Introduction
The establishment of the placenta in humans begins
with the invasion and migration of fetal trophoblast cells into the
maternal decidua (1). During this
process, the decidual environment is infiltrated by a large number
of leukocytes, which predominantly include
CD56+CD16− NK cells (~70%), macrophages
(~10%) and T cells (~10%) (2,3).
Previous studies have demonstrated that T cells have an important
physiological role in early pregnancy (4). T cells may be subdivided into
CD45RA+ T cells and CD45RO+ T cells according
to the surface molecules that are expressed. The percentage of
CD45RO+ T cells has been demonstrated to increase in
first trimester human decidua (5,6).
However, the expansion mechanisms of decidual CD45RO+ T
cells remain unclear. The expansion of leukocytes in first
trimester human decidua may be explained by two mechanisms: i)
decidual leukocytes are recruited from the peripheral blood by
hormones, cytokines and chemokines (7–10);
and ii) the generation of decidual leukocytes occurs in the
decidual microenvironment (2,11).
Trophoblast cells are primary fetal cells, which are in close
contact with maternal immune cells in human decidua (1). Although the effects of trophoblast
cells on the recruitment of immune cells have been extensively
investigated (8,12,13),
the effect of trophoblast cells on the expansion and
differentiation of decidual leukocytes remains unknown (14). A previous study demonstrated that
the interactions between trophoblast cells and monocytes
significantly increased the secretion and production of cytokines
and chemokines (15), including
monocyte chemoattractant protein-1 (MCP-1), which is important in
the generation and survival of CD45RO+ T cells (16). Whether the crosstalk between
trophoblast cells and monocytes participates in the expansion of
decidual CD45RO+ T cells remains to be elucidated.
Carcinoembryonic antigen-related cell adhesion
molecule 1 (CEACAM1), is a multifunctional cellular adhesion
molecule, and is a member of the carcinoembryonic antigen family
and the immunoglobulin superfamily (17). It is well-established that CEACAM1
is significant in regulating the functions of T cells (18). Numerous studies have demonstrated
that CEACAM1 may be expressed at low levels on the surface of
resting T cells. However, the expression of CEACAM1 may be rapidly
upregulated following T cell activation, implying that CEACAM1 acts
as an activation-induced cell surface molecule of T cells (18–21).
Previous studies have demonstrated that a fraction of T cells
infiltrating the lamina propria of the small intestine in celiac
disease and the large intestine in inflammatory bowel disease
express CEACAM1. This suggests that CAECAM1 may be expressed at
inflammatory sites and participate in modulating the immune
response in vivo(21,22).
Studies have also indicated that the first trimester of pregnancy
is a pro-inflammatory phase (23,24).
T cells in first trimester human decidua have been demonstrated to
express cell surface activation markers, including CD69 and HLA-DR,
which implies that decidual T cells are regionally activated
(25). To date, the mechanism of
CEACAM1 expression on the surface of decidual T cells at an early
stage of pregnancy remains unknown. We therefore investigated the
expression of CEACAM1 on the surface of T cells in first trimester
human decidua, and the effect of the crosstalk between trophoblast
cells and monocytes on the expression of CEACAM1 on the surface of
CD45RO+ T cells.
In conclusion, in the present study, we analyzed the
percentage of CD45RO+ T cells and the expression of
CEACAM1 on the surface of T cells in first trimester human decidua
and in the peripheral blood, and identified that these percentages
were significantly increased in the decidua. Using the model to
generate CD45RO+ T cells in vitro, we
demonstrated that conditioned medium from the coculture of the
extravillous trophoblast HTR-8/SVneo cell line and monocytes (MHM)
increased the percentage of CD45RO+ T cells in an MCP-1
dependent manner, and increased the expression of CEACAM1 on the
surface of CD4+CD45RO+ T cells. These data
implied that decidual CD45RO+ T cells were activated in
an early stage of pregnancy, and suggested that trophoblasts and
monocytes may be involved in the increase of CD45RO+ T
cells and the high expression of CEACAM1 on their surface.
Materials and methods
Sample collection
Twenty-one healthy nonpregnant females and seventeen
healthy pregnant females in their first trimester (from Qilu
Hospital, Shandong University, Shandong, China) volunteered to
participate in this study (Table
I). The use of human tissues was approved by the Ethics
Committee of Qilu Hospital (Shandong University, Jinan, China), and
written informed consent was obtained from all participants.
Peripheral blood samples and decidual tissues were obtained
following elective termination of the pregnancy. Cases without
maternal or fetal complications were selected for tissue sampling.
Peripheral blood samples from nonpregnant females, who were not
taking systemic hormonal contraception, were without medical
complications and were in the secretory phase of the menstrual
cycle, were included as controls. To obtain the decidual
mononuclear cells, the decidual tissue was macroscopically
separated from the villi, washed twice with phosphate-buffered
saline (PBS) to remove the contaminated blood, dissected into small
pieces, washed twice again, and then passed through a 120- and
75-μm stainless steel mesh. The decidual mononuclear cells were
isolated using Ficoll Histopaque®-1077 (Sigma-Aldrich,
St. Louis, MO, USA) by density gradient centrifugation.
 | Table ICharacteristics of the study
groups. |
Table I
Characteristics of the study
groups.
| Variable | Secretory phase of
menstrual cycle | Pregnant |
|---|
| No. of
subjects | 21 | 17 |
| Age (years) | 27.7±2.2 | 29.5±4.4 |
| Height (cm) | 163.9±3.5 | 162.5±3.1 |
| BMI
(kg/m2) | 21.8±1.7 | 20.7±2.5 |
| Gestational age
(weeks) | | 6.6±0.8 |
Reagents
Fluorescein isothiocyanate (FITC)-conjugated mouse
anti-human CD4 monoclonal antibody (mAb) (Jingmei Biological, Co.,
Beijing, China), CD8 mAb (Becton-Dickinson, Franklin Lakes, NJ,
USA), phycoerythrin (PE)-conjugated mouse anti-human CEACAM1 mAb
(R&D Systems, Minneapolis, MN, USA), PE-cyanine (Cy)
5-conjugated mouse anti-human CD45RO mAb (Jingmei Biological Co.)
and their isotype- and fluorochrome-matched control antibodies,
were used for flow cytometry. Human recombinant MCP-1 (rhMCP-1) and
monoclonal anti-MCP-1 antibodies were obtained from R&D
Systems.
Conditioned medium from the coculture of
monocytes and HTR8/SVneo cell line (MHM)
HTR8/SVneo cells were grown in RPMI-1640 medium,
supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 mg/ml streptomycin. CD14+ monocytes were
isolated from the peripheral blood with a magnetic cell sorting
system [CD14+ microbeads (#130-050-201) and an LS column
(#130-042-401)] according to the manufacturer’s instructions
(Miltenyi Biotech, Bergisch Gladbach, Germany). The HTR8/SVneo
cells were cocultured at a ratio of 1:1
(5×105/5×105 cells). The conditioned medium
was collected after 40 h.
Enzyme-linked immunosorbent assay
(ELISA)
The MCP-1 levels in the culture supernatant were
measured with the Human CCL2/MCP-1 Quantikine ELISA kit (R&D
Systems), and assays were conducted according to the manufacturer’s
instructions. All measurements were performed in triplicate to
avoid technical error and intra-assay variants.
Flow cytometric analysis
The expression of CD4, CD8, CD45RO and CEACAM1 on
the surface of T cells was determined by extracellular staining
with a specific monoclonal antibody. The background fluorescence
was assessed using the appropriate isotype-and fluorochrome-matched
control mAbs. The FACSCalibur flow cytometer and the CellQuest
software program of the FACSCalibur system (Becton-Dickinson) were
used for the measurement and analysis of the stained cells.
Model to generate CD45RO+ T
cells
Peripheral blood mononuclear cells (PBMCs) were
isolated from the peripheral blood of healthy donors using gradient
centrifugation over Histopaque®-1077 (Sigma-Aldrich).
PBMCs from one donor were treated with 25 mg/ml mitomycin C (Roche,
Basel, Switzerland) for 20 min, and washed twice with warmed
RPMI-1640 supplemented with 10% FBS. PBMCs were reseeded into
24-well plates at a density of 500 μl/well and a concentration of
1×106 cells. PBMCs from another donor, without
treatment, were cocultured with the pretreated PBMCs, (density, 500
μl/well; concentration, 1×106 cells). The supernatant
and control medium pretreated with anti-MCP-1 mAb (10 μg/ml) or
rhMCP-1 (5 μg/ml) for 30 min were added to the 24-well plates
(density, 1 ml/well). A mixed lymphocyte reaction (MLR) was
conducted for 8 days, cells were collected, and the medium was
changed every 3 days.
Statistical analysis
Statistical analysis was performed using SPSS
version 11.5. Normality of the data was tested using the
Shapiro-Wilk test. Data were normally distributed, and the results
are presented as the mean ± standard deviation (SD). A one-way
analysis of variance (ANOVA) test was used for statistical
comparisons between groups (where number of groups ≥3) and a
Fisher’s least significant difference test was used for post hoc
analysis of the significant ANOVA results. A paired student’s
t-test was used for the statistical analysis of the differences in
the percentages of CD45RO+ T cells in the model, in
vitro, on days 0 and 8. P<0.05 was considered to indicate a
statistically significant difference.
Results
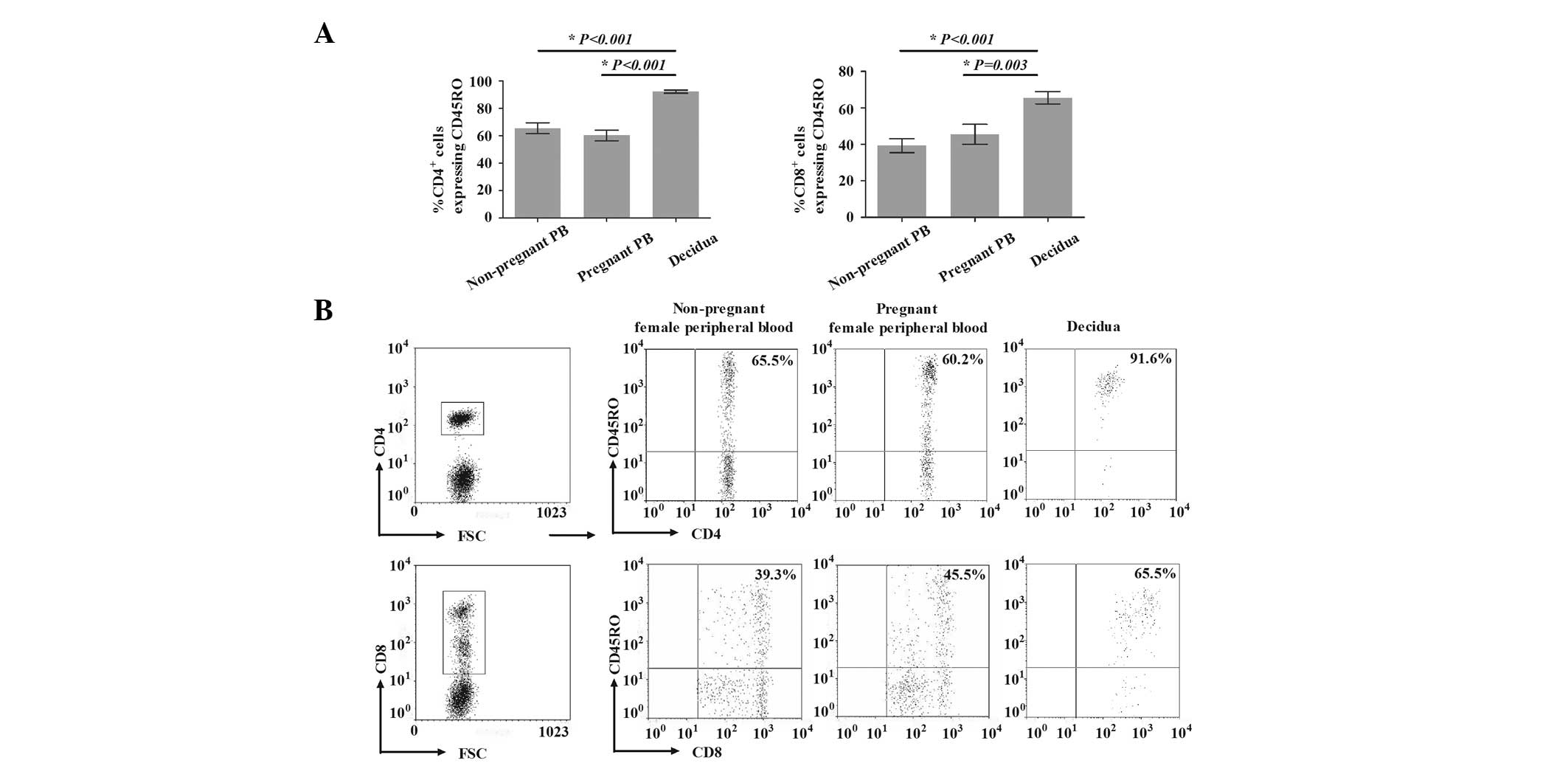
Percentage of CD45RO+ T cells
in first trimester human decidua
The percentages of CD45RO+ T cells in
first trimester human decidua and in the peripheral blood of
healthy nonpregnant and pregnant females are presented in Fig. 1. For healthy nonpregnant and
pregnant females, the percentages of
CD4+CD45RO+ T cells among CD4+ T
cells in the peripheral blood were 65.5±12.6 and 60.2±12.3%,
respectively. The percentage of CD4+CD45RO+ T
cells among CD4+ T cells in the decidua was
significantly higher (91.6±4.8%) compared with that in the
peripheral blood from nonpregnant (P<0.001) and pregnant
(P<0.001) females. Similarly, the percentage of
CD8+CD45RO+ T cells among CD8+ T
cells in the decidua (65.5±10.7%) was also significantly higher,
compared with that in the peripheral blood of nonpregnant
(39.3±12.2%) and pregnant (45.5±17.2%) females (P<0.001 and
P=0.003, respectively). However, the percentages of
CD4+CD45RO+ T cells among CD4+ and
CD8+CD45RO+ T cells among CD8+ T
cells in the peripheral blood were not significantly different
between nonpregnant and pregnant females (P=0.265 and P=0.317,
respectively).
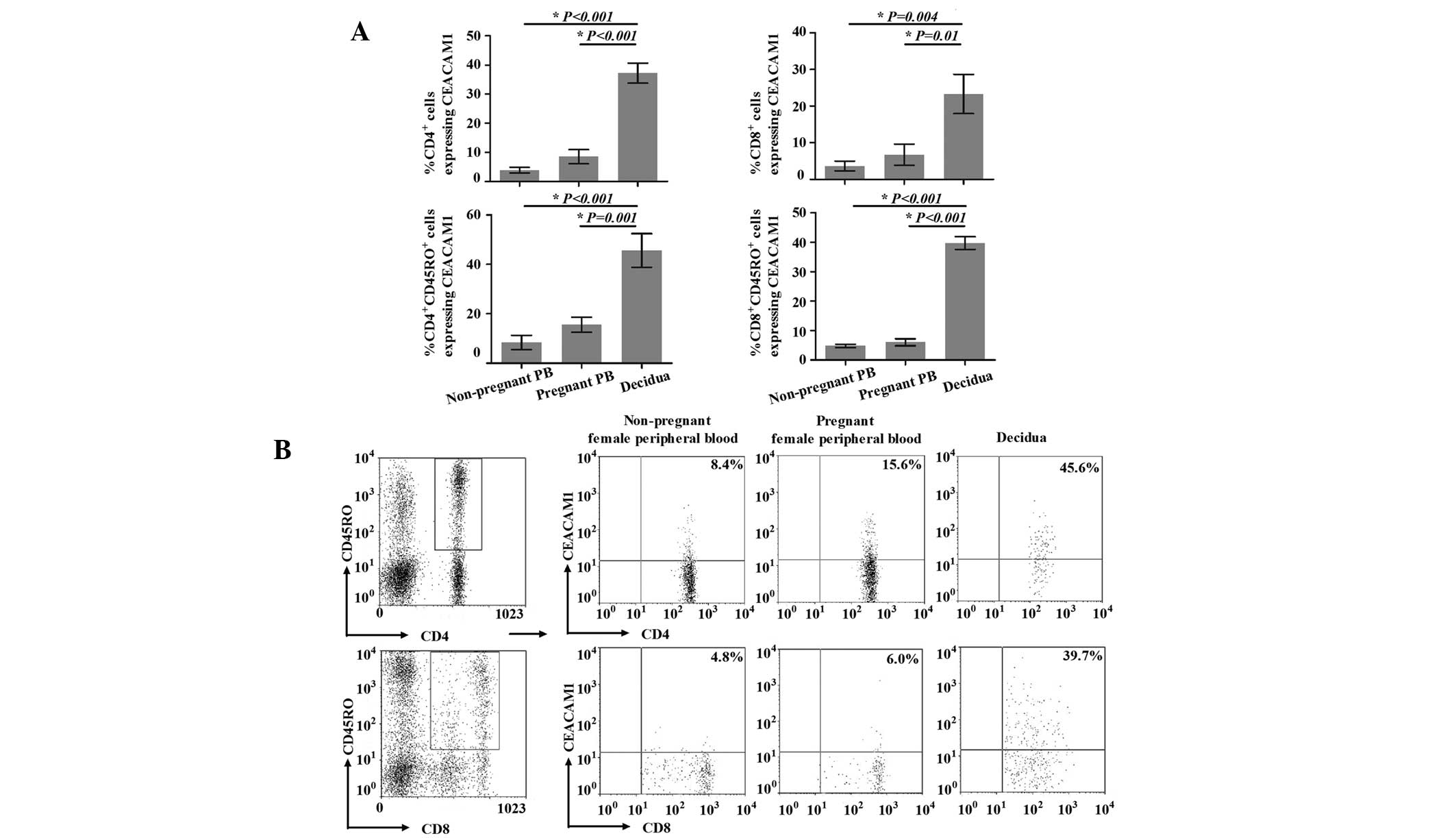
Expression of CEACAM1 on the surface of
peripheral and decidual T cells
A number of studies have demonstrated that CEACAM1,
an activation-induced cell surface molecule of T cells, inhibits
the cytokine production, proliferation and cytotoxic activity of
activated T cells by homophilic (CEACAM1-CEACAM1) and heterophilic
(CEACAM1-CEACAM5 and CEACAM1-Opa) interactions (26–29).
As numerous studies have demonstrated that decidual T cells are
regionally activated (25,30,31),
we detected the expression of CEACAM1 on the surface of decidual T
cells. As shown in Fig. 2A, for
healthy nonpregnant and pregnant females, the percentages of
CD4+CEACAM1+ T cells in the peripheral blood
(3.9±2.7 and 8.5±6.8%, respectively) were not significantly
different (P=0.204). However, the percentage of
CD4+CEACAM1+ T cells in the decidua
(37.2±9.8%) was significantly higher compared with that in the
peripheral blood (P<0.001 vs. peripheral blood from nonpregnant
females and P<0.001 vs. peripheral blood from pregnant females).
The percentages of CD8+CEACAM1+ T cells
showed the same trends as those of the
CD4+CEACAM1+ T cells, and those in the
peripheral blood of nonpregnant and pregnant females, and in the
decidua were 3.6±2.6, 6.7±5.8 and 23.3±10.7%, respectively
(P=0.004, peripheral blood from nonpregnant females vs. decidua;
P=0.01, peripheral blood from pregnant females vs. decidua; P=0.56,
peripheral blood from nonpregnant females vs. pregnant
females).
Further, we identified the expression of CEACAM1 on
the surface of decidual and peripheral CD45RO+ T cells
(Fig. 2). The expression of
CEACAM1 on the surface of peripheral
CD4+CD45RO+T cells from healthy nonpregnant
females was identified in 8.4±7.1% of cells, in contrast to
15.6±7.4% of such cells from healthy pregnant females; however,
this difference was not significant (P=0.436). Notably,
CEACAM1-expressing cells were present at a significantly higher
level (45.6±21.4%) in the decidua compared with the peripheral
blood from nonpregnant (P<0.001) and pregnant (P=0.001) females.
The expression of CEACAM1 on the surface of decidual and peripheral
CD8+CD45RO+ T cells demonstrated the same
trends, with expression rates of 4.8±1.0 and 6.0±2.0% in healthy
nonpregnant and pregnant females, respectively (P=0.586, peripheral
blood from nonpregnant females vs. pregnant females). In addition,
the expression of CEACAM1 on the surface of decidual
CD8+CD45RO+ T cells (39.7±3.8%) was
significantly higher than that in the peripheral blood (P<0.001
vs. peripheral blood from nonpregnant females and P<0.001 vs.
peripheral blood from pregnant females).
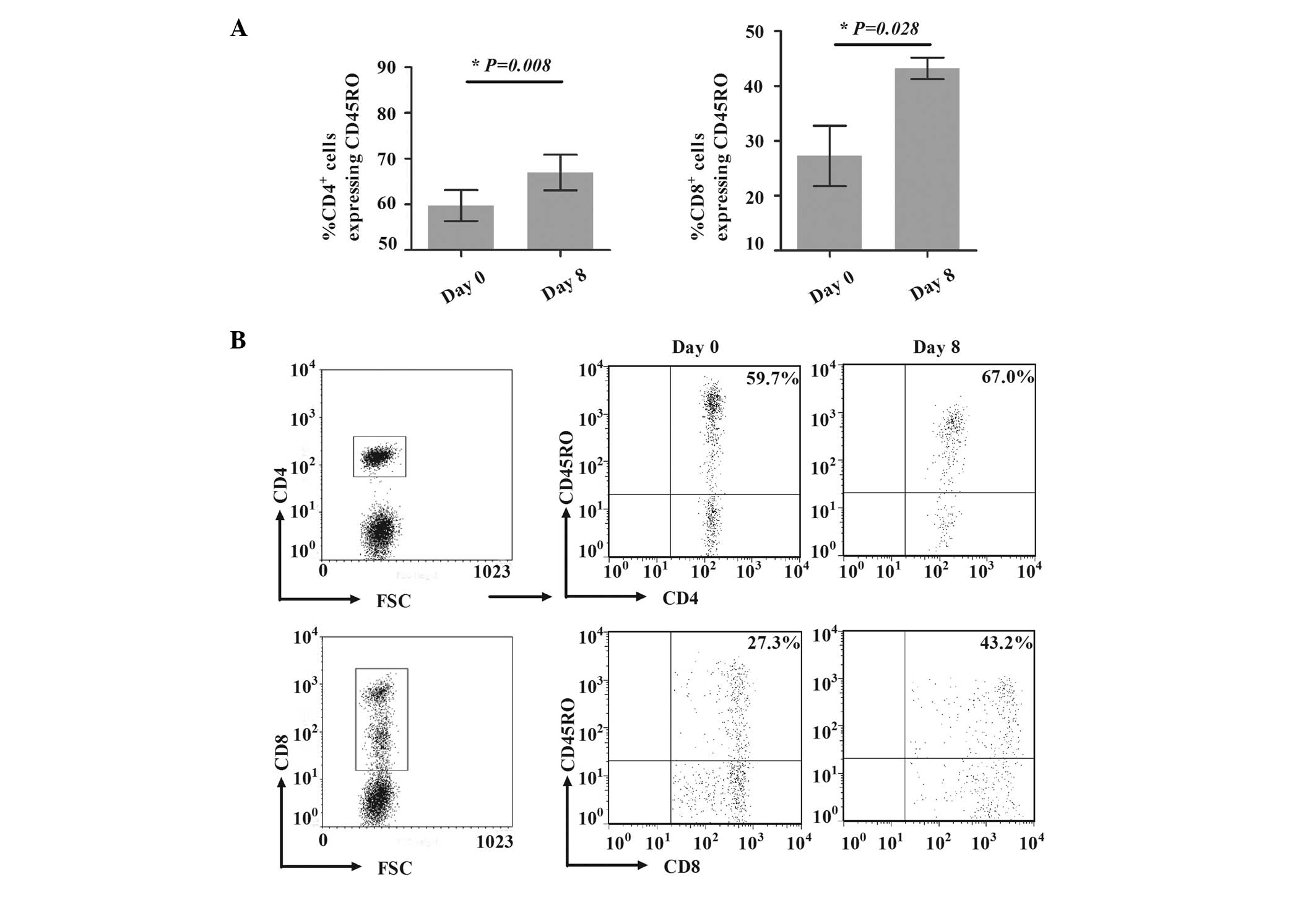
Expansion of CD45RO+ T cells
by MHM is MCP-1 dependent
To investigate the potential expansion mechanisms of
CD45RO+ T cells, the model to generate
CD45RO+ T cells was established in vitro, as
described in Materials and methods. Compared with the percentages
on day 1, the percentages of CD4+CD45RO+ T
cells among CD4+ T cells and of CD8+CD45RO+ T
cells among CD8+ T cells were significantly increased on
day 8, from 59.7±6.8 to 67.0±7.7% (P=0.008) and from 27.3±11.1 to
43.2±3.9% (P=0.028; Fig. 3),
respectively, indicating that the induced model was successful.
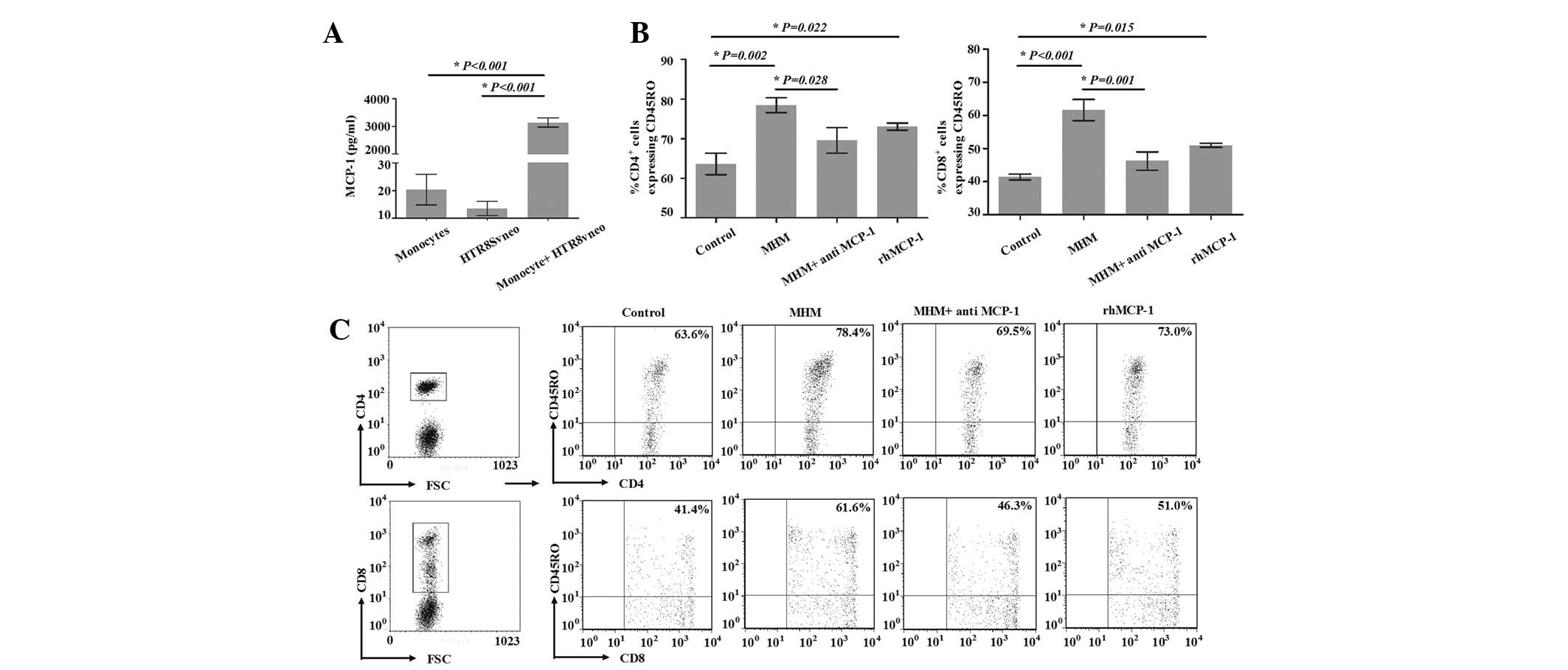
To clarify the effect of the interaction between
trophoblast cells and monocytes on the expansion of
CD45RO+ T cells, MHM was added to the model, and the
percentage of CD4+CD45RO+ T cells among
CD4+ T cells on day 8 was significantly increased from
63.6±4.7 to 78.4±3.2% (P=0.002). Similarly, the percentage of
CD8+CD45RO+ T cells among CD8+ T
cells on day 8 was increased from 41.4±1.6 to 61.6±5.6%
(P<0.001; Fig. 4B and C). These
data suggested that trophoblasts and monocytes were likely to be
involved in the generation of CD45RO+ T cells. The
secretion of MCP-1 was determined by ELISA, as it has been
demonstrated to be significant in the generation of
CD45RO+ T cells (16).
Consistent with a previous study (15), we observed that the production of
MCP-1 in the MHM was notably higher than that in the monocyte or
human trophoblast HTR8/SVneo cell line cultures (Fig. 4A). In order to clarify the effect
of MCP-1 from the MHM on the increase of CD45RO+ cells
in the model, neutralizing antibody against MCP-1 was added to the
MHM; the percentage of CD4+CD45RO+ T cells
among CD4+ T cells on day 8 was significantly decreased
in the MHM + anti-MCP-1 group compared with that in the MHM group
(69.5±5.6 vs. 78.4±3.2, P=0.028; Fig.
4B and C). Compared with the control group, the percentage of
CD4+CD45RO+ T cells among CD4+ T
cells on day 8 was significantly increased in the rhMCP-1 group
(63.6±4.7 vs. 73.0±1.5, respectively, P=0.022). The percentage of
CD8+CD45RO+ T cells among CD8+ T
cells in the MHM + anti-MCP-1 group was significantly decreased
compared with that of the MHM group (46.3±4.8 vs. 61.6±5.6%,
P=0.001; Fig. 4B and C). Compared
with the control group, the percentage of
CD8+CD45RO+ T cells among CD8+ T
cells on day 8 was significantly increased in the rhMCP-1 group
(41.4±1.6 vs. 51.0±1.0%, P=0.015). These results suggested that
MCP-1 was involved in the increase of CD45RO+ T cells
through the addition of MHM.
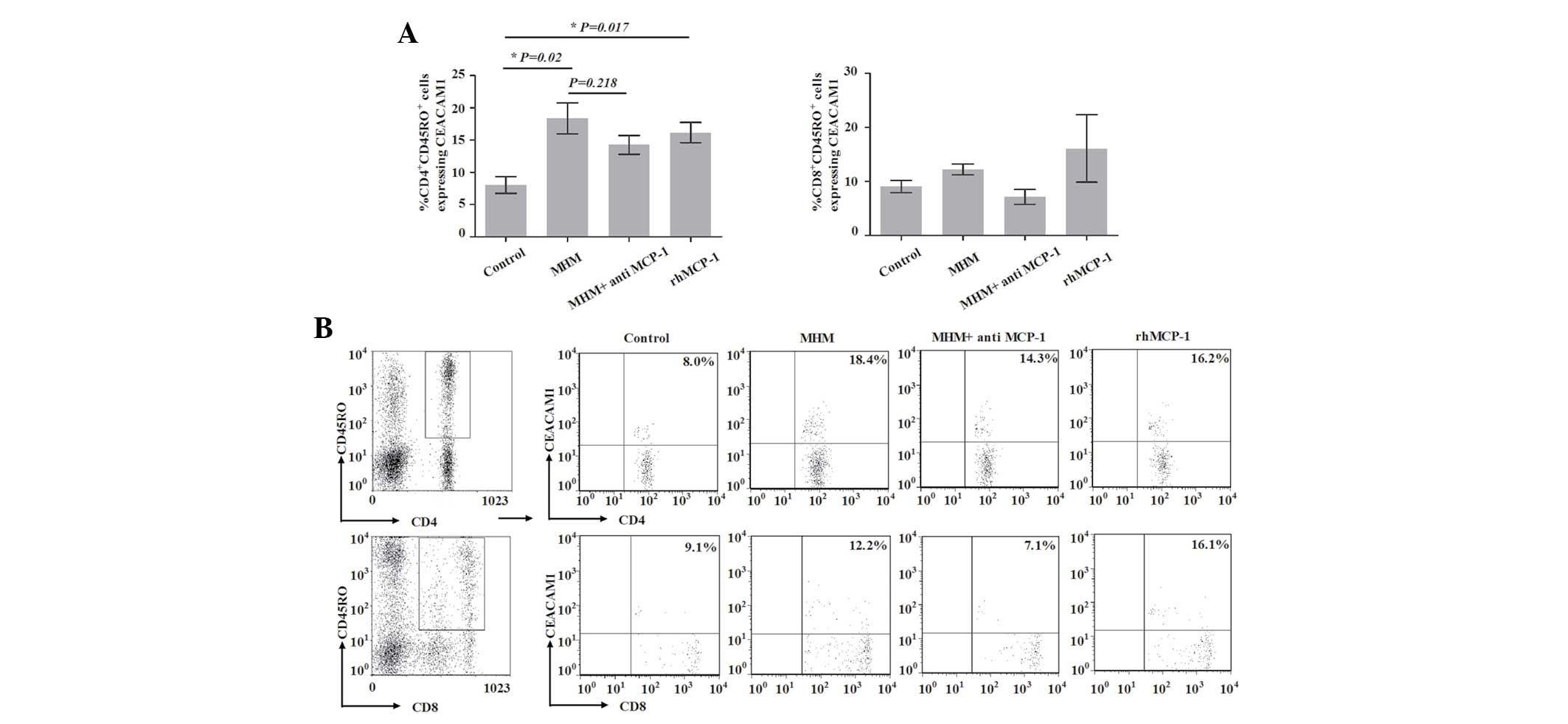
Proportion of CEACAM1-expressing
CD4+CD45RO+ T cells is increased by MHM and
rhMCP-1
To investigate the potential involvement of the
interaction of trophoblast cells and monocytes in the expression of
CEACAM1 on the surface of CD45RO+ T cells in first
trimester human decidua, we analyzed the expression of CEACAM1 on
the surface of such T cells in the model. As shown in Fig. 5, the proportion of
CEACAM1-expressing CD4+CD45RO+ T cells in the
control group on day 8 was 8.04±1.31% (Fig. 5). However, when cells in the model
were treated with MHM, the proportion of CEACAM1-expressing
CD4+CD45RO+ T cells on day 8 was
significantly increased (18.38±2.4%) compared with that of the
control group (P=0.02). Compared with the MHM group, the proportion
of CEACAM1-expressing CD4+CD45RO+ T cells was
not significantly decreased in the MHM + anti-MCP-1 group
(P=0.218); however, that proportion was significantly increased in
the rhMCP-1 group compared with that in the control group
(P=0.017). These results implied that MCP-1 was involved in the
increase in the proportion of CEACAM1-expressing
CD4+CD45RO+ T cells induced by MHM. The
proportion of CEACAM1-expressing CD8+CD45RO+
T cells did not exhibit a statistically significant difference
among the four groups (P=0.303). These data implied that the
proportion of CEACAM1-expressing CD4+CD45RO+
T cells increased with the expansion of
CD4+CD45RO+ T cells, and that the interaction
of trophoblast cells and monocytes may be involved in the
process.
Discussion
In the present study, we demonstrated that the
percentages of CD45RO+ T cells were significantly higher
in first trimester human decidua than in the peripheral blood,
which is concordant with previous studies (5,6).
A number of studies have indicated that T cells in
first trimester human decidua are regionally activated (5,30,31).
As CEACAM1 has been demonstrated to be important in modulating the
functions of T cells and is regarded as an activation-induced cell
surface molecule of T cells (20,32),
we measured the expression of CEACAM1 on the surface of T cells in
an early stage of pregnancy. Notably, we identified
CEACAM1-expressing cells in significantly higher numbers among
freshly isolated CD4+ and CD8+ T cells in
first trimester human decidua than in the peripheral blood. The
data also suggested that these decidual T cells were activated. We
analyzed the expression of CEACAM1 on the surface of
CD45RO+ T cell subsets and identified the proportion of
cells expressing CEACAM1 among CD4+CD45RO+
and CD8+CD45RO+ T cells to be significantly
higher in first trimester human decidua than in the peripheral
blood. These data implied that a high percentage of decidual
CD45RO+ T cells were in an active state, and that
CEACAM1 may participate in the regulation of the activation and
functions of the decidual CD45RO+ T cells. In further
experiments, we determined which factors induce the increase in the
percentage of CD45RO+ T cells, and in the percentage of
CD45RO+ T cells expressing CEACAM1, in first trimester
human decidua.
Previous studies have demonstrated that during the
invasion and migration of fetal trophoblast cells into the maternal
decidua, the two come into close contact with maternal leukocytes
(1). Previous studies have focused
on the recruitment of leukocytes by trophoblast cells (8,12,13),
and limited data are available on the ability of trophoblast cells
to modulate the expansion and activation of decidual T cells
(14). It has been demonstrated
that the interaction of trophoblast cells and monocytes markedly
enhances the expression of cytokines/chemokines, including MCP-1
(15). MCP-1, the main monocyte
chemoattractant, has been demonstrated to be associated with the
generation and survival of CD45RO+ T cells (16). In the present study, to investigate
the possible expansion and activation mechanisms of decidual
CD45RO+ T cells, we developed an effective in
vitro model to generate these cells. We identified that
CD45RO+ T cells were greatly expanded when the model was
supplemented with MHM, suggesting that the direct interaction
between trophoblast cells and monocytes may contribute to the
increase in CD45RO+ T cells in first trimester human
decidua. Further studies are required to determine whether this
increase in CD45RO+ T cells is a result of the
proliferation of inherent memory T cells in vitro, or the
conversion from naïve T cells (or other subsets). Our results also
demonstrated that the increase in CD45RO+ T cells was
dependent on the increased expression of MCP-1 in the MHM.
In addition, we demonstrated that in the induced
model used to generate CD45RO+ T cells, as the
percentage of CD45RO+ T cells increased, the expression
of CEACAM1-expressing CD4+CD45RO+ T cells
also increased (from 8.0±1.3 to 18.4±2.4%). Notably, we identified
that the number of CEACAM1-expressing
CD4+CD45RO+ T cells significantly increased
when the model was supplemented with MHM; however, the expression
of CEACAM1-expressing CD8+CD45RO+ T cells did
not show a statistically significant difference between the groups.
These data further implied that the soluble immune mediators
resulting from the direct interaction between trophoblast cells and
monocytes may contribute to the increase in the expression of
CEACAM1 on the surface of CD4+CD45RO+ T
cells, but not on that of CD8+CD45RO+ T
cells, in first trimester human decidua. CEACAM1 has been detected
on the surface of extravillous trophoblast cells, and is
hypothesized to promote the invasion of these cells (33–35).
In addition, numerous studies have demonstrated that CEACAM1
inhibits the cytokine production, proliferation and cytotoxic
activity of activated T cells, by homophilic and heterophilic
interactions (26–29). The effect of the homophilic
interaction of CEACAM1 on the surface of decidual T cells and
extravillous trophoblast cells in the induction of maternal-fetal
tolerance remains to be elucidated.
In conclusion, our data indicated that during the
invasion and migration of fetal trophoblast cells into the maternal
decidua, the soluble immune mediators that are secreted as a result
of the interaction between trophoblasts and monocytes may be
involved in the increase of decidual CD45RO+ T cells and
the expression of CEACAM1 on their surfaces. Our results provide
insights into the interaction between maternal immune cells and
fetal antigens.
Acknowledgements
The authors wish to acknowledge Dr Mumtaz Virji
(University of Bristol, UK) and Dr Markus Neckenig (University of
Sheffield, UK) for their critiques of the manuscript. The authors
would like to thank Dr Charles H. Graham (Department of Anatomy and
Cell Biology, Queen’s University, Kingston, ON Canada) for
providing the HTR8/SVneo cell line and Ms. Zhen Li for support with
the statistical analysis (Shandong Academy of Medical Sciences,
Shandong, China). This study was supported by grants from the
National Natural Science Foundation of China (grant nos. 30872321,
81072406 and 31100650), the Natural Science Foundation of Shandong
Province (grant no. Y2008C02) and the Independent Innovation
Foundation of Shandong University (grant no. 2012TS143).
References
|
1
|
Burrows TD, King A and Loke YW:
Trophoblast migration during human placental implantation. Hum
Reprod Update. 2:307–321. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
King A, Gardner L and Loke YW:
Co-stimulation of human decidual natural killer cells by
interleukin-2 and stromal cells. Hum Reprod. 14:656–663. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sindram-Trujillo A, Scherjon S, Kanhai H,
Roelen D and Claas F: Increased T-cell activation in decidua
parietalis compared to decidua basalis in uncomplicated human term
pregnancy. Am J Reprod Immunol. 49:261–268. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trundley A and Moffett A: Human uterine
leukocytes and pregnancy. Tissue Antigens. 63:1–12. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saito S, Nishikawa K, Morii T, et al: A
study of CD45RO, CD45RA and CD29 antigen expression on human
decidual T cells in an early stage of pregnancy. Immunol Lett.
40:193–197. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slukvin II, Merkulova AA, Vodyanik MA and
Chernyshov VP: Differential expression of CD45RA and CD45RO
molecules on human decidual and peripheral blood lymphocytes at
early stage of pregnancy. Am J Reprod Immunol. 35:16–22.
1996.PubMed/NCBI
|
|
7
|
Schumacher A, Brachwitz N, Sohr S, et al:
Human chorionic gonadotropin attracts regulatory T cells into the
fetal-maternal interface during early human pregnancy. J Immunol.
182:5488–5497. 2009. View Article : Google Scholar
|
|
8
|
Tsuda H, Michimata T, Hayakawa S, et al: A
Th2 chemokine, TARC, produced by trophoblasts and endometrial gland
cells, regulates the infiltration of CCR4+ T lymphocytes
into human decidua at early pregnancy. Am J Reprod Immunol. 48:1–8.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carlino C, Stabile H, Morrone S, et al:
Recruitment of circulating NK cells through decidual tissues: a
possible mechanism controlling NK cell accumulation in the uterus
during early pregnancy. Blood. 111:3108–3115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qu X, Yang M, Zhang W, et al: Osteopontin
expression in human decidua is associated with decidual natural
killer cells recruitment and regulated by progesterone. In Vivo.
22:55–61. 2008.PubMed/NCBI
|
|
11
|
Vacca P, Cantoni C, Vitale M, et al:
Crosstalk between decidual NK and CD14+ myelomonocytic
cells results in induction of Tregs and immunosuppression. Proc
Natl Acad Sci USA. 107:11918–11923. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanna J, Wald O, Goldman-Wohl D, et al:
CXCL12 expression by invasive trophoblasts induces the specific
migration of CD16-human natural killer cells. Blood. 102:1569–1577.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang Y, Zhu XY, Du MR and Li DJ: Human
trophoblasts recruited T lymphocytes and monocytes into decidua by
secretion of chemokine CXCL16 and interaction with CXCR6 in the
first-trimester pregnancy. J Immunol. 180:2367–2375. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
King A, Gardner L and Loke YW: Human
decidual leukocytes do not proliferate in response to either
extravillous trophoblast or allogeneic peripheral blood
lymphocytes. J Reprod Immunol. 30:67–74. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fest S, Aldo PB, Abrahams VM, et al:
Trophoblast-macrophage interactions: a regulatory network for the
protection of pregnancy. Am J Reprod Immunol. 57:55–66. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang T, Dai H, Wan N, Moore Y and Dai Z:
The role for monocyte chemoattractant protein-1 in the generation
and function of memory CD8+ T cells. J Immunol.
180:2886–2893. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hammarström S: The carcinoembryonic
antigen (CEA) family: structures, suggested functions and
expression in normal and malignant tissues. Semin Cancer Biol.
9:67–81. 1999.PubMed/NCBI
|
|
18
|
Nagaishi T, Iijima H, Nakajima A, Chen D
and Blumberg RS: Role of CEACAM1 as a regulator of T cells. Ann NY
Acad Sci. 1072:155–175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moller MJ, Kammerer R, Grunert F and von
Kleist S: Biliary glycoprotein (BGP) expression on T cells and on a
natural-killer-cell sub-population. Int J Cancer. 65:740–745. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kammerer R, Hahn S, Singer BB, Luo JS and
von Kleist S: Biliary glycoprotein (CD66a), a cell adhesion
molecule of the immunoglobulin superfamily, on human lymphocytes:
structure, expression and involvement in T cell activation. Eur J
Immunol. 28:3664–3674. 1998. View Article : Google Scholar
|
|
21
|
Donda A, Mori L, Shamshiev A, et al:
Locally inducible CD66a (CEACAM1) as an amplifier of the human
intestinal T cell response. Eur J Immunol. 30:2593–2603. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morales VM, Christ A, Watt SM, et al:
Regulation of human intestinal intraepithelial lymphocyte cytolytic
function by biliary glycoprotein (CD66a). J Immunol. 163:1363–1370.
1999.PubMed/NCBI
|
|
23
|
Koga K and Mor G: Toll-like receptors at
the maternal-fetal interface in normal pregnancy and pregnancy
disorders. Am J Reprod Immunol. 63:587–600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mor G and Cardenas I: The immune system in
pregnancy: a unique complexity. Am J Reprod Immunol. 63:425–433.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saito S, Nishikawa K, Morii T, Narita N,
Enomoto M and Ichijo M: Expression of activation antigens CD69,
HLA-DR, interleukin-2 receptor-alpha (IL-2R alpha) and IL-2R beta
on T cells of human decidua at an early stage of pregnancy.
Immunology. 75:710–712. 1992.PubMed/NCBI
|
|
26
|
Markel G, Wolf D, Hanna J, et al: Pivotal
role of CEACAM1 protein in the inhibition of activated decidual
lymphocyte functions. J Clin Invest. 110:943–953. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakajima A, Iijima H, Neurath MF, et al:
Activation-induced expression of carcinoembryonic antigen-cell
adhesion molecule 1 regulates mouse T lymphocyte function. J
Immunol. 168:1028–1035. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boulton IC and Gray-Owen SD: Neisserial
binding to CEACAM1 arrests the activation and proliferation of
CD4+ T lymphocytes. Nat Immunol. 3:229–236. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen CJ and Shively JE: The cell-cell
adhesion molecule carcinoembryonic antigen-related cellular
adhesion molecule 1 inhibits IL-2 production and proliferation in
human T cells by association with Src homology protein-1 and
down-regulates IL-2 receptor. J Immunol. 172:3544–3552. 2004.
View Article : Google Scholar
|
|
30
|
Geiselhart A, Dietl J, Marzusch K, et al:
Comparative analysis of the immunophenotypes of decidual and
peripheral blood large granular lymphocytes and T cells during
early human pregnancy. Am J Reprod Immunol. 33:315–322. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ho HN, Chao KH, Chen CK, Yang YS and Huang
SC: Activation status of T and NK cells in the endometrium
throughout menstrual cycle and normal and abnormal early pregnancy.
Hum Immunol. 49:130–136. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gray-Owen SD and Blumberg RS: CEACAM1:
contact-dependent control of immunity. Nat Rev Immunol. 6:433–446.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bamberger AM, Sudahl S, Löning T, et al:
The adhesion molecule CEACAM1 (CD66a, C-CAM, BGP) is specifically
expressed by the extravillous intermediate trophoblast. Am J
Pathol. 156:1165–1170. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Briese J, Oberndörfer M, Pätschenik C, et
al: Osteopontin is colocalized with the adhesion molecule CEACAM1
in the extravillous trophoblast of the human placenta and enhances
invasion of CEACAM1-expressing placental cells. J Clin Endocrinol
Metab. 90:5407–5413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bamberger AM, Minas V, Kalantaridou SN, et
al: Corticotropin-releasing hormone modulates human trophoblast
invasion through carcinoembryonic antigen-related cell adhesion
molecule-1 regulation. Am J Pathol. 168:141–150. 2006. View Article : Google Scholar
|