Introduction
Adeno-associated virus (AAV) is a small,
single-stranded DNA-containing, non-pathogenic, human parvovirus.
AAV has been widely utilized as a vector for gene therapy in
various cell and tissue types (1–5).
However, a number of non- and less-permissive cell types have been
identified (6,7) and these cell types were not infected
efficiently by AAV.
Mouse fibroblast NIH/3T3 cells have been used to
develop induced pluripotent stem (iPS) cells (8). The iPS cells may provide new
opportunities for modeling human diseases and the potential for
personalized regenerative cell therapies (9). However, gene therapy in NIH/3T3 cells
that is mediated by the AAV vector is limited, as NIH/3T3 cells
have been identified to be a less-permissive cell type (10,11).
Ultrasound-targeted microbubble destruction (UTMD)
has efficiently and safely enhanced AAV-mediated gene transduction
in certain permissive cell types (12–15).
However, there are no studies concerning the increased AAV-mediated
gene transduction with the application of UTMD in the
less-permissive cell type, NIH/3T3.
The exact mechanism whereby UTMD enhances cellular
uptake has not yet been elucidated. However, the theory of
sonoporation is generally accepted (16–18),
in which microbubbles exposed to ultrasound generate microstreams
or microjets, which create shear stress on cells and open transient
pores in cell membranes (19).
These transient pores are suggested to facilitate the cellular
uptake of extracellular material, and entry through such pores is
proposed to be direct and rely on endocytosis (17). However, it has also been suggested
that endocytosis is essential for UTMD enhancement in cellular
uptake, which is not concordant with the theory of sonoporation
(20–23).
NIH/3T3 cells were identified to be a
less-permissive cell type due to their defective endosomal
processing (10,11). It is assumed that if UTMD is able
to greatly enhance the AAV-mediated gene transduction in the
NIH/3T3 cells, this would suggest that UTMD influences the
rate-limiting steps of NIH/3T3 cells. This is significant when
investigating the correlation between UTMD and endosomal
processing.
The present study applied UTMD as a technique to
enhance the gene transduction of AAV in a less-permissive cell
type, NIH/3T3. The UTMD parameters were optimized and the gene
transduction enhancement, dose dependence and cell viability in
NIH/3T3 cells was compared with a permissive cell type, HeLa.
Studies of the two cell types would aid in determining the value of
UTMD in AAV-mediated gene transduction, and in elucidating the
mechanism of UTMD facilitation in cellular uptake.
Materials and methods
Cell culture
NIH/3T3 and HeLa cells were maintained in Dulbecco's
modified Eagle's medium (DMEM; Gibco, Carlsbad, CA, USA) at 37°C
and 5% CO2. The medium was supplemented with 10% fetal
bovine serum (Gibco). The cells were seeded into alternative wells
of 24-well plates, 24 h prior to infection. To achieve 90%
confluency, 1×105 HeLa cells and 5×104
NIH/3T3 cells were seeded in each well.
Virus infection
When the cells had been infected, the medium was
replaced with 150 μl complete DMEM containing recombinant AAV
serotype 2 (rAAV2) vector encoding the enhanced green fluorescent
protein (EGFP) gene (rAAV2-EGFP; Beijing FivePlus Molecular
Medicine Institute, Beijing, China). Fresh DMEM (350 μl) was added
to the wells 2 h post-infection, and the medium containing the
virus was replaced with 500 μl complete DMEM 24 h following
treatment.
In the dose-effect experiments, the cells were
infected with rAAV2-EGFP only. The doses of the virus were
expressed as the multiplicity of infection (MOI). Six MOIs were
investigated for each cell type following several preliminary
tests. For HeLa cells, 0, 1,000, 2,000, 4,000 and 16,000 vector
genome (v.g.)/cell were investigated. For NIH/3T3 cells, 0, 1,000,
10,000, 100,000 and 500,000 v.g./cell were investigated. In the
remaining experiments, various MOIs were selected for respective
purposes.
UTMD protocols
A therapeutic ultrasound machine (Physioson-Basic;
Physioson Elektromedizin AG, Laipersdorf, Germany) was used to emit
ultrasound at a frequency of 1 MHz. The adjustable ultrasound
parameters included the ultrasound intensity, exposure time and
pulse output ratio. The ultrasound transducer was placed at the
bottom of the 24-well plates with a small amount of coupling medium
on the surface of the probe, which had an area of 2.5
cm2.
Microbubbles (Sonovue, Bracco, Milan, Italy) were
lipid-shelled ultrasound contrast agents containing sulfur
hexafluoride gas (diameter, 2.5–6.0 μm) and used at a concentration
of ~2×108 bubbles/ml. The volumetric ratio of
microbubbles to medium dictated the dose of the contrast agent to
be used.
To optimize the parameters, six combinations of
ultrasound and microbubble parameters were investigated according
to the results of a previous study (13). The parameters were combined
according to the following sequence: Ultrasound intensity, exposure
time, pulse output ratio and volume ratio of microbubbles.
Gene transfection efficiency assays
At 48 h post-treatment, the EGFP transfection
efficiency was evaluated by fluorescence microscopy and flow
cytometry. Green fluorescence was detected using inverted
fluorescence microscopy (Zeiss Axiovert S100; Carl Zeiss, Jena,
Germany). The percentage of infected cells was measured by flow
cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA)
following trypsinization and centrifugation. The treatment groups
comprised AAV (treatment with rAAV2-EGFP alone) and UTMD+AAV
(treatment with rAAV2-EGFP followed by UTMD). The measurements for
each group were conducted in triplicate (one well per
replicate).
Cell viability assays
WST-8 was the effective constituent of the Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) in the cell viability tests, as it is reduced to
yellow water-soluble formazan by the dehydrogenase released from
mitochondria. The quantity of formazan is in proportion to the
number of living cells. Optical density (OD) values of the cell
medium were detected to quantify the formazan levels and the
corresponding cell viability.
The medium of the cells was changed to 500 μl fresh
DMEM 2 h following infection and UTMD treatment. Immediately, the
cells were added to 50 μl CCK-8 reagent, and then incubated for 2
h. Following this, 100 μl medium from each well of the 24-well
plates was extracted and transferred to 96-well plates. The
resulting color was analyzed using a microplate absorbance reader
(iMark, Bio-Rad, Hercules, CA, USA) and the OD values were measured
at 450 nm. Cells that were not infected or treated with UTMD served
as the control group. The measurements for each group were
conducted in quadruplicate (one well per replicate).
Reverse transcription PCR (RT-PCR) and
real-time PCR (qPCR) assay
Cells were trypsinized and harvested from the
24-well plates (six wells per group) 48 h following infection and
UTMD treatment. The cells were lysed, and the total RNA was
extracted from respective samples using TRIzol® reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer's instructions. The quantity and purity of the
isolated RNA was measured by spectrophotometry. Reverse
transcription to synthesize cDNA was conducted using the First
Strand cDNA Synthesis kit (Promega Corporation, Madison, WI, USA).
Quantitative PCR was performed on a 7500 Real-Time PCR System
(Applied Biosystems, Inc., Foster City, CA, USA) using
GoTaq®qPCR Master mix (Promega Corporation) and primers
were designed against EGFP. The primer sequences used were:
Forward: 5′-AGAAGAACGGCATCAAGGTG-3′ and reverse:
5′-GAACTCCAGCAGGACCATGT-3′. The conditions used for the reaction
were: One cycle at 50°C for 2 min and 95°C for 10 min, and 40
cycles at 95°C for 15 sec and 60°C for 15 sec. Quantification,
using the 2−ΔΔCT analytical method, was performed in
triplicate with β-actin as the internal standard.
Western blot analysis
At 48 h following infection and UTMD treatment,
cells were harvested from 24-well plates (24 wells per group). To
detect the EGFP protein, the samples were separated on a sodium
dodecyl sulfate-polyacrylamide gel (10% acrylamide) and blotted
onto a nitrocellulose membrane. The membrane was then blocked with
0.2% I-Block (Sigma-Aldrich, St. Louis, MO, USA) in Tris-buffered
saline supplemented with 0.1% Tween 20 (TBST) for 1 h at room
temperature. Following incubation with goat polyclonal anti-EGFP
antibody (1:1,000 in TBST ab111258; Abcam, Cambridge, UK) overnight
at 4°C, the membrane was washed three times in TBST and incubated
for 2 h with a peroxidase-conjugated anti-goat immunoglobulin G
antibody (1:5,000 in TBST). The membrane was washed again,
incubated for 1 min with SuperSignal West Pico Chemiluminescent
Substrate (Pierce Biotechnology, Inc., Rockford, IL, USA) and
exposed to Biomax Light Film (Kodak-Industrie, Chalon-sur-Saône,
France).
Statistical analysis
Data were expressed as mean ± standard deviation.
One-way analysis of variance (ANOVA) with Bonferroni adjustment was
used to determine the differences among groups in the UTMD
parameter optimization and cell viability experiments. The
independent samples t-test was used to detect differences between
the treatment and control groups in the gene transduction
efficiency detection and PCR experiments. P<0.05 was considered
to indicate a statistically significant difference. Statistical
analyses were performed using SPSS software (version 13.0; SPSS
Inc., Chicago, IL, USA).
Results
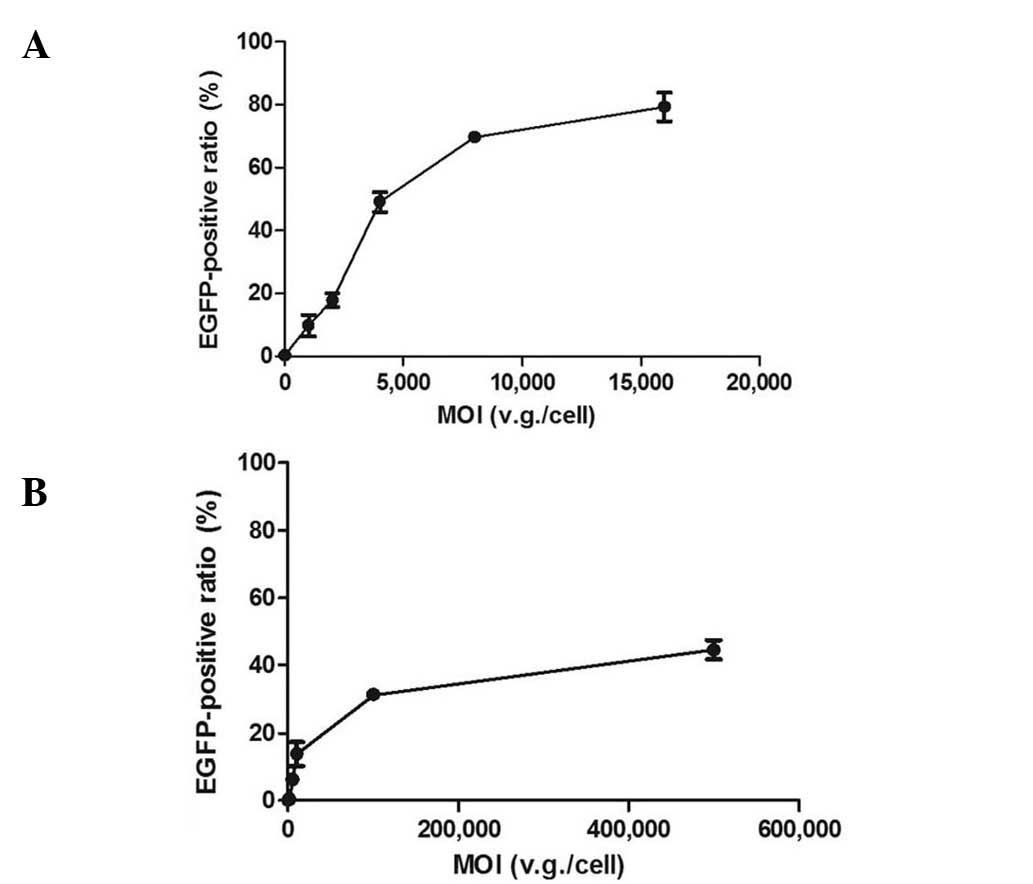
UTMD parameter optimization
When the cells were infected with rAAV2-EGFP only,
the transduction efficiency increased as the MOIs were elevated in
both cell types. In the dose-effect curves, the effects markedly
increased in the ascending curve segment and gradually in the plain
segment (Fig. 1). To obtain the
MOI in the later enhancement experiments, the approximate midpoint
of the ascending segment was selected. To explore the dose
dependence of UTMD enhancement, a lower and higher dose were also
selected. Therefore, the MOIs of 1,000, 2,000 and 4,000 v.g./cell
were selected for HeLa cells, and 1,000, 10,000 and 50,000
v.g./cell for NIH/3T3 cells. To confirm the UTMD enhancement of
gene transcription and protein expression, the MOI of 2,000
v.g./cell was selected for HeLa cells and 10,000 v.g./cell for
NIH/3T3 cells.
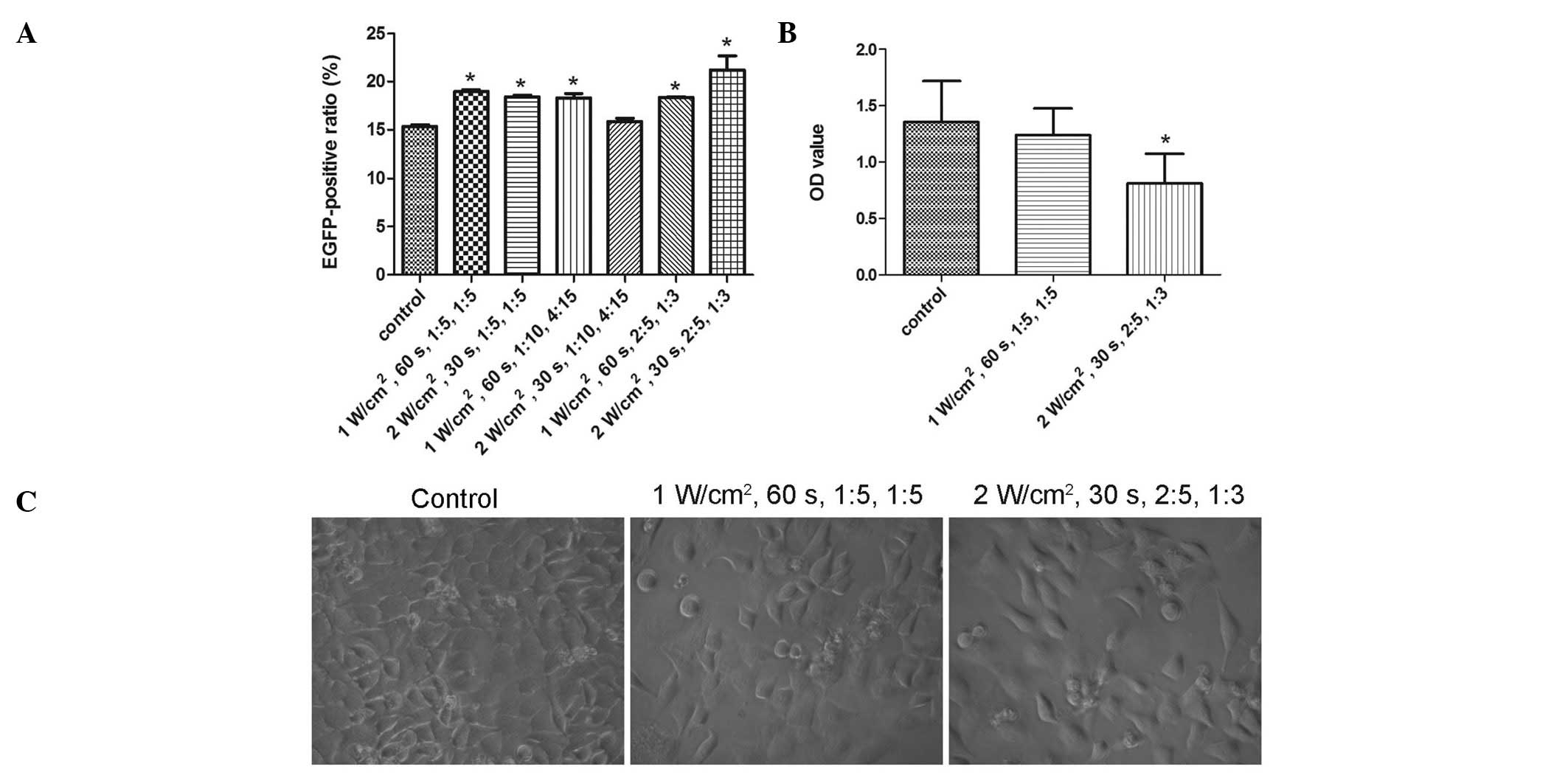
All the combinations of UTMD parameters, with the
exception of 2 W/cm2, 30 sec, 1:10, 4:15, significantly
enhanced the transduction efficiency of rAAV2-EGFP (P=0.000,
P=0.001, P=0.001, P=1.000, P=0.001 and P=0.000, from the first to
the last combination, respectively) (Fig. 2A). The combination of 2
W/cm2, 30 sec, 2:5, 1:3 demonstrated the greatest
increase in transduction efficiency, while that of 1
W/cm2, 60 sec, 1:5, 1:5 demonstrated the second greatest
increase. However, due to the extreme parameters, such as 2
W/cm2 and 2:5, in the former combination, the two
combinations were investigated in the cell viability tests.
A comparison of treated and untreated cells in the
CCK-8 cell viability assays showed the combination of 2
W/cm2, 30 sec, 2:5, 1:3 to be harmful (P=0.007), whereas
that of 1 W/cm2, 60 sec, 1:5, 1:5 was shown to be safe
(P=1.000) (Fig. 2B). Results of
the light microscopy examinations demonstrated that fewer cells
remained after being treated with 2 W/cm2, 30 sec, 2:5,
1:3 compared with 1 W/cm2, 60 sec, 1:5, 1:5 (Fig. 2C). Therefore, the optimized UTMD
parameter combination was defined as 1 W/cm2, 60 sec,
1:5, 1:5.
Enhancement and dose dependence
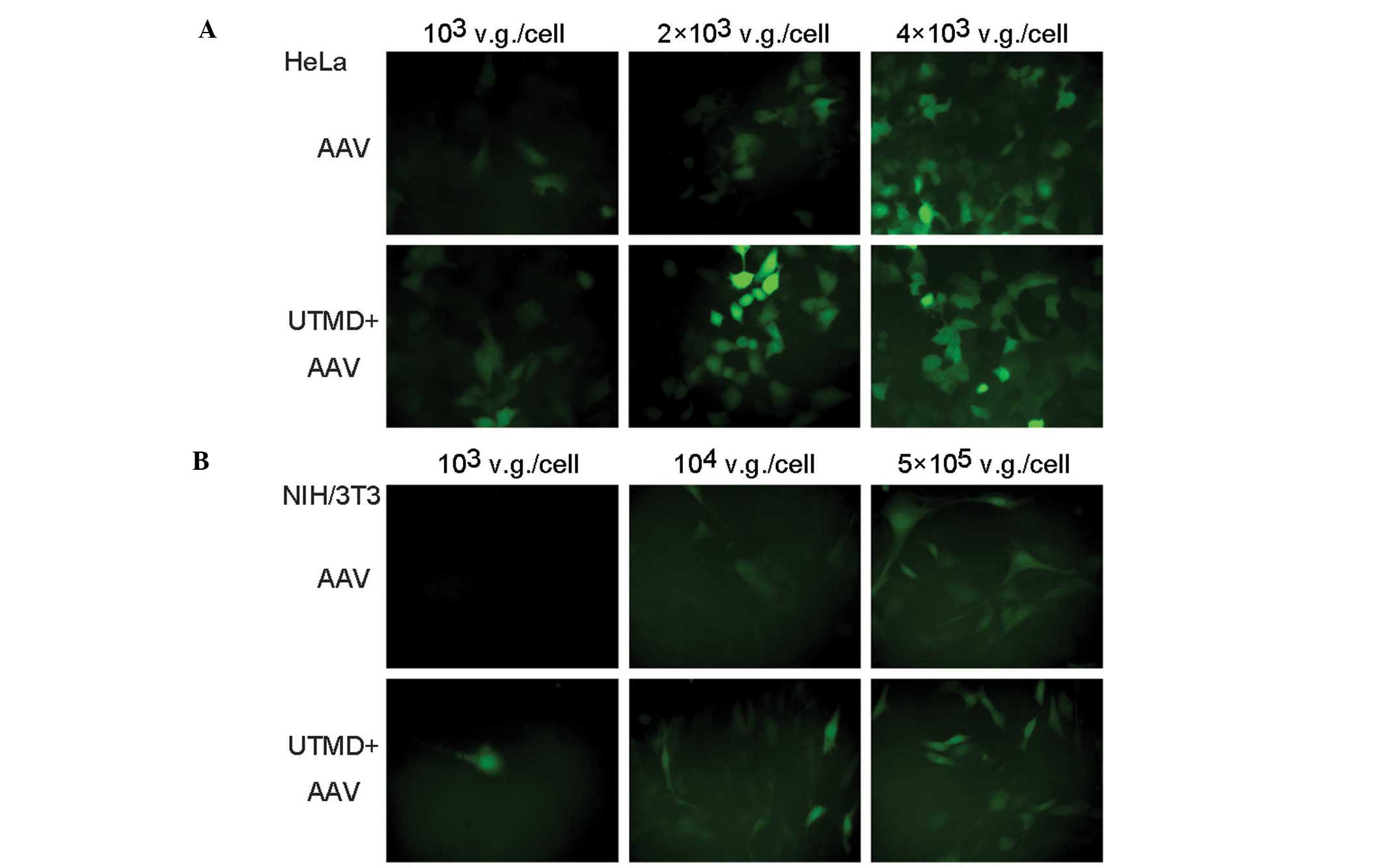
In the fluorescence microscopic images, the number
of green fluorescent HeLa cells in the UTMD+AAV group was greater
than that in the AAV group when low and medium AAV doses were
applied. The fluorescence intensity of the cells in the UTMD+AAV
group was stronger than that in the AAV group. In addition, the
UTMD enhancement was not as obvious when low and medium AAV doses
were used as opposed to a high AAV dose (Fig. 3A). Similar results were observed in
the NIH/3T3 cells (Fig. 3B).
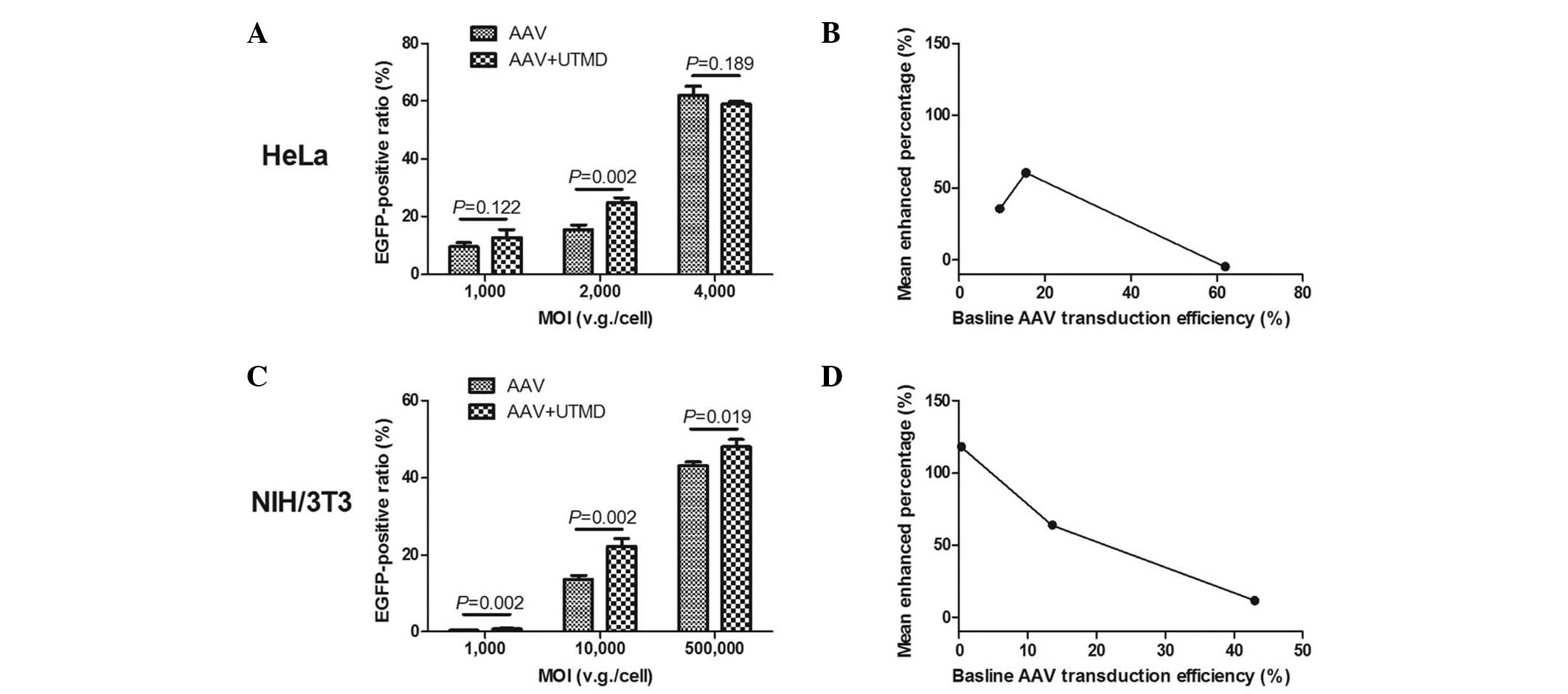
Data from the flow cytometry investigation
demonstrated that UTMD enhancement was greatest in the HeLa and
NIH/3T3 cells when medium doses of AAV were applied. The mean ratio
of green fluorescent HeLa cells in the UTMD+AAV group was
24.96±1.42% at a MOI of 2,000 v.g./cell, which was significantly
higher than that in the AAV group (15.56±1.64%) (P=0.002). In the
HeLa cells, the absolute mean enhanced ratio was 9.4% and the UTMD
enhanced transfection by 1.60-fold (Fig. 4A). The mean ratio of green
fluorescent NIH/3T3 cells in the UTMD+AAV group was 22.28±1.89% at
a MOI of 10,000 v.g./cell, which was also significantly higher than
that in the AAV group (13.59±1.05%) (P=0.002). The absolute mean
enhanced ratio was 8.69% and the UTMD enhanced transfection by
1.64-fold in the NIH/3T3 cells (Fig.
4C). As the baseline AAV transduction efficiency increased, the
mean enhanced percentage of transduction efficiency initially
increased and then decreased in the HeLa cells (Fig. 4B), but continued decreasing in the
NIH/3T3 cells (Fig. 4D).
Enhanced gene transcription and
expression
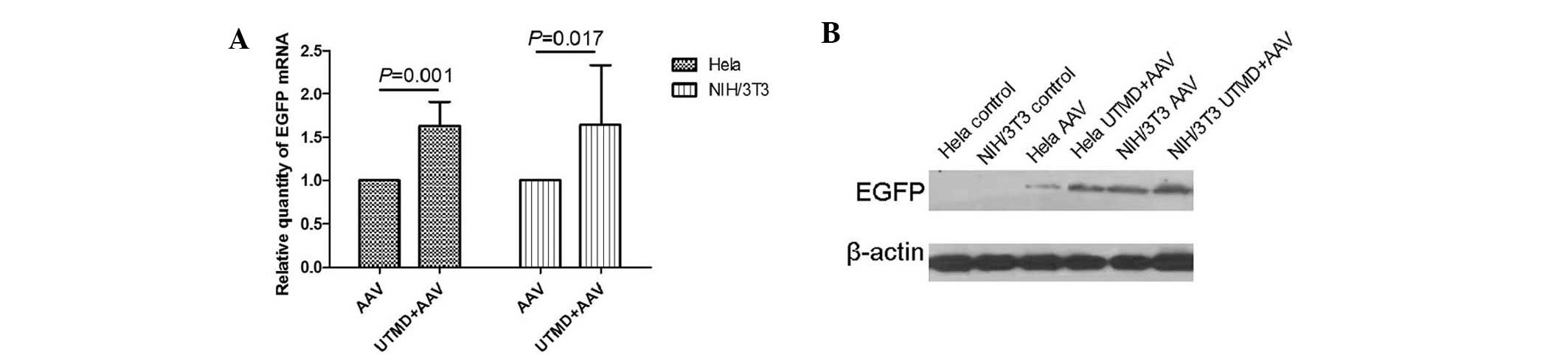
The PCR results revealed that, the relative quantity
of EGFP gene transcription in the UTMD+AAV group was significantly
greater than that in the AAV group in HeLa and NIH/3T3 cells
(P=0.001 and P=0.017, respectively). The mean UTMD enhancement was
1.62±0.11-fold in the HeLa cells and 1.64±0.28-fold in the NIH/3T3
cells (Fig. 5A). The western blot
analysis demonstrated that the relative quantity of EGFP gene
expression in the UTMD+AAV group was greater than that in the AAV
group in HeLa and NIH/3T3 cells (Fig.
5B).
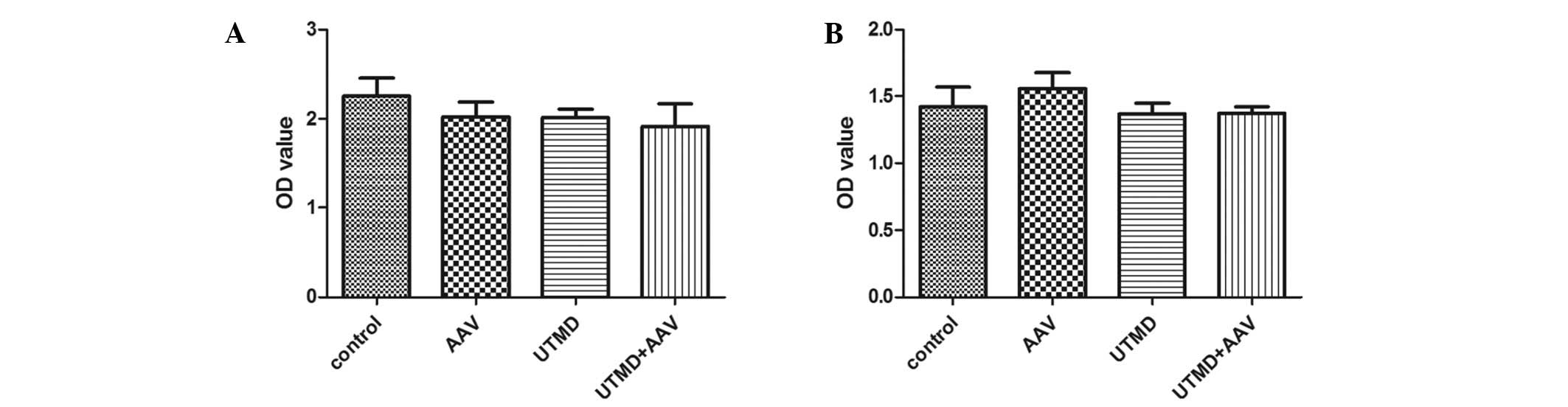
Cell viability
The cell viability assays indicated that AAV
infection, UTMD treatment and AAV infection with UTMD treatment did
not significantly affect proliferation in the HeLa cells (P=0.640,
P=0.558 and P=0.150, respectively) (Fig. 6A). Similarly, there was no
significant difference in cell viability among the AAV, UTMD and
UTMD+AAV groups (P=0.610, P=1.000 and P=1.000, respectively) in the
NIH/3T3 cells (Fig. 6B).
Discussion
In the present study, UTMD efficiently and safely
enhanced the gene transduction of AAV in the less-permissive cell
type, NIH/3T3. The enhancement effect of UTMD was 1.6 fold with
regard to transduction efficiency and gene transcription. The
results were similar to those of a previous study that demonstrated
a 1.75-fold enhancement effect in another permissive cell type,
retinal pigment epithelium (13).
Furthermore, when UTMD along with corresponding parameters was
applied in vivo, the enhancement effects were higher and
sustained (13,15). Therefore, applying UTMD-mediated
AAV transduction in in vivo studies of iPS cells in the gene
therapy of inherited and acquired disorders may be beneficial
(8,24,25).
The dose dependence of UTMD enhancement in the two
cell types demonstrated inefficiency at high AAV doses. The
inefficiency suggested saturation of the mechanism. The generally
accepted mechanism of sonoporation is considered to facilitate
direct entry of extracellular material through transit pores
(17), and this route of entry
would not become saturated as the extracellular material increased.
Thus, sonoporation is not able to explain the dose dependence of
the UTMD-enhanced AAV-mediated transduction. Other mechanisms may
therefore be involved in the UTMD enhancement.
When using UTMD, the transduction efficiency was
enhanced by 1.64-fold in the NIH/3T3 cells, and by 1.60 fold in the
HeLa cells. Similarly, the enhanced gene transcription in the
NIH/3T3 cells (1.64-fold) was also slightly greater than that in
the HeLa cells (1.62-fold). Therefore, UTMD did not greatly enhance
the gene transduction of AAV in the endosomal processing-defective
cell type, NIH/3T3. In previous studies on NIH/3T3 cells, the
enhanced gene transduction of AAV suggested an effect on the
rate-limiting steps (10,26). However, the present study did not
indicate that UTMD could bypass the defect in AAV trafficking in
NIH/3T3 cells. The results were not able to identify a correlation
between the mechanism of UTMD enhancement in cellular uptake and
endosomal processing. Additional studies in other non- or
less-permissive cell types with different AAV trafficking defects
(27–30) are required to elucidate the exact
mechanism of UTMD enhancement in AAV cellular uptake.
The parameters of UTMD used in the present study
were taken from various studies (12,13,15,31).
Therefore, the application of the UTMD parameters was determined by
cell type, to a certain degree. The HeLa cell type was utilized to
optimize the parameters. This was due to only one UTMD parameter
combination being used in the enhancement in two cell types, in
favor of comparison. HeLa cells were more tolerant than NIH/3T3
cells in the optimization experiment, and the optimized UTMD
parameter combination significantly enhanced the gene transduction
of AAV. Although enhancement was achieved utilizing optimized
parameters for UTMD, there may be a greater increase in enhancement
with the implementation of other methods, such as multiple and
repeating operations of UTMD (32).
Overall, UTMD enhanced the gene transduction of AAV
in the less-permissive cell type, NIH/3T3. UTMD-enhanced
AAV-mediated gene transduction may be beneficial in iPS cell
application. The degree of UTMD enhancement and the mode of dose
dependence in NIH/3T3 cells were similar to those of the permissive
cell type, HeLa. UTMD did not bypass the rate-limiting steps of AAV
cellular trafficking in NIH/3T3 cells. Additional studies are
required to elucidate the mechanism of UTMD enhancement and thus
enable an increased enhancement.
Acknowledgements
The authors would like to thank Xueqian Xie for the
critical reading of the manuscript, and Xiaomei Wu, Zhongming Xiao
and Huiming Li for their technical assistance. This study was
supported by the National Natural Science Foundations of China
(grant nos. 81000687, 81000617 and 81171352).
Abbreviations:
|
AAV
|
adeno-associated virus
|
|
iPS
|
induced pluripotent stem
|
|
UTMD
|
ultrasound-targeted microbubble
destruction
|
|
MOI
|
multiplicity of infection
|
|
CCK-8
|
cell counting kit-8
|
|
OD
|
optical density
|
References
|
1
|
Moscioni D, Morizono H, McCarter R, et al:
Long-term correction of ammonia metabolism and prolonged survival
in ornithine transcarbamylase-deficient mice following
liver-directed treatment with adeno-associated viral vectors. Mol
Ther. 14:25–33. 2006. View Article : Google Scholar
|
|
2
|
Hauswirth WW, Aleman TS, Kaushal S, et al:
Treatment of leber congenital amaurosis due to RPE65 mutations by
ccular subretinal injection of adeno-associated virus gene vector:
short-term results of a phase I trial. Hum Gene Ther. 19:979–990.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manno CS, Chew AJ, Hutchison S, et al:
AAV-mediated factor IX gene transfer to skeletal muscle in patients
with severe hemophilia B. Blood. 101:2963–2972. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaplitt MG, Feigin A, Tang C, et al:
Safety and tolerability of gene therapy with an adeno-associated
virus (AAV) borne GAD gene for Parkinson's disease: an open label,
phase I trial. Lancet. 369:2097–2105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brantly ML, Spencer LT, Humphries M, et
al: Phase I trial of intramuscular injection of a recombinant
adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector
in AAT-deficient adults. Hum Gene Ther. 17:1177–1186. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartlett JS, Kleinschmidt J, Boucher RC
and Samulski RJ: Targeted adeno-associated virus vector
transduction of nonpermissive cells mediated by a bispecific
F(ab'gamma)2 antibody. Nat Biotechnol. 17:181–186. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hansen J, Qing K and Srivastava A:
Infection of purified nuclei by adeno-associated virus 2. Mol Ther.
4:289–296. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Robinton DA and Daley GQ: The promise of
induced pluripotent stem cells in research and therapy. Nature.
481:295–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hansen J, Qing K and Srivastava A:
Adeno-associated virus type 2-mediated gene transfer: altered
endocytic processing enhances transduction efficiency in murine
fibroblasts. J Virol. 75:4080–4090. 2001. View Article : Google Scholar
|
|
11
|
Hansen J, Qing K, Kwon HJ, Mah C and
Srivastava A: Impaired intracellular trafficking of
adeno-associated virus type 2 vectors limits efficient transduction
of murine fibroblasts. J Virol. 74:992–996. 2000. View Article : Google Scholar
|
|
12
|
Müller OJ, Schinkel S, Kleinschmidt JA,
Katus HA and Bekeredjian R: Augmentation of AAV-mediated cardiac
gene transfer after systemic administration in adult rats. Gene
Ther. 15:1558–1565. 2008.PubMed/NCBI
|
|
13
|
Li HL, Zheng XZ, Wang HP, Li F, Wu Y and
Du LF: Ultrasound-targeted microbubble destruction enhances
AAV-mediated gene transfection in human RPE cells in vitro and rat
retina in vivo. Gene Ther. 16:1146–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie W, Liu S, Su H, Wang Z, Zheng Y and Fu
Y: Ultrasound microbubbles enhance recombinant adeno-associated
virus vector delivery to retinal ganglion cells in vivo. Acad
Radiol. 17:1242–1248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng X, Du L, Wang H and Gu Q: A novel
approach to attenuate proliferative vitreoretinopathy using
ultrasound-targeted microbubble destruction and recombinant
adeno-associated virus-mediated RNA interference targeting
transforming growth factor-β2 and platelet-derived growth factor-B.
J Gene Med. 14:339–347. 2012.PubMed/NCBI
|
|
16
|
Mehier-Humbert S, Bettinger T, Yan F and
Guy RH: Plasma membrane poration induced by ultrasound exposure:
implication for drug delivery. J Control Release. 104:213–222.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Wamel A, Kooiman K, Harteveld M, et
al: Vibrating microbubbles poking individual cells: drug transfer
into cells via sonoporation. J Control Release. 112:149–155.
2006.PubMed/NCBI
|
|
18
|
Taniyama Y, Tachibana K, Hiraoka K, et al:
Local delivery of plasmid DNA into rat carotid artery using
ultrasound. Circulation. 105:1233–1239. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzuki R, Oda Y, Utoguchi N and Maruyama
K: Progress in the development of ultrasound-mediated gene delivery
systems utilizing nano- and microbubbles. J Control Release.
149:36–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meijering BD, Juffermans LJ, van Wamel A,
et al: Ultrasound and microbubble-targeted delivery of
macromolecules is regulated by induction of endocytosis and pore
formation. Circ Res. 104:679–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hauser J, Ellisman M, Steinau HU, Stefan
E, Dudda M and Hauser M: Ultrasound enhanced endocytotic activity
of human fibroblasts. Ultrasound Med Biol. 35:2084–2092. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lionetti V, Fittipaldi A, Agostini S,
Giacca M, Recchia FA and Picano E: Enhanced caveolae-mediated
endocytosis by diagnostic ultrasound in vitro. Ultrasound Med Biol.
35:136–143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paula DM, Valero-Lapchik VB,
Paredes-Gamero EJ and Han SW: Therapeutic ultrasound promotes
plasmid DNA uptake by clathrin-mediated endocytosis. J Gene Med.
13:392–401. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhong L, Zhao W, Wu J, Maina N, Han Z and
Srivastava A: Adeno-associated virus-mediated gene transfer in
hematopoietic stem/progenitor cells as a therapeutic tool. Curr
Gene Ther. 6:683–698. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stender S, Murphy M, O'Brien T, et al:
Adeno-associated viral vector transduction of human mesenchymal
stem cells. Eur Cell Mater. 13:93–99. 2007.PubMed/NCBI
|
|
26
|
Li M, Jayandharan GR, Li B, et al:
High-efficiency transduction of fibroblasts and mesenchymal stem
cells by tyrosine-mutant AAV2 vectors for their potential use in
cellular therapy. Human Gene Ther. 21:1527–1543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duan D, Yue Y, Yan Z, Yang J and
Engelhardt JF: Endosomal processing limits gene transfer to
polarized airway epithelia by adeno-associated virus. J Clin
Invest. 105:1573–1587. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qing K, Hansen J, Weigel-Kelley KA, Tan M,
Zhou S and Srivastava A: Adeno-associated virus type 2-mediated
gene transfer: role of cellular FKBP52 protein in transgene
expression. J Virol. 75:8968–8976. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qing K, Mah C, Hansen J, Zhou S, Dwarki V
and Srivastava A: Human fibroblast growth factor receptor 1 is a
co-receptor for infection by adeno-associated virus 2. Nat Med.
5:71–77. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ponnazhagan S, Wang XS, Woody MJ, et al:
Differential expression in human cells from the p6 promoter of
human parvovirus B19 following plasmid transfection and recombinant
adeno-associated virus 2 (AAV) infection: human megakaryocytic
leukaemia cells are non-permissive for AAV infection. J Gen Virol.
77:1111–1122. 1996. View Article : Google Scholar
|
|
31
|
Xie W, Liu S, Su H, Wang Z, Zheng Y and Fu
Y: Ultrasound microbubbles enhance recombinant adeno-associated
virus vector delivery to retinal ganglion cells in vivo. Acad
Radiol. 17:1242–1248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stride E and Saffari N: Investigating the
significance of multiple scattering in ultrasound contrast agent
particle populations. IEEE Trans Ultrason Ferroelectr Freq Control.
52:2332–2345. 2005. View Article : Google Scholar : PubMed/NCBI
|