Introduction
Paclitaxel and docetaxel belong to the taxane family
and are based on the backbone structure of baccatin III. These
drugs have been widely used as antineoplastic agents for almost two
decades and are effective for the treatment of a wide spectrum of
cancer forms (1–3). However, the development of tumor
resistance to these agents has limited their clinical success
(4). Therefore, an approach to
enhance the cytotoxicities of paclitaxel and docetaxel while
minimizing resistance is required.
Gap junctions (GJs) are plasma membrane channels
between adjacent cells that may be open or closed (gating
function). GJs are composed of two hemichannels, also known as
connexons. Each connexon contains six transmembrane connexin (Cx)
monomers and docks to its counterpart in the coupled cell membrane
to form a GJ channel. GJs provide a direct method of intercellular
communication via the transfer of ions, metabolites and other small
molecules. GJ intercellular communication (GJIC) is crucial for a
number of physiological processes, including cell proliferation,
differentiation, synchronization and maintenance of homeostasis
(5,6). In addition, a number of studies have
demonstrated that GJIC is important for cancer biology (7–9).
Previous studies have demonstrated that the toxicity
of cisplatin and oxaliplatin is increased by the presence of GJIC
in transformed cell lines (10,11).
The enhanced cytotoxicity may be due to the intercellular
transmission of a ‘death signal’ via GJ channels to neighboring
cells. This effect has been reported in radiation treatment, where
unirradiated cells adjacent to irradiated cells also underwent cell
death (12,13). In addition, the engagement of GJs
may represent an approach for enhancing the efficacy of cancer
therapeutics, whereas the inhibition of GJs is likely to decrease
their efficacy.
The sensitivity of human glioblastoma cells to
paclitaxel is increased by Cx43 expression, in part, by
downregulation of BCL-2 (14).
Paclitaxel has an inhibitory effect on GJ function in lens
epithelial cells, while GJIC is augmented by docetaxel in murine
salivary gland carcinoma cells (15,16).
However, the link between the cytotoxicity of paclitaxel/docetaxel
and GJIC has not been determined. In addition, while the structures
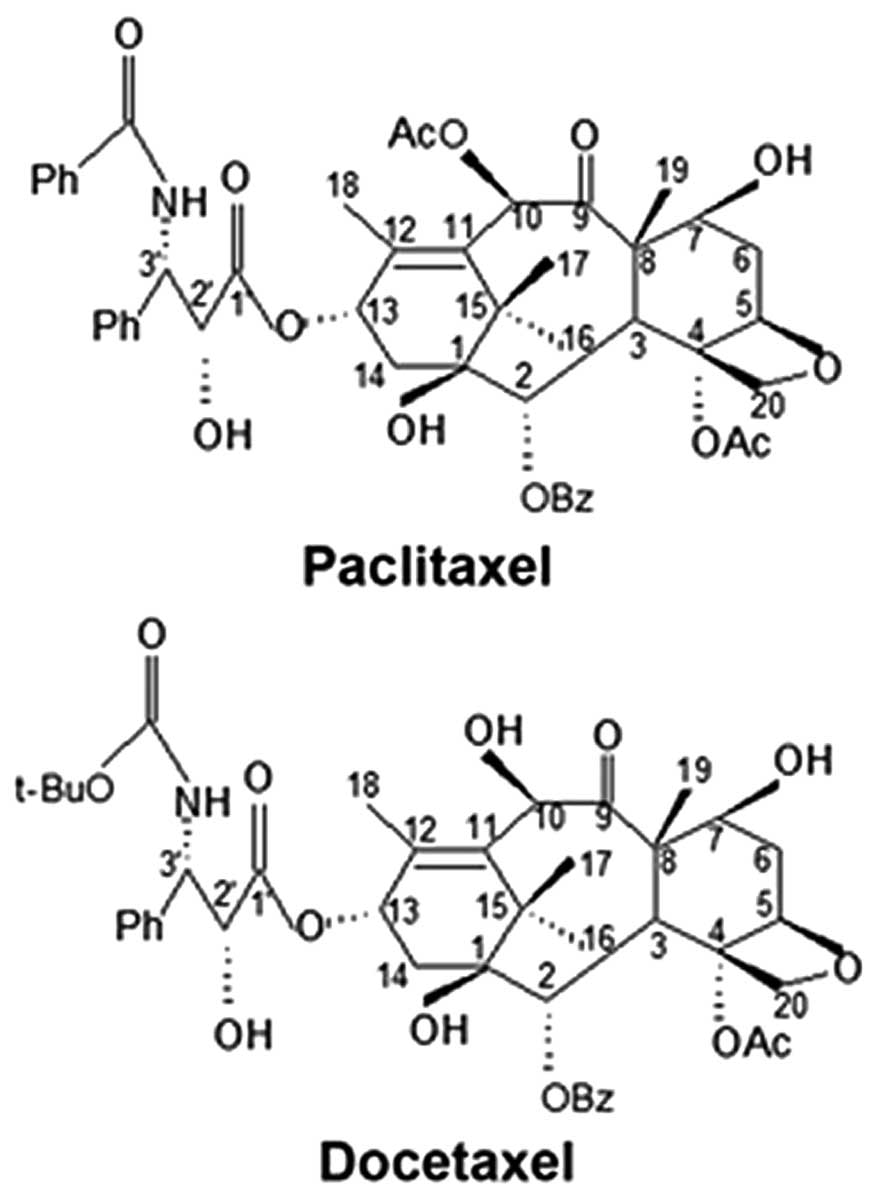
of paclitaxel and docetaxel exhibit only minor differences
(Fig. 1), their effects on
junctional channels are distinguishable. The comprehensive
mechanisms by which these two agents affect Cx channels remain
largely unknown (14,16).
In the present study, functional GJs were found to
enhance paclitaxel- and docetaxel-induced cytotoxicity in HeLa
cells transfected with the Cx32 gene (Cx32 HeLa cells). Docetaxel
has a modest effect on GJIC, while paclitaxel suppresses GJ
function in Cx32 HeLa cells. The inhibition of Cx32 channels by
paclitaxel was found to be associated, in part, with the closure of
their gating function following short-term exposure to paclitaxel.
The differential effects of paclitaxel and docetaxel on GJs affect
their own toxicities in Cx32 HeLa cells.
Materials and methods
Materials
Paclitaxel, docetaxel, doxycycline and
18-α-glycyrrhetinic acid (18-α-GA) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Calcein acetoxymethyl ester
(Calcein-AM) and cell culture reagents were purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA). Primary and
secondary antibodies for western blot analysis were obtained from
Sigma-Aldrich. Secondary antibodies for immunofluorescence were
purchased from Invitrogen Life Technologies. All other reagents
were purchased from Sigma-Aldrich unless otherwise stated.
Cell culture
The HeLa cell line used in this study expressed the
Cx32 gene under the control of a bidirectional
tetracycline-inducible promoter and was previously described
(17). The Cx32 coding sequence
was followed by an influenza hemagglutinin (HA) epitope tag at the
C-terminus and Cx32 expression was induced by doxycycline (1 μg/ml)
for 48 h. Cells were grown in DMEM supplemented with 10% (v/v)
fetal bovine serum, 200 μg/ml hygromycin B and 100 μg/ml G418
sulfate.
Paclitaxel/docetaxel and 18-α-GA
treatment
Paclitaxel/docetaxel and 18-α-GA were dissolved in
dimethylsulfoxide (DMSO) and diluted in cell culture medium where
required. The final DMSO concentration did not exceed 0.1% (v/v).
18-α-GA (10 μM) was added to the cells 1 h prior to treatment.
18-α-GA was not removed from the cells during paclitaxel/docetaxel
treatment.
Assay to measure the cytotoxicity of
paclitaxel and docetaxel
The toxicity of paclitaxel/docetaxel was evaluated
using a standard colony-forming assay (10). Briefly, cells were seeded at high
density (~3×104 cells/cm2) to ensure cells
were 70–100% confluent at the time of drug treatment. Following
exposure to paclitaxel/docetaxel for 6 h at 37ºC, cells were rinsed
twice with PBS, harvested by trypsinization, counted, diluted and
reseeded into 6-well plates at a density of 500 cells/well. The
cells were cultured for 5–8 days and then stained with 4% crystal
violet in ethanol. Colonies containing ≥50 cells were counted. For
the low cell density condition, cells were seeded at ~100
cells/cm2. GJs are not formed under these conditions as
the cells were not able to form contacts with each other. Cells
were incubated with paclitaxel/docetaxel for 6 h following
attachment and processed as described. Plating efficiency was
normalized against the number of colonies formed by vehicle-treated
cells. The colony forming efficiency of the untreated cells grown
under the low- and high-density conditions were not found to be
significantly different from each other (data not shown).
Dye-coupling assay
A ‘parachute’ dye-coupling assay was used to test
the functional GJs, as described previously (17,18).
Donor cells were dual-labeled in culture medium supplemented with 5
μM CM-Dil and 5 μM Calcein-AM. CM-Dil is a red membrane dye that
does not permeate through GJs to spread to adjacent cells.
Calcein-AM is transformed intracellularly into the GJ-permeable dye
calcein. The donor cells were washed with PBS, trypsinized and
seeded on a monolayer of receiver cells at a ratio of 1:150
(donor/receiver) for 4 h at 37ºC. Receiver cells containing calcein
were monitored by fluorescence microscopy and the average number of
receiver cells containing calcein/donor cell was scored and
normalized to that of the control.
Western blot analysis
Western blot analysis was performed as described
previously (11). In brief,
whole-cell lysates (20 μg) were fractionated by 10% SDS-PAGE and
transferred onto a nitrocellulose membrane. Cx32 protein was
detected by exposure to mouse anti-HA clone HA-7 IgG in
Tris-buffered saline and Tween-20 (dilution, 1:1,000), followed by
the addition of alkaline phosphatase-conjugated goat anti-mouse IgG
(secondary; dilution, 1:2,000). The anti-β-actin primary and
secondary detection antibodies were diluted to 1:10,000. All
western blot exposures were within the linear range of detection
and the intensities of immunopositive bands were quantified using
Quantity One software (Bio-Rad, Hercules, CA, USA).
Immunofluorescence of Cx32
Following treatment with paclitaxel/docetaxel for 1
h, cells were fixed with 4% paraformaldehyde and then blocked with
2% BSA. Mouse anti-HA (dilution, 1:200) was applied for 4 h at room
temperature, followed by an FITC-conjugated goat anti-mouse
secondary antibody (dilution, 1:400) for 1 h in the dark. Hoechst
33258 was applied for 5 min to stain the nuclei of the cells.
Following washing three times with PBS, cells were visualized and
images were captured using an Olympus IX71 fluorescence
microscope.
Dye uptake assay
A dye uptake assay was used for evaluating the
hemichannel activity. Cx32 HeLa cells were plated at low densities
such that the majority of the cells were not in physical contact
with each other. The cells were exposed to paclitaxel/docetaxel in
Ca2+-free DMEM for 1 h, followed by incubation with 1%
lucifer yellow (LY) and 1% rhodamine dextran (RD) in PBS for 10
min. LY is a GJ-permeable dye and serves as a tracer for
hemichannel activity. RD is too large to spread via GJs and was
therefore used as a negative control. Following treatment, the
cells were rinsed with culture medium containing normal calcium and
observed under a fluorescence microscope. Dye uptake was measured
using the percentage of fluorescent cells containing LY normalized
to that of the solvent control.
Statistical analysis
Data were analyzed with the Sigma Plot software
(Jandel Scientific, San Rafael, CA, USA) using the unpaired
Student's t test and presented as the mean ± SEM. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of cell density on the
cytotoxicity of paclitaxel/docetaxel
HeLa cells expressing Cx32 were cultured under
low-density conditions, in which GJs did not form as the cells were
not in physical contact and high-density conditions, which
permitted GJ formation. Following exposure to paclitaxel/docetaxel
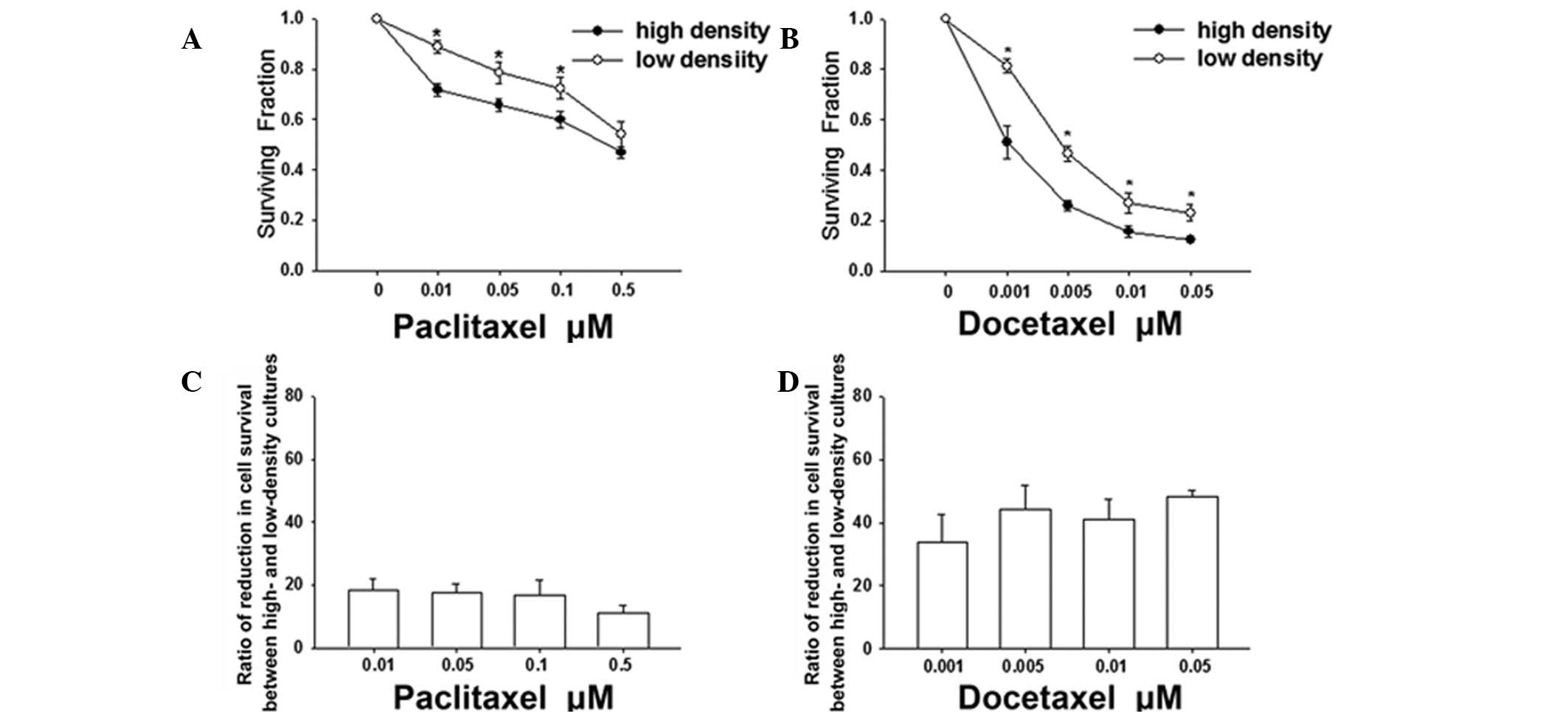
for 6 h, cell survival was assessed using a standard colony
formation assay. At the two densities, paclitaxel and docetaxel
reduced the clonogenic survival in a dose-dependent manner
(Fig. 2). However, the survival of
cells plated at a high density was significantly less than that of
cells plated at a low density at concentrations of paclitaxel up to
0.1 μM (Fig. 2A) or at
concentrations of docetaxel up to 0.05 μM (Fig. 2B). These results indicate that the
cytotoxicity of these agents is greater when cells are plated at
high densities. In addition, Fig. 2C
and D demonstrate that the reduction ratios of the cell
survival between high- and low-density cultures were 18.5±3.5,
17.7±2.7, 16.8±4.9 and 11.11±2.5% at concentrations of paclitaxel
between 0.01 and 0.5 μM, respectively and 33.8±9.0, 44.2±7.7,
41.0±6.4 and 48.3±2.0% at concentrations of docetaxel between 0.001
and 0.05 μM, respectively. These results indicate that the enhanced
sensitivity of docetaxel attributable to the high-density culture
is higher than that of paclitaxel at the tested concentrations.
Specifically, cell death at low density was ~20% at 0.05 μM
paclitaxel and 0.001 μM docetaxel. However, cell death at high
density was 34 and 49% at the same concentrations of paclitaxel and
docetaxel, which represent increases by factors of 1.7 and 2.5,
respectively. Thus, the toxic effect of docetaxel was higher than
that of paclitaxel when cells were grown at high-cell density.
Together, these results demonstrate that the
cytotoxicities of paclitaxel and docetaxel are dependent on cell
density and indicate that docetaxel induces a greater increase in
toxicity to high-density culture than paclitaxel.
Effect of cell density on paclitaxel and
docetaxel response is associated with GJIC
The difference between the cytotoxicity of
paclitaxel and docetaxel in Cx32 HeLa cells at low- and
high-density conditions indicates a possible role for intercellular
communication. GJIC is a major pathway for such intercellular
communication. To investigate the effect of GJIC on
paclitaxel/docetaxel sensitivity, two methods were adopted to
modulate Cx expression and GJ function, including the induction of
Cx32 expression with doxycycline and the inhibition of GJIC using a
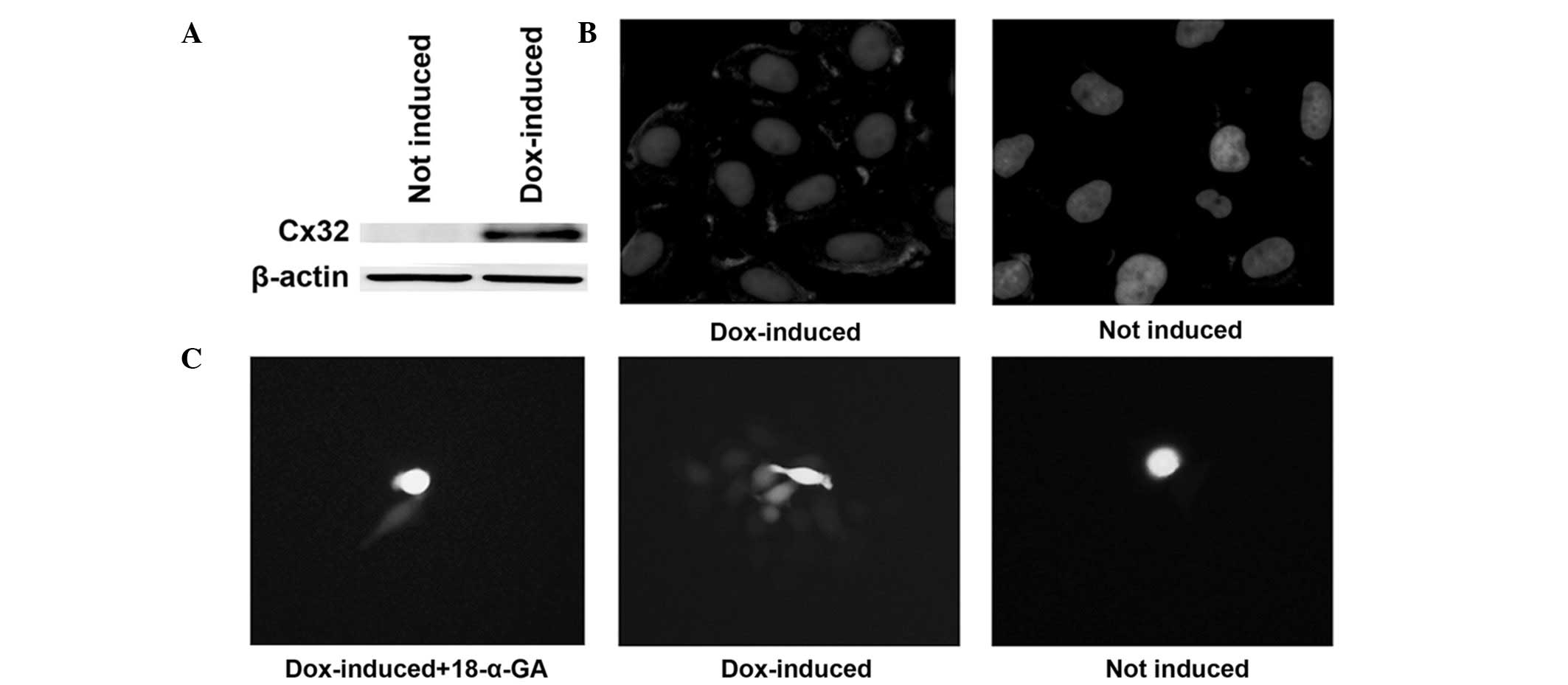
chemical inhibitor. The induction of Cx32 expression with
doxycycline and its localization on the cell membrane were
confirmed by western blot analysis and immunofluorescence (Fig. 3A and B). The emergence of GJIC in
Cx32 HeLa cells and the inhibitory effect of 18-α-GA, a known GJ
blocker (19), were detected using
a ‘parachute’ dye-coupling assay (Fig.
3C).
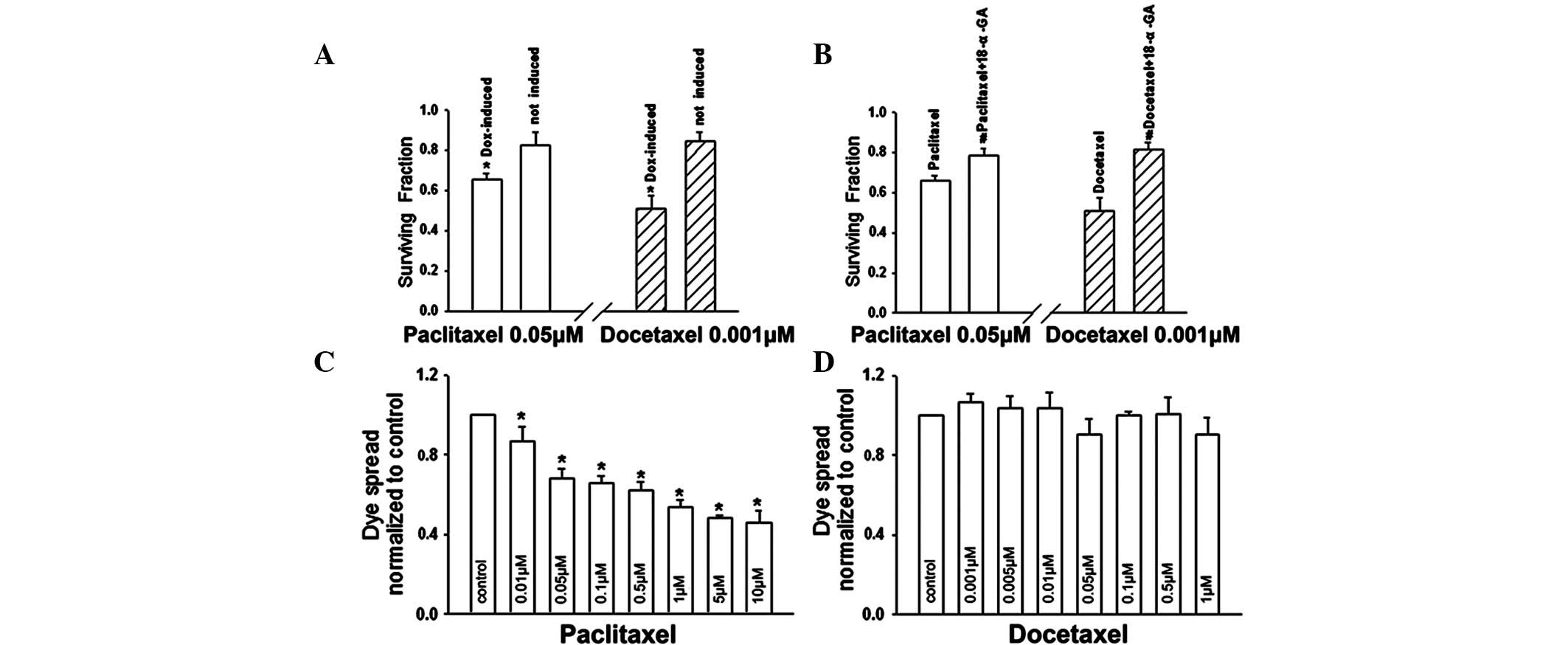
At high densities, the survival of
doxycycline-induced (Cx32 expression) cells was substantially
decreased at 0.05 μM paclitaxel or 0.001 μM docetaxel, compared
with the survival of uninduced (no Cx32 expression) cells.
Specifically, cell survival induced by paclitaxel or docetaxel was
significantly reduced by 20.3 and 39.3%, respectively (Fig. 4A). Incubation of Cx32-expressing
cells with 10 μM 18-α-GA under high cell-density conditions
increased survival from 65.7 to 79.0% in the presence of 0.05 μM
paclitaxel (P<0.05) and from 51.0 to 81.2% in the presence of
0.001 μM docetaxel (P<0.05; Fig.
4B). These results indicate that the toxicities of paclitaxel
and docetaxel are significantly increased when cells are plated at
high density and Cx32 is expressed, or when junctional channels are
not blocked. These results indicate that enhanced paclitaxel and
docetaxel cytotoxicities at high cell densities are mediated by
GJIC.
Effect of paclitaxel and docetaxel on GJ
function
As described, paclitaxel/docetaxel toxicity is
regulated by GJIC at high cell densities. Cell death is likely to
markedly reduce the valid cell density for forming GJIC. Therefore,
if paclitaxel or docetaxel affected channel function by influencing
exclusion from cell death, the toxicity of the agents would be
altered. To verify this hypothesis, a ‘parachute’ dye-coupling
assay was performed to determine the effects of paclitaxel and
docetaxel on Cx32 channels. Following treatment of Cx32 HeLa cells
with paclitaxel/docetaxel for 1 h, which did not lead to cell
death, paclitaxel markedly reduced dye coupling (Fig. 4C), while docetaxel had no effect on
the spread of dye among cells (Fig.
4D). As demonstrated in Fig.
2, at a concentration range over which the cell density
affected paclitaxel/docetaxel toxicity, the sensitivity of
docetaxel was higher than that of paclitaxel in the high-density
culture (with GJIC) compared with the low-density culture (without
GJIC). These observations indicate that the effect of paclitaxel
and docetaxel on GJIC may affect their own toxicities.
Effects of paclitaxel and docetaxel on
Cx32 expression and its membrane localization
Changes in the number of GJs by affecting Cx32
expression or its membrane localization is one of the mechanisms by
which paclitaxel and docetaxel have been hypothesized to alter GJ
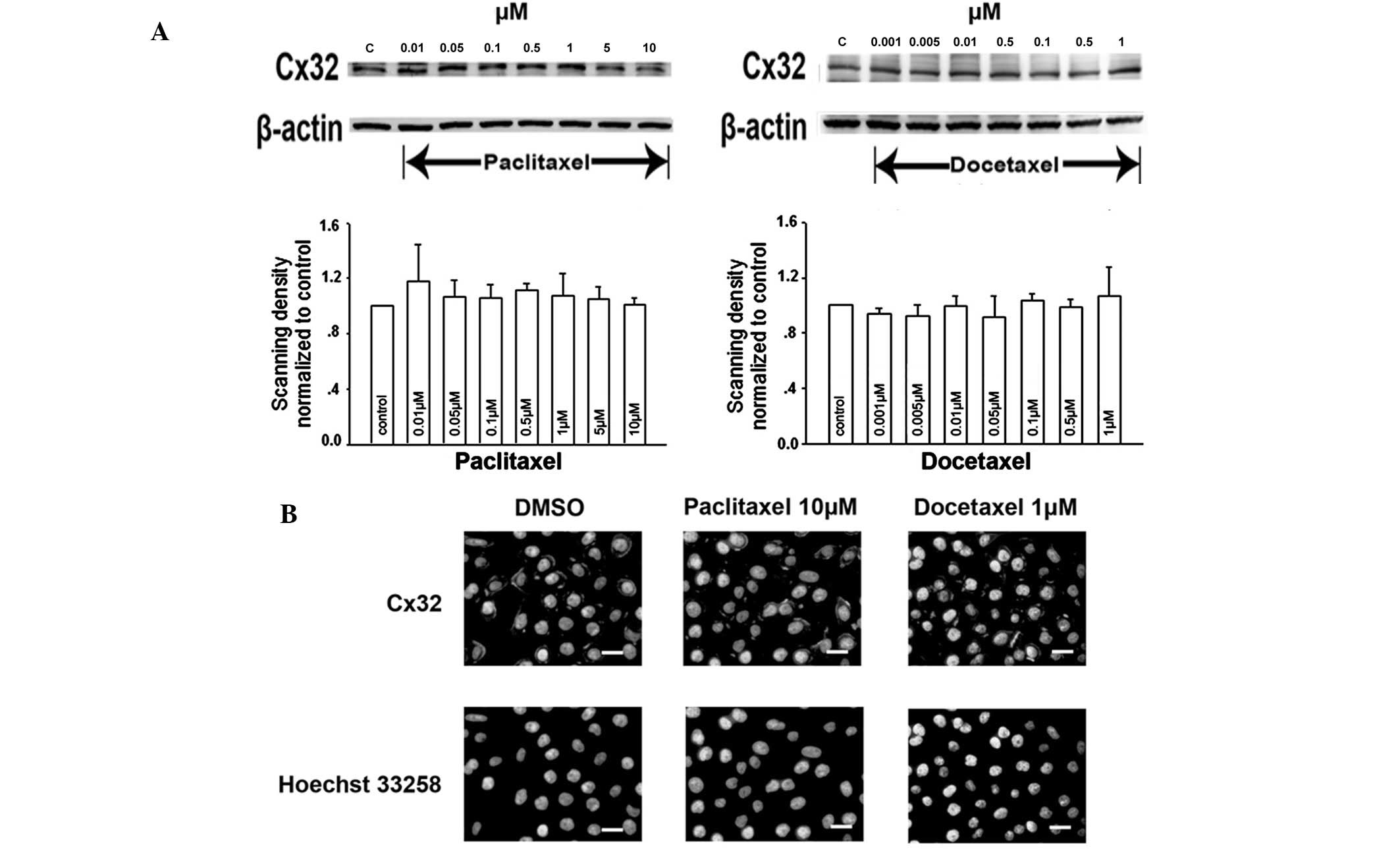
function. The expression of Cx32 was determined by western blot
analysis. Treatment of Cx32 HeLa cells with a range of
paclitaxel/docetaxel concentrations for 1 h did not alter Cx32
expression levels compared with that of the vehicle control
(Fig. 5A).
Following this, Cx32 localization on the cell
membrane was analyzed using immunofluorescence analysis. In control
cells (treated with DMSO), Cx32-specific immunoreactivity was
predominantly localized to the plasma membrane at cell-cell
junctions (Fig. 5B). Cells treated
with various concentrations of paclitaxel/docetaxel for 1 h
revealed an almost invariant level of Cx32 immunoreactive foci at
the cell membrane, even at 10 μM paclitaxel and 1 μM docetaxel
(Fig. 5B).
These observations indicate that, following
short-term (1 h) treatment, the effects of paclitaxel/docetaxel on
junctional function are not a result of affecting Cx32 expression
or its membrane localization.
Effects of paclitaxel and docetaxel on
Cx32 hemichannel activity
Treatment with paclitaxel/docetaxel for 1 h did not
alter the expression and localization of Cx32, indicating that
these agents may inhibit dye coupling by affecting channel gating.
To test this hypothesis, the permeability of the hemichannel was
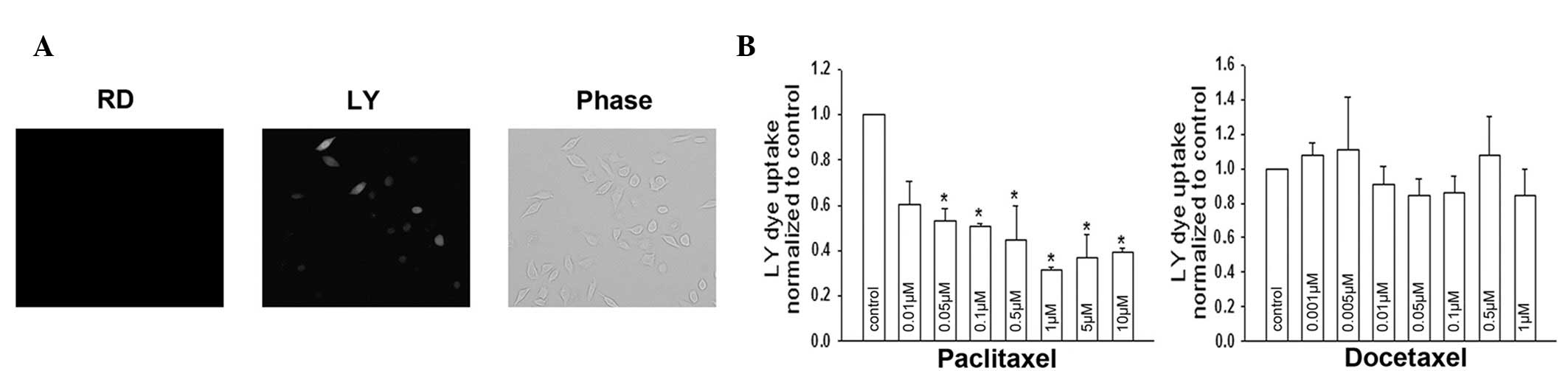
examined by a dye uptake assay. LY uptake was observed in control
Cx32 HeLa cells (Fig. 6A). Dye
uptake activity was altered in cells treated with paclitaxel for 1
h. As demonstrated in Fig. 6B,
docetaxel did not affect Cx32 hemichannel activity; however,
paclitaxel had an inhibitory effect. The results are consistent
with the effect of these agents on junctional function (Fig. 4C and D); however, specific values
exhibited small deviations. These deviations may be due to
differences in the two experimental approaches. Together, the
observations demonstrate that paclitaxel-induced closure of
‘gating’ is largely responsible for its GJIC inhibition.
Discussion
The present study indicates that GJIC is an
important constituent of paclitaxel- and docetaxel-induced
cytotoxicity in Cx32 HeLa cells. The toxicity of
paclitaxel/docetaxel was increased in cell cultures at high density
(i.e., cells contact each other) compared with those at low density
(i.e., cells lack junctional contacts). To exclude other potential
differences, besides GJIC, which exist when cells are grown at low-
vs. high-cell density that may account for the increased
cytotoxicity, two methods to suppress the GJs in high-density
culture were performed. The results confirm that the activities of
paclitaxel and docetaxel were lower when Cx32 was not induced or
when the GJ was blocked by 18-α-GA, indicating that Cx32-composed
GJIC may be targeted to enhance the cytotoxicity of paclitaxel and
docetaxel.
In addition, these results indicate that paclitaxel
inhibits GJIC, which leads to an attenuation of its own
cytotoxicity. The two agents have similar toxicities in the absence
of GJIC. However, docetaxel is more active than paclitaxel in the
presence of GJIC as it does not significantly affect the Cx32
channels, unlike paclitaxel (Fig. 4C
and D). Although a number of studies have reported that GJIC is
lost in numerous types of carcinoma (8,20–22),
the expression of Cx and GJIC are preserved in specific forms of
cancer or increase during the invasion and metastatic stages with
nominally defective GJs (23–27).
In these GJs, which are derived from Cx32 or Cx43, one must
consider the differential effects of paclitaxel/docetaxel on GJIC
and how these effects are likely to impact their therapeutic
efficacy.
Paclitaxel and docetaxel promote microtubule
assembly, inhibit depolymerization and block mitosis in
proliferating cells (28).
However, their effects on Cx channels are distinguishable. Results
of the current study indicate that paclitaxel impaired GJIC;
however, this effect was not observed following short-term (1 h)
treatment of Cx32 HeLa cells by docetaxel. In longer-term
treatments (48 h) of various cell lines with Cx43 derived GJs,
paclitaxel suppressed dye spread (15), while docetaxel had the opposite
effect (16). Although channels
are composed of different Cx isoforms with diverse permeabilities
and sensitivities to regulatory agents (29), paclitaxel and docetaxel treatment
had different effects on Cx32 or Cx43 channels.
Paclitaxel treatment for 1 h appears to block Cx32
channels primarily by blocking the gating function, thus, resulting
in a closed channel and not by decreasing Cx32 expression or
promoting its translocation from the cell membrane to the
cytoplasm. Paclitaxel may have a direct effect on channel gating.
Cx's are hypothesized to belong to the same family of proteins
based on sequence similarity. These proteins have four
predominantly transmembrane hydrophobic regions and two
extracellular loops with the amino- and carboxyl-terminals (CT)
located in the cytoplasm (5,30).
Modulators may induce a conformational change in Cx to directly
alter GJIC. For example, quinine blocks Cx36 and Cx50 channels by
binding to an intracellular site, possibly within the channel pore
(31) and 2-APB directly inhibits
Cx26 and/or Cx32 channels with the involvement of the CT domain of
the Cx (32).
The structural differences of paclitaxel and
docetaxel lie in the C3′-substituents of the C13 side chain and an
acetyl or a hydroxyl substituted at the C10 position (Fig. 1). These changes may affect the
conformation of Cx32 to cause gate closure. The differential
effects of paclitaxel and docetaxel indicate that the hydrophobic
group at the β-position in the amide group of the C3′-substituents
in the C13 side chain that bonds with the conjugated double-bonds
(e.g., phenyl) may induce channel inhibition by: i) π-π stacking
interactions with the phenyl rings of aromatic amino acid side
chains or the imidazolyl group of histidine or ii) a hydrophobic
interaction entering the hydrophobic region of the protein
structure. The acetyl (paclitaxel) or hydroxyl (docetaxel) groups
at C10 may enhance the interaction with amino acid residues by
hydrogen bonding; however, docetaxel had no impact on channel
activity. This result indicates that the moiety may not enter the
inner part of the protein and is therefore not directly involved in
non-covalent interactions with amino acid residues. These results,
as well as the structural diversity of taxanes and the conformation
of Cx proteins (33,34) indicate that the specific
interactions between taxanes and Cx must be studied further.
Results of the present study indicate that the
different actions of paclitaxel and docetaxel on the intercellular
communication mediated by GJs that are, in turn, derived from Cx32,
affects their own cytotoxic activities. In addition, short-term
exposure to paclitaxel appears to primarily affect the function of
the junction by affecting the gating mechanism of the channel.
These observations indicate a promising new approach in which the
appropriate taxane may be selected for the treatment of cancer
based on the presence or absence of GJs in a specific
carcinoma.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 30901807 and
30973434), the Fundamental Research Funds for the Central
Universities (no. 10YKPY32) and Grant for Development of Important
New Drugs from Xinjiang province, China (no. 201230045).
References
|
1
|
Gelmon K: The taxoids: paclitaxel and
docetaxel. Lancet. 344:1267–1272. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
King KM, Lupichuk S, Baig L, Webster M,
Basi S, Whyte D and Rix S: Optimal use of taxanes in metastatic
breast cancer. Curr Oncol. 16:8–20. 2009.PubMed/NCBI
|
|
3
|
Shepherd FA, Dancey J, Ramlau R, et al:
Prospective randomized trial of docetaxel versus best supportive
care in patients with non-small-cell lung cancer previously treated
with platinum-based chemotherapy. J Clin Oncol. 18:2095–2103.
2000.
|
|
4
|
Galletti E, Magnani M, Renzulli ML and
Botta M: Paclitaxel and docetaxel resistance: molecular mechanisms
and development of new generation taxanes. ChemMedChem. 2:920–942.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maeda S and Tsukihara T: Structure of the
gap junction channel and its implications for its biological
functions. Cell Mol Life Sci. 68:1115–1129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vinken M, Vanhaecke T, Papeleu P, Snykers
S, Henkens T and Rogiers V: Connexins and their channels in cell
growth and cell death. Cell Signal. 18:592–600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holder JW, Elmore E and Barrett JC: Gap
junction function and cancer. Cancer Res. 53:3475–3485.
1993.PubMed/NCBI
|
|
8
|
Mesnil M, Crespin S, Avanzo JL and
Zaidan-Dagli ML: Defective gap junctional intercellular
communication in the carcinogenic process. Biochim Biophys Acta.
1719:125–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kandouz M and Batist G: Gap junctions and
connexins as therapeutic targets in cancer. Expert Opin Ther
Targets. 14:681–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jensen R and Glazer PM:
Cell-interdependent cisplatin killing by Ku/DNA-dependent protein
kinase signaling transduced through gap junctions. Proc Natl Acad
Sci USA. 101:6134–6139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Q, You T, Yuan D, et al: Cisplatin
and oxaliplatin inhibit gap junctional communication by direct
action and by reduction of connexin expression, thereby
counteracting cytotoxic efficacy. J Pharmacol Exp Ther.
333:903–911. 2010. View Article : Google Scholar
|
|
12
|
Prise KM and O'Sullivan JM:
Radiation-induced bystander signalling in cancer therapy. Nat Rev
Cancer. 9:351–360. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harada K, Nonaka T, Hamada N, et al:
Heavy-ion-induced bystander killing of human lung cancer cells:
role of gap junctional intercellular communication. Cancer Sci.
100:684–688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang RP, Hossain MZ, Huang R, Gano J, Fan
Y and Boynton AL: Connexin 43 (cx43) enhances chemotherapy-induced
apoptosis in human glioblastoma cells. Int J Cancer. 92:130–138.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giessmann D, Theiss C, Breipohl W and
Meller K: Decreased gap junctional communication in neurobiotin
microinjected lens epithelial cells after taxol treatment. Anat
Embryol (Berl). 209:391–400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piechocki MP, Lonardo F, Ensley JF, Nguyen
T, Kim H and Yoo GH: Anticancer activity of docetaxel in murine
salivary gland carcinoma. Clin Cancer Res. 8:870–877.
2002.PubMed/NCBI
|
|
17
|
Koreen IV, Elsayed WA, Liu YJ and Harris
AL: Tetracycline-regulated expression enables purification and
functional analysis of recombinant connexin channels from mammalian
cells. Biochem J. 383:111–119. 2004. View Article : Google Scholar
|
|
18
|
Goldberg GS, Bechberger JF and Naus CC: A
preloading method of evaluating gap junctional communication by
fluorescent dye transfer. Biotechniques. 18:490–497.
1995.PubMed/NCBI
|
|
19
|
Davidson JS, Baumgarten IM and Harley EH:
Reversible inhibition of intercellular junctional communication by
glycyrrhetinic acid. Biochem Biophys Res Commun. 134:29–36. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Loewenstein WR and Kanno Y: Intercellular
communication and the control of tissue growth: lack of
communication between cancer cells. Nature. 209:1248–1249. 1966.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naus CC and Laird DW: Implications and
challenges of connexin connections to cancer. Nat Rev Cancer.
10:435–441. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leithe E, Sirnes S, Omori Y and Rivedal E:
Downregulation of gap junctions in cancer cells. Crit Rev Oncog.
12:225–256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanna EA, Umhauer S, Roshong SL, et al:
Gap junctional intercellular communication and connexin43
expression in human ovarian surface epithelial cells and ovarian
carcinomas in vivo and in vitro. Carcinogenesis. 20:1369–1373.
1999. View Article : Google Scholar
|
|
24
|
Rüttinger C, Bergmann M, Fink L, et al:
Expression of connexin 43 in normal canine testes and canine
testicular tumors. Histochem Cell Biol. 130:537–548.
2008.PubMed/NCBI
|
|
25
|
Kanczuga-Koda L, Sulkowska M, Koda M,
Rutkowski R and Sulkowski S: Increased expression of gap junction
protein - connexin 32 in lymph node metastases of human ductal
breast cancer. Folia Histochem Cytobiol. 45(Suppl 1): S175–S180.
2007.PubMed/NCBI
|
|
26
|
Zhang W, DeMattia JA, Song H and Couldwell
WT: Communication between malignant glioma cells and vascular
endothelial cells through gap junctions. J Neurosurg. 98:846–853.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saito-Katsuragi M, Asada H, Niizeki H, et
al: Role for connexin 26 in metastasis of human malignant melanoma:
communication between melanoma and endothelial cells via connexin
26. Cancer. 110:1162–1172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lavelle F, Bissery MC, Combeau C, Riou JF,
Vrignaud P and André S: Preclinical evaluation of docetaxel
(Taxotere). Semin Oncol. 22:3–16. 1995.
|
|
29
|
Harris AL: Emerging issues of connexin
channels: biophysics fills the gap. Q Rev Biophys. 34:325–472.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beyer EC, Paul DL and Goodenough DA:
Connexin family of gap junction proteins. J Membr Biol.
116:187–194. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Srinivas M, Hopperstad MG and Spray DC:
Quinine blocks specific gap junction channel subtypes. Proc Natl
Acad Sci USA. 98:10942–10947. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tao L and Harris AL: 2-aminoethoxydiphenyl
borate directly inhibits channels composed of connexin26 and/or
connexin32. Mol Pharmacol. 71:570–579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maeda S, Nakagawa S, Suga M, et al:
Structure of the connexin 26 gap junction channel at 3.5 A
resolution. Nature. 458:597–602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bouvier D, Spagnol G, Chenavas S, et al:
Characterization of the structure and intermolecular interactions
between the connexin40 and connexin43 carboxyl-terminal and
cytoplasmic loop domains. J Biol Chem. 284:34257–34271. 2009.
View Article : Google Scholar : PubMed/NCBI
|