Introduction
Inflammatory bowel disease (IBD) is comprised of two
distinct disorders, ulcerative colitis (UC) and Crohn’s disease
(CD), which exhibit independent clinical features and are fairly
distinct in their organ specificity and histopathological
characteristics. Their etiologies are unknown. In the majority of
cases, the onset of IBD occurs at a young age during the most
productive phase of life (1,2).
Anticipation, the development of the disease at an earlier age in
offspring than in their parents, has been discussed, but remains
controversial (3–7). UC and CD are chronic diseases, whose
clinical courses differ considerably, with frequent relapses or
chronically active disease in certain patients, whereas others have
years of virtually complete remission (1,2).
IBD remains a major clinical and therapeutic
challenge and animal models are required for research into the
complexity of the condition and to test the efficacy of
anti-inflammatory agents. There are several animal models for IBD,
including chemically induced colitis and mutant mice, such as
interleukin (IL)-2 and IL-10 knockout mice (8,9). No
model reproduces human IBD perfectly, but these models have
provided valuable information applicable to human IBD (8,9).
Trinitrobenzene sulfonic acid (TNBS) may be used to induce colitis
in experimental animals, yielding clinical and morphological
features that closely mimic those of Crohn’s disease, with the peak
of inflammation occurring 2–3 days following the induction of
colitis and continuing for a duration of 8 weeks (8,10,11).
It has been demonstrated that the total colonoscopy
of a rat is feasible and that the endoscopic inflammation scores
correlate well with those obtained using macroscopic and
microscopic assessments (11,12).
Colonic biopsies may also be obtained successfully during this
procedure (12). However, it is
unclear as to whether small tissue samples obtained in biopsies,
together with a colonoscopy, are sufficient in order to follow the
course of the inflammatory processes in experimental animals.
Therefore, the present study was undertaken in order to determine
the reliability of following the inflammatory course of
TNBS-induced colitis using a colonoscopy together with biopsy
samples obtained during the procedure.
Materials and methods
Rats
In the present study, we used 20 adult male Wistar
rats (Hannover GALAS™; Taconic Farms, Inc., Lille Skensved,
Denmark), with a mean weight of 201.9 g (range 166–246 g). The rats
were housed singly in Makrolon III cages and fed a standard diet
ad libitum (B&K Universal AS, Nittedal, Norway),
consisting of cereal products (88.5%), soy protein (6%), animal
protein (2.5%) and soy oil (0.5%), supplemented with vitamins,
minerals and amino acids (2.5%). Water was also provided ad
libitum. The rats were maintained at 21±1ºC, in a relative
humidity of 55±5% with a 12/12 h light/dark cycle. The rats were
left in the animal house for at least seven days to allow them to
acclimatize prior to the experiment. When the rats were fasting, a
grid floor was used so that their feces would fall down, thus
preventing the rats from eating them. The rats were randomized into
two groups, control and TNBS, with ten rats in each. The control
group was treated in the same way as the TNBS group, with the
exception of the introduction of TNBS intracolonically.
Induction of colitis with TNBS
Rats were deprived of food for 24 h prior to the
administration of TNBS. A single TNBS dose (Sigma-Aldrich Logistik,
Steinheim, Germany) was introduced into the colon of each rat (25
mg/rat in 50% ethanol solution; 0.5 ml/rat), followed by 2 ml of
air, at a distance of 8 cm from the anal margin via a probe (an 8.5
cm long, 2.5 mm round-tip Teflon tube) under isoflurane anesthesia.
Following the insertion of TNBS, the rats remained prone with their
hind legs raised for at least 1 min. The animals were supervised
until recovery and were monitored several times daily. If a rat
exhibited signs of pain, it was injected subcutaneously with 0.2 ml
Temgesic® (0.3 mg Temgesic/ml, Schering-Plough,
Whitehouse Station, NJ, USA).
Colonoscopy and mucosal biopsies
Colonoscopy and mucosal biopsies were performed on
the control and TNBS rats, three days following the introduction of
TNBS in the latter. The bowel was prepared as described previously
(12). The rats were fasted for 24
h and received a gastric dose of 1 and 2 ml Picoprep®
(Ferring, West Drayton, UK) followed by 2 ml water at 24 and 12 h,
respectively, prior to the colonoscopy. Picoprep consists of a
volume of 150 ml containing 10 mg of sodium sulfates, 3.5 g of
magnesium oxide and 12 g of citric acid. Picoprep was introduced
via an 8.5 cm long, 2.5 mm round-tip Teflon feeding gauge
(AngTheo’s AB, Lidingö, Sweden).
The rats were anesthetized by inhalation of
isoflurane (Schering-Plough) prior to and during colonoscopy. They
were then placed in a supine position and secured to an acrylic
surgical table (World Precision Instruments, Sarasota, FL, USA)
upon a warming pad (Gaymar T/Pad; Gaymar Industries, Orchard Park,
NY, USA) using a heat therapy pump (TP500 t/Pump; Gaymar) to
maintain normothermia during the procedure. A video gastroscope
(GIF-N180; Olympus, Tokyo, Japan) was used. Biopsies were obtained
with the aid of disposable biopsy forceps (EndoJaw FB-231K; Olympus
Medical System, Tokyo, Japan). The tip of the scope was lubricated
with 2% lidocaine (Xylocaine®; AstraZeneca, London, UK)
and introduced gently into the anus.
The endoscopic grading scale of inflammation used
was the same as that used by Vermeulen et al(11). This scale comprises the following
five subscales (with a total score ranging from 0–19): degree of
inflammation (0–6), extent of disease (0–10), stenosis (0–1), edema
(0–1) and active bleeding (0–1).
Following the procedure, the rats were allowed to
recover from anesthesia and were monitored for ~1 h. They were then
sacrificed by inhalation of CO2 and a post-mortem
laparotomy was performed in which the abdomen and colon were
examined. The colon was opened and washed with saline solution and
the degree of inflammation was assessed using an adaptation of the
Wallace and Keenan macroscopic scoring system (13). Using this scoring system,
inflammation is assessed on a scale from 0–10 based on the degree
of ulceration, inflammation and extent of disease: 0, normal aspect
of the mucosa; 1, localized hyperaemia without ulcerations; 2,
ulceration; 3, ulceration with thickening of bowel wall at one
site; 4, two or more sites of ulceration and thickening of the
bowel wall; 5, major sites of damage extending >1 cm along the
length of the colon; 6–10, damage extending >2 cm (the score
increases by one for each centimeter of damaged tissue). Tissue
samples from the colon were collected for histological
examination.
Histopathological examination
Biopsy samples obtained during colonoscopy and
whole-wall colon tissues removed during the post-mortem laparotomy
were fixed in 4% buffered paraformaldehyde overnight, embedded in
paraffin and cut into 5 μm sections. The sections were HE stained.
Inflammation was evaluated using the scoring system of Hunter et
al(14), in which the total
score is a summation of four parameters: inflammatory infiltration
(0–3), the number of gut walls engaged (0–3), damage to the mucosal
architecture (0–1) and edema (0–1). For biopsy specimens, the
number of gut wall engagements was graded 0–2. The total score of
this scale ranged from 0–10 for whole-wall colon tissue and 0–9 for
the biopsies.
This study was performed in accordance with the
Directive for the Protection of Vertebrate Animals used for
Experimental and other Scientific Purposes (86/609/EEC), in
compliance with the Helsinki Declaration. The local ethical
committee for experimental animals at the University of Bergen
approved the protocols of the study.
Statistical analysis
The paired t-test and non-parametric Spearman’s
correlation test were used. P<0.5 was considered to indicate a
statistically significant difference. The data are expressed as the
means ± SEM values.
Results
Prior to the endpoint of the experiment,
three rats in the TNBS group died
Post-mortem macroscopic examination of the abdomen
and colon of these rats revealed severe colonic inflammation with
perforation. Total colonoscopy was performed successfully in the
remaining rats, who recovered well from the procedure.
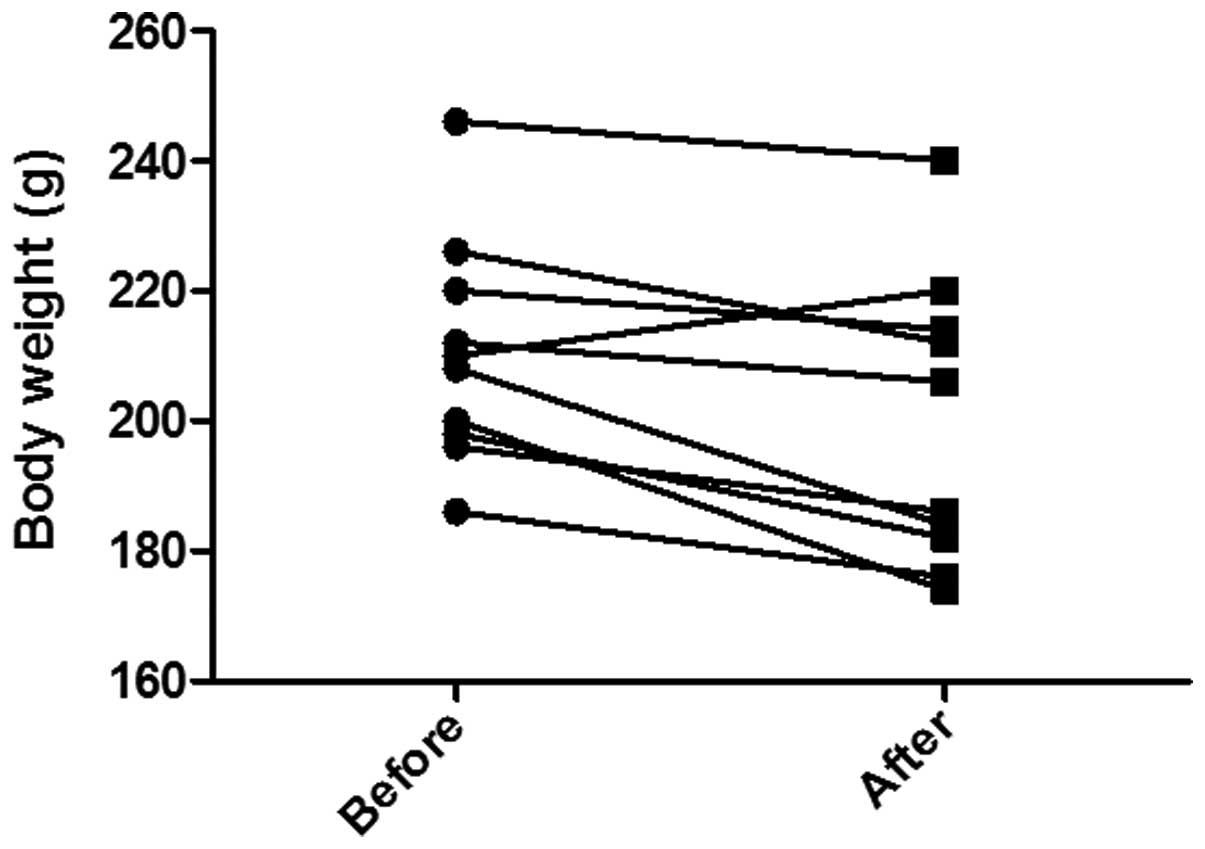
The body weight in the control group was 191.5±9.8 g
at the beginning and end of the experiment. In the TNBS group, the
body weight was 210.2±5.5 g at the start of the experiment and
199.4±7.0 g at its end point (Fig.
1). This weight loss was considered to be statistically
significant (P=0.009).
Colonoscopy findings
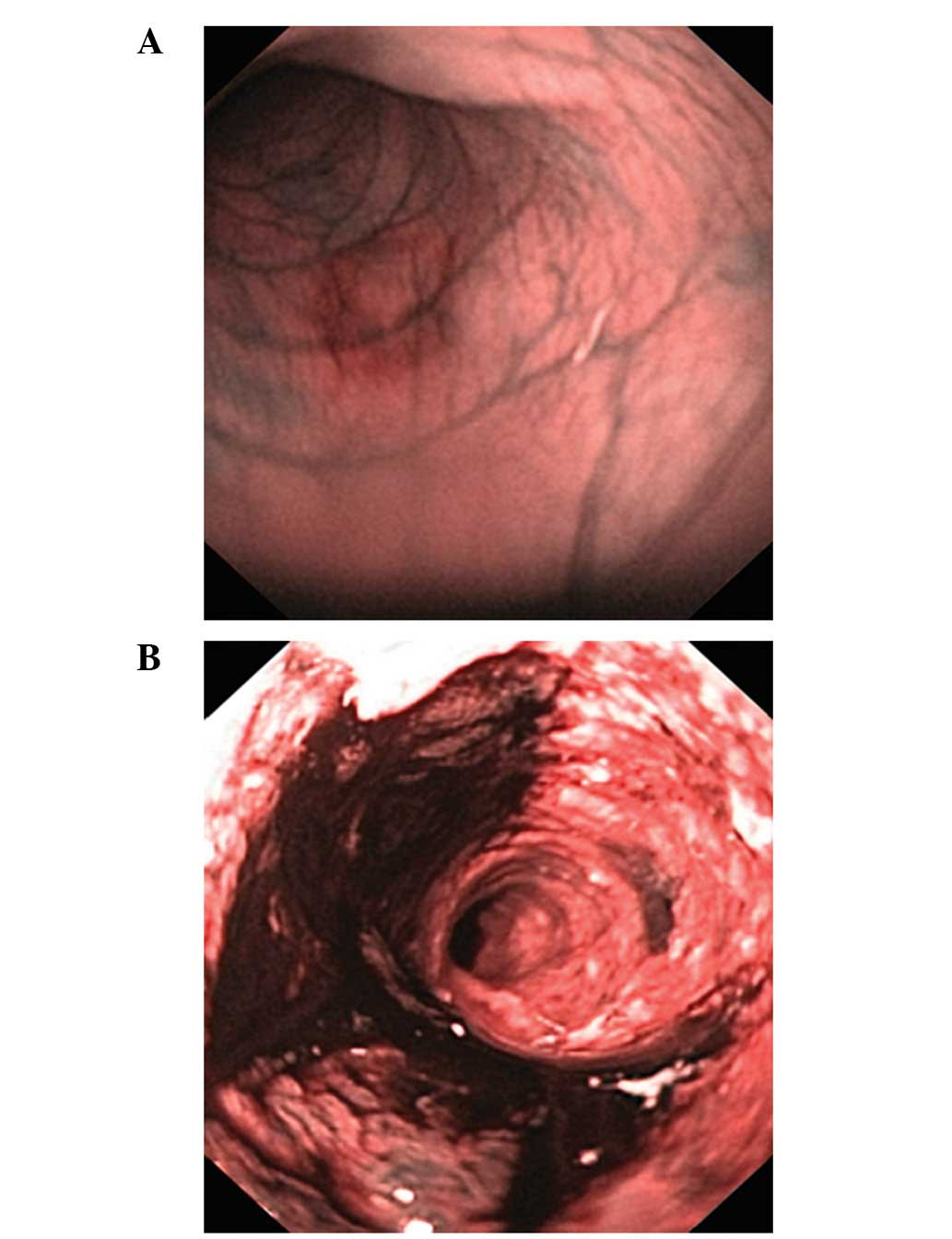
Colonoscopy examinations of the control group did
not reveal any inflammation. Severe colitis was observed in every
TNBS-induced colitis rat, with a Vermeulen inflammation score of
12.2±1.0 (Fig. 2).
Macroscopic appearance
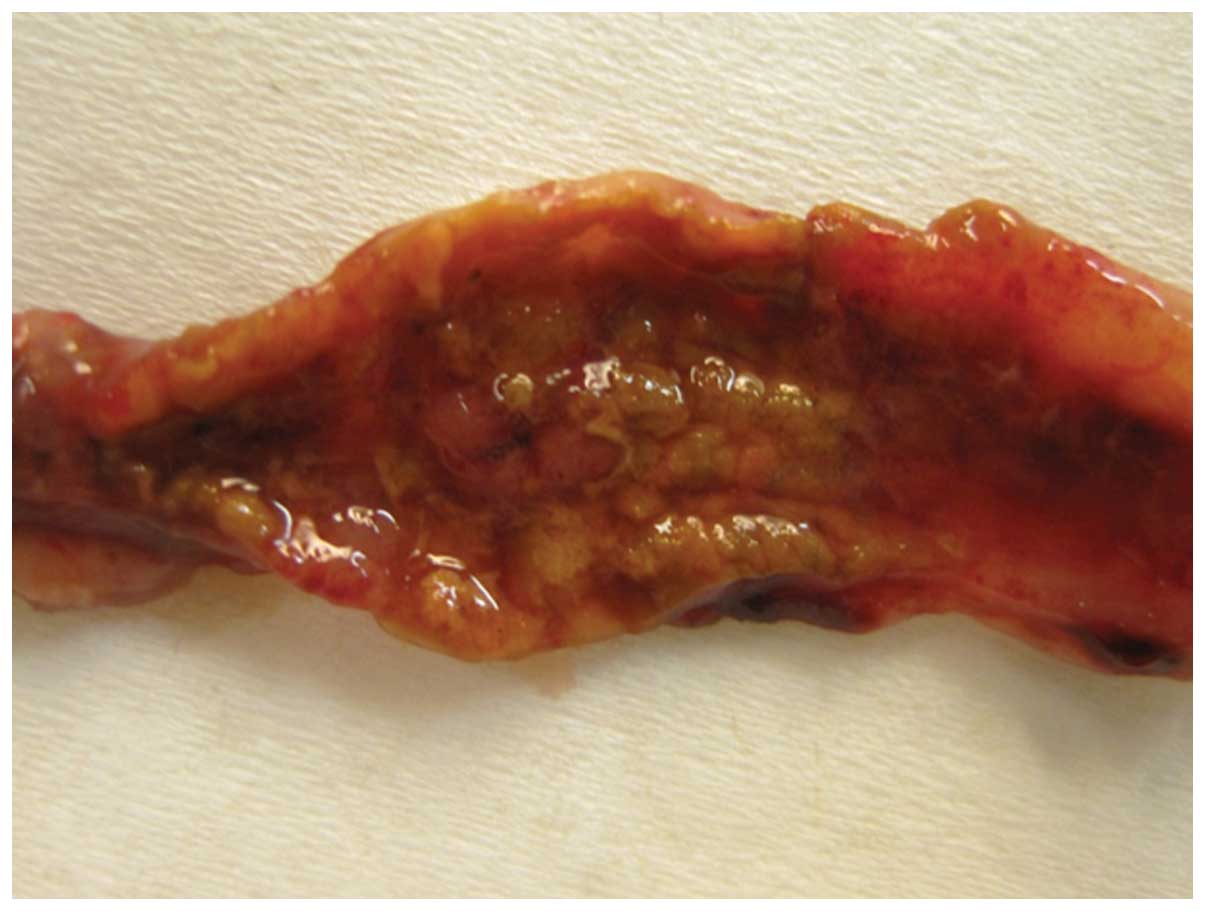
Post-mortem macroscopic examination of the abdomens
and colons of the control group did not reveal any complications or
inflammation in the colon. The Wallace and Keenan inflammation
score for the TNBS group was 5.7±0.4 (Fig. 3).
Histopathological findings
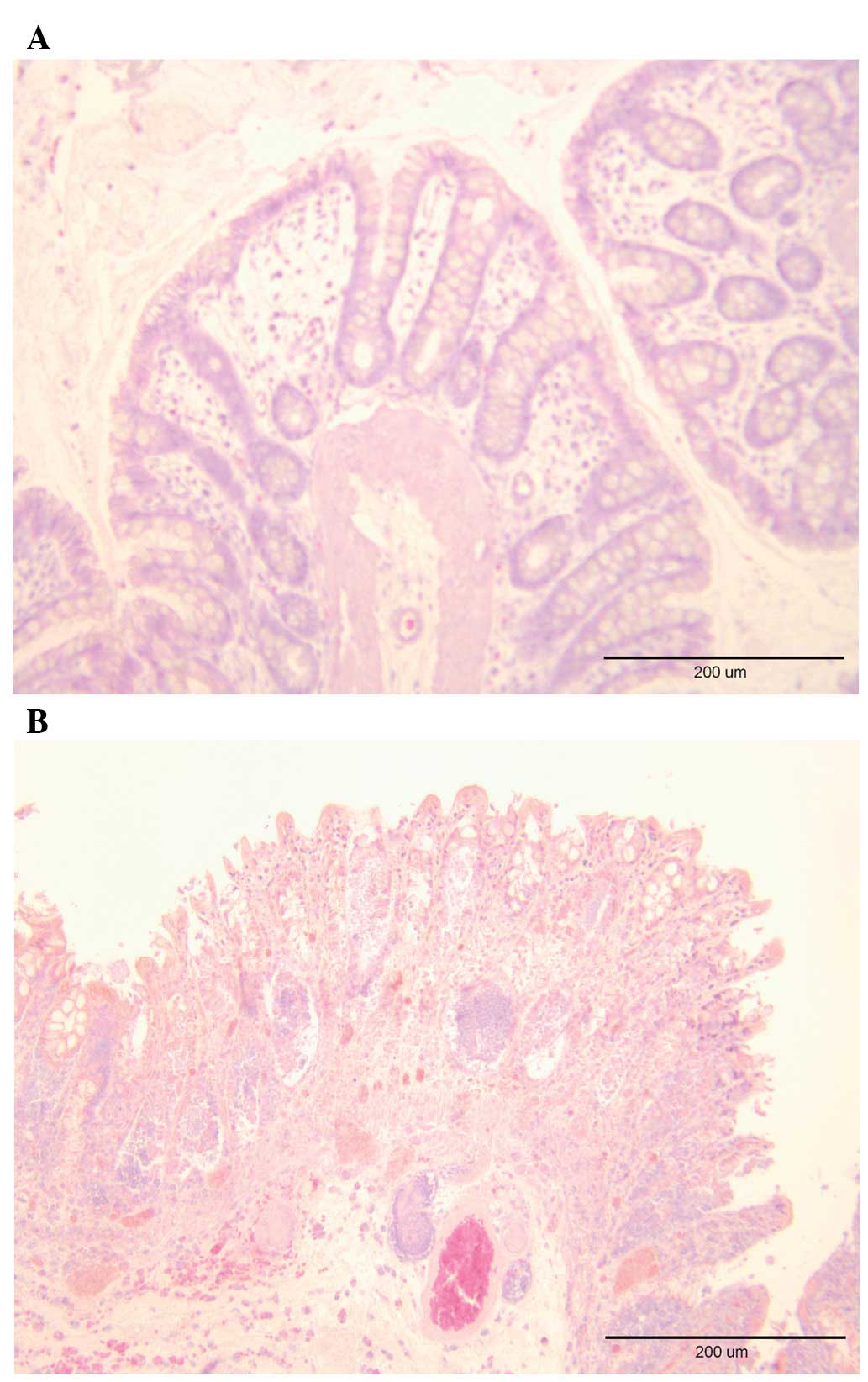
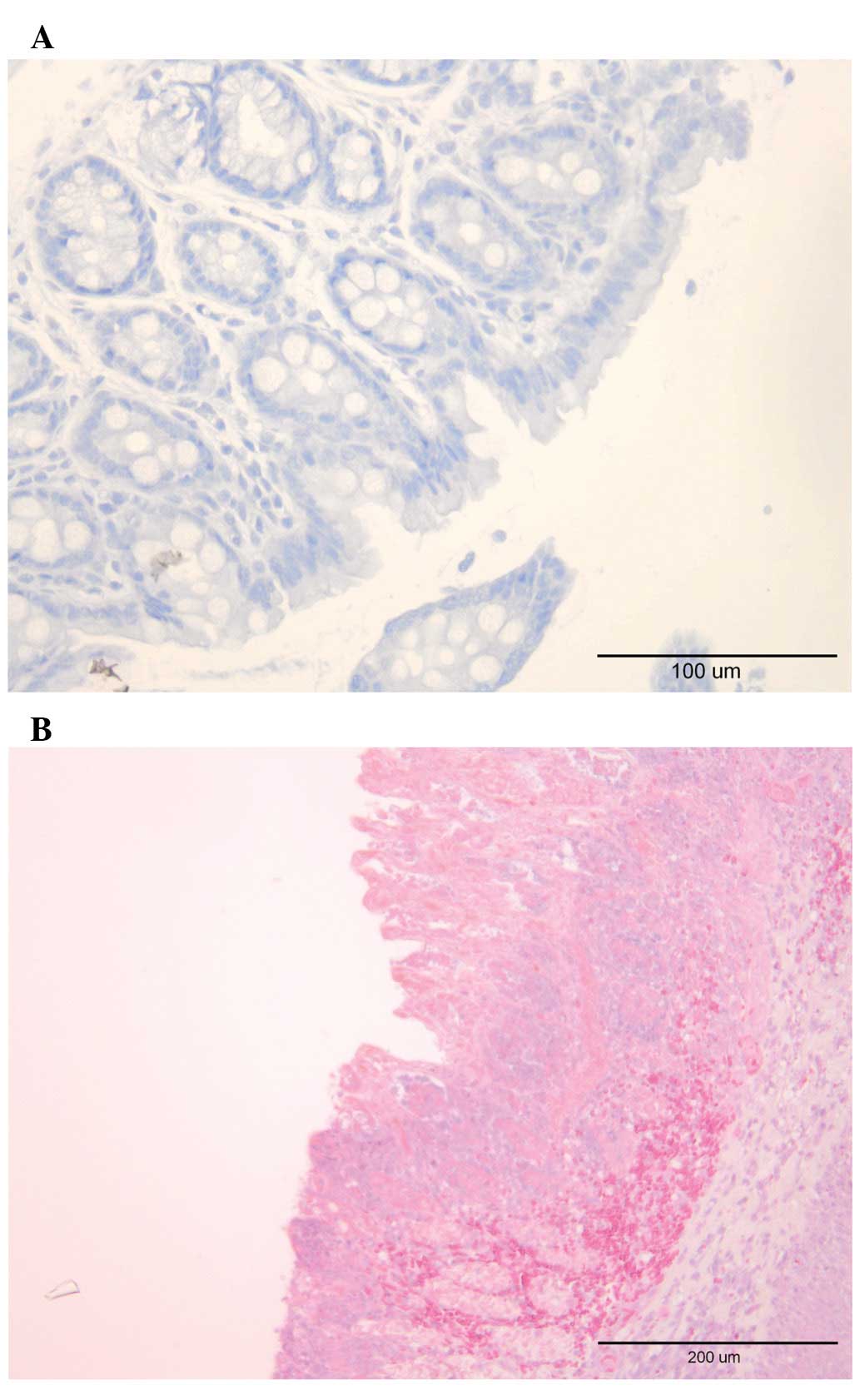
Examination of the whole-wall colonic tissue samples
from the control group did not reveal any significant signs of
inflammation. The same was true for biopsies obtained during
colonoscopy examination. In rats with TNBS-induced colitis, the
inflammation scores in whole-wall colonic samples and biopsies
collected during colonoscopy were 6.50±0.27 and 4.80±0.25,
respectively (Figs. 4 and 5).
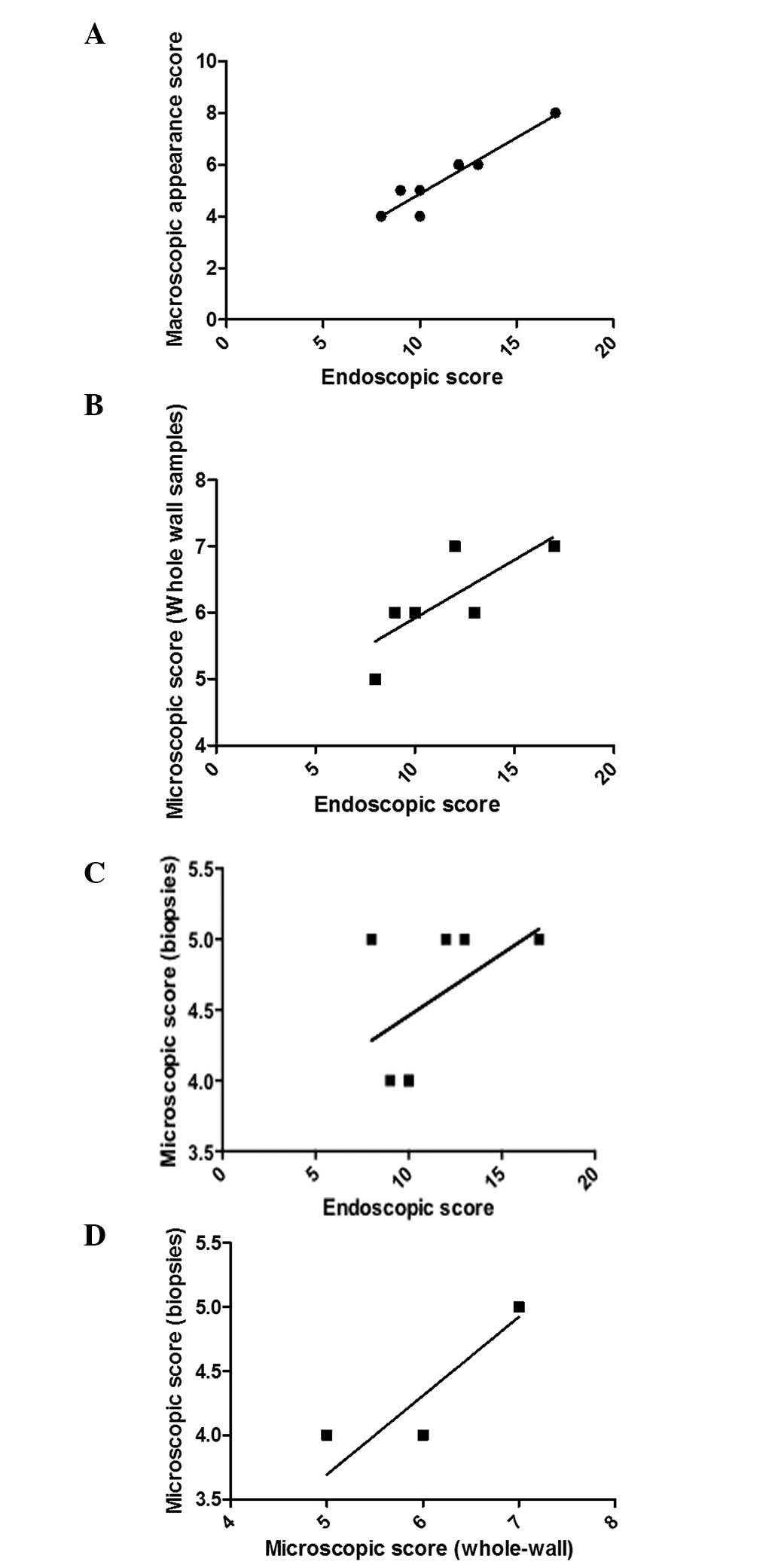
The endoscopic inflammation score obtained through
colonoscopy examinations correlated with that established
macroscopically (P<0.0001, r=0.9), and those obtained
microscopically from the score obtained from the whole-wall colonic
(P=0.01, r=0.8) and biopsy samples collected during colonoscopy
(P=0.02, r=0.8). The inflammation scores from whole-wall colonic
and biopsy samples collected during colonoscopy correlated markedly
(P=0.004, r=0.9; Fig. 6).
Discussion
Crohn’s disease exhibits endoscopically deep
aphthoid ulcerations on otherwise normal mucosa, edema, and
fibrotic and inflammatory stenoses (15). Histopathologically, transmural
inflammatory ulceration and aphthoid ulceration [small foci with
superficial ulcerations deep in the crypts over a lymphoid follicle
(16)]. Colonoscopy examination of
rats following the induction of TNBS-induced colitis revealed deep
widespread ulcers, edema and inflammatory stenoses. The
corresponding histopathological findings indicated transmural
ulceration, inflammatory cell infiltration and mucosal damage.
These findings emphasize the similarities between TNBS-induced
colitis and Crohn’s disease.
The inflammation was evaluated three days following
the induction of colitis. There was a significant weight loss and
the inflammatory scores were 64.2, 57, 65 and 53.3% of the maximum
score as assessed by endoscopy, macroscopic appearance and the
histological examination of whole-wall colonic and biopsy samples,
respectively. As three rats died due to severe inflammation and
perforation, the inflammation that occurred at the time of
evaluation should be considered as severe. These findings are in
agreement with earlier observations regarding TNBS-induced colitis
in rats, of which revealed the maximal inflammation 2–3 days
following induction (8,14).
The degree of inflammation, as assessed by
endoscopic examinations, correlated markedly with that assessed
macroscopically and with the histopathological examination of the
colonic whole-wall and biopsy samples obtained during endoscopy.
Thus, colonoscopy may be used to follow the inflammatory course in
rats with induced colitis, in accordance with previously reported
observations (14). Furthermore,
the present study revealed that biopsy samples collected during
colonoscopy are useful in assessing the degree of inflammation in
addition to endoscopic findings. However, the degree of
inflammation assessed by endoscopy, expressed as a percentage of
the maximum score, is more consistent with the histopathological
assessment of whole-wall colonic samples than with the biopsy
samples obtained during colonoscopy. This may be due to the fact
that i) the whole-wall samples provide a larger area for
examination, which is important in TNBS-induced colitis for the
spread of pathological changes in otherwise normal mucosa, and/or
ii) biopsy samples only include two of the wall layers, namely the
mucosa and submucosa, whereas whole-wall samples include a third
layer, the muscularis, which is particularly important as the
severity of inflammation is dependent upon the depth of
ulceration.
In conclusion, colonoscopy provides a reliable
method for following up on the course of inflammation in
experimentally induced colitis. Although biopsy samples obtained
during colonoscopy may be used as well in the assessment of the
degree of inflammation, whole-wall samples are superior in this
regard.
Acknowledgements
This study was supported by a grant from
Helse-Fonna.
References
|
1
|
Danese S and Fiocchi C: Etiopathogenesis
of inflammatory bowel diseases. World J Gastroenterol.
12:4807–4812. 2006.PubMed/NCBI
|
|
2
|
Nunes T, Fiorino G, Danese S and Sans M:
Familial aggregation in inflammatory bowel disease: Is it genes or
environment? World J Gastroenterol. 17:2715–2722. 2011.PubMed/NCBI
|
|
3
|
Heresbach D, Gulwani-Akolkar B, Lesser M,
et al: Anticipation in Crohn’s disease may be influenced by gender
and ethnicity of the transmitting parent. Am J Gastroenterol.
93:2368–2372. 1998.
|
|
4
|
Grandbastien B, Peeters M, Franchimont D,
et al: Anticipation in familial Crohn’s disease. Gut. 42:170–174.
1998.
|
|
5
|
Lee JC, Bridger S, McGregor C, Macpherson
AJ and Jones JE: Why children with inflammatory bowel disease are
diagnosed at a younger age than their affected parent. Gut.
44:808–811. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Faybush EM, Blanchard JF, Rawsthorne P and
Bernstein CN: Generational differences in the age at diagnosis with
Ibd: genetic anticipation, bias, or temporal effects. Am J
Gastroenterol. 97:636–640. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hampe J, Heymann K, Kruis W, Raedler A,
Fölsch UR and Schreiber S: Anticipation in inflammatory bowel
disease: a phenomenon caused by an accumulation of confounders. Am
J Med Genet. 92:178–183. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elson CO, Sartor RB, Tennyson GS and
Riddell RH: Experimental models of inflammatory bowel disease.
Gastroenterology. 109:1344–1367. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saleh M and Elson CO: Experimental
inflammatory bowel disease: insights into the host-microbiota
dialog. Immunity. 34:293–302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Milde AM and Murison R: A study of the
effects of restraint stress on colitis induced by dextran sulphate
sodium in singly housed rats. Integr Physiol Behav Sci. 37:140–150.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vermeulen W, De Man JG, Nullens S,
Pelckmans PA, De Winter BY and Moreels TG: The use of colonoscopy
to follow the inflammatory time course of TNBS colitis in rats.
Acta Gastroenterol Belg. 74:304–311. 2011.PubMed/NCBI
|
|
12
|
El-Salhy M, Wendelbo I, Gundersen D,
Hatlebakk JG and Husken T: Colonoscopy in young rats with mucosa
biopsies: A model for experimental gastroenterology. Mol Med Rep.
7:1757–1760. 2013.PubMed/NCBI
|
|
13
|
Wallace JL and Keenan CM: An orally active
inhibitor of leukotriene synthesis accelerates healing in a rat
model of colitis. Am J Physiol. 258:G527–G534. 1990.PubMed/NCBI
|
|
14
|
Hunter MM, Wang A, Hirota CL and McKay DM:
Neutralizing anti-IL-10 antibody blocks the protective effect of
tapeworm infection in a murine model of chemically induced colitis.
J Immunol. 174:7368–7375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Modigliani R: Endoscopy. Inflammatory
bowel disease. Järnerot G, Lennard-Jones J and Truelove S: Corona
Astra; Malmö, Sweden: pp. 243–267. 1992
|
|
16
|
Modigliani R: The pathology. Inflammatory
bowel disease. Järnerot G, Lennard-Jones J and Truelove S: Corona
Astra; Malmö, Sweden: pp. 269–293. 1992
|