial growth factor in human
hepatocellular carcinoma. Hepatology. 28:68–77. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salgia R: Role of c-Met in cancer:
emphasis on lung cancer. Semin Oncol. 36(2 Suppl 1): S52–S58. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitra AK: Ligand-independent activation of
c-Met by fibronectin and α(5)β(1)-integrin regulates ovarian cancer
invasion and metastasis. Oncogene. 30:1566–1576. 2011.PubMed/NCBI
|
|
5
|
Sourbier C: Met and the microenvironment:
new insights for ovarian cancer metastasis. Cell Adh Migr.
5:209–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawakami Y, Kawakami K, Steelant WF, et
al: Tetraspanin CD9 is a ‘proteolipid,’ and its interaction with
alpha 3 integrin in microdomain is promoted by GM3 ganglioside,
leading to inhibition of laminin-5-dependent cell motility. J Biol
Chem. 277:34349–34358. 2002.
|
|
7
|
Sorokin AV, Mikhailov AM, Kachko AV, et
al: Human recombinant laminin-binding protein: isolation
purification and crystallization. Biochemistry (Mosc). 65:546–553.
2000.PubMed/NCBI
|
|
8
|
Toledo MS, Suzuki E, Handa K and Hakomori
S: Effect of ganglioside and tetraspanins in microdomains on
interaction of integrins with fibroblast growth factor receptor. J
Biol Chem. 280:16227–16234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Birchmeier C, Birchmeier W, Gherardi E and
Vande Woude GF: Met, metastasis, motility and more. Nat Rev Mol
Cell Biol. 4:915–925. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun P, Wang XQ, Lopatka K, Bangash S and
Paller AS: Ganglioside loss promotes survival primarily by
activating integrin-linked kinase/Akt without phosphoinositide 3-OH
kinase signaling. J Invest Dermatol. 119:107–117. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu H, Fukami K, Itoh T and Takenawa T:
Phosphorylation of phospholipase Cgamma1 on tyrosine residue 783 by
platelet-derived growth factor regulates reorganization of the
cytoskeleton. Exp Cell Res. 243:113–122. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeffers M, Rong S and Vande Woude GF:
Hepatocyte growth factor/scatter factor-Met signaling in
tumorigenicity and invasion/metastasis. J Mol Med (Berl).
74:505–513. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J and Geng X: HGF/cMet signaling
pathway in hepatocellular carcinoma invasion and metastasis. J Clin
Invest. 18:731–732. 2002.
|
|
14
|
Liu WM and Zhang XA: KAI1/CD82, a tumor
metastasis suppressor. Cancer Lett. 240:183–194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyake M, Koyama M, Seno M and Ikeyama S:
Identification of the motility-related protein (MRP-1), recognized
by monoclonal antibody M31-15, which inhibits cell motility. J Exp
Med. 174:1347–1354. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Todeschini AR, Dos Santos JN, Handa K and
Hakomori SI: Ganglioside GM2-tetraspanin CD82 complex inhibits met
and its cross-talk with integrins, providing a basis for control of
cell motility through glycosynapse. J Biol Chem. 282:8123–8133.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ono M, Handa K, Sonnino S, et al: GM3
ganglioside inhibits CD9-facilitated haptotatic cell motility:
coexpression of GM3 and CD9 is essential in the downregulation of
tumor cell motility and malignancy. Biochemistry. 40:6414–6421.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang X, Li Y, Zhang J, Xu Y, Tian Y and
Ma K: Ganglioside GM3 inhibits hepatoma cell motility via
down-regulating activity of EGFR and PI3K/AKT signaling pathway. J
Cell Biochem. 114:1616–1624. 2013. View Article : Google Scholar : PubMed/NCBI
|


















