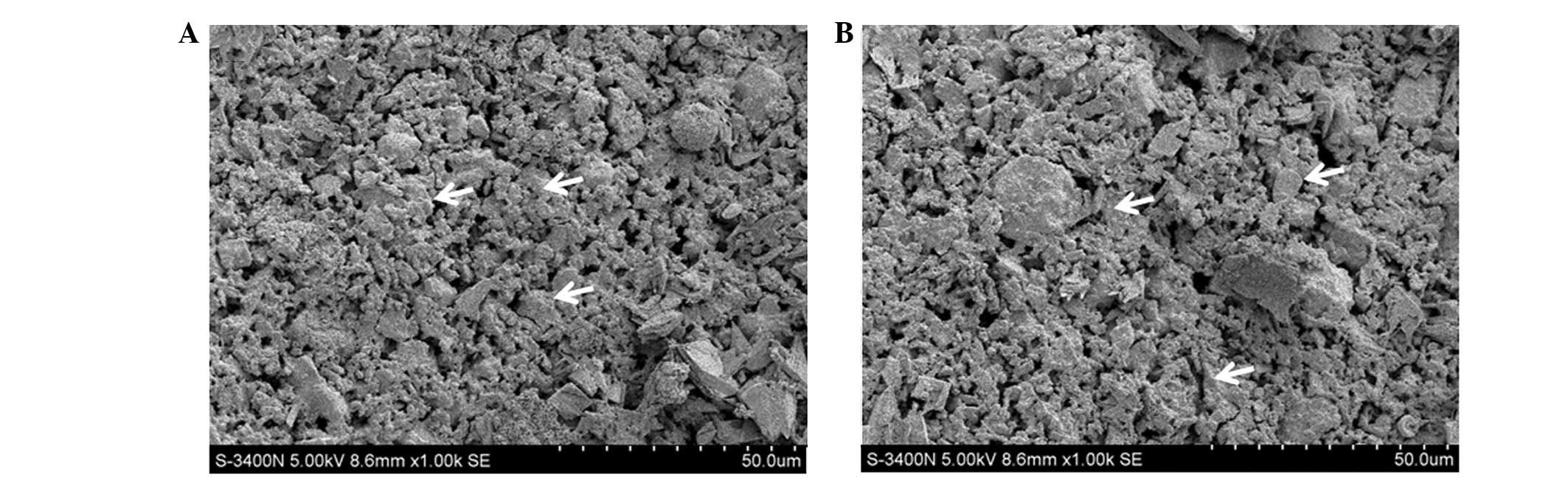
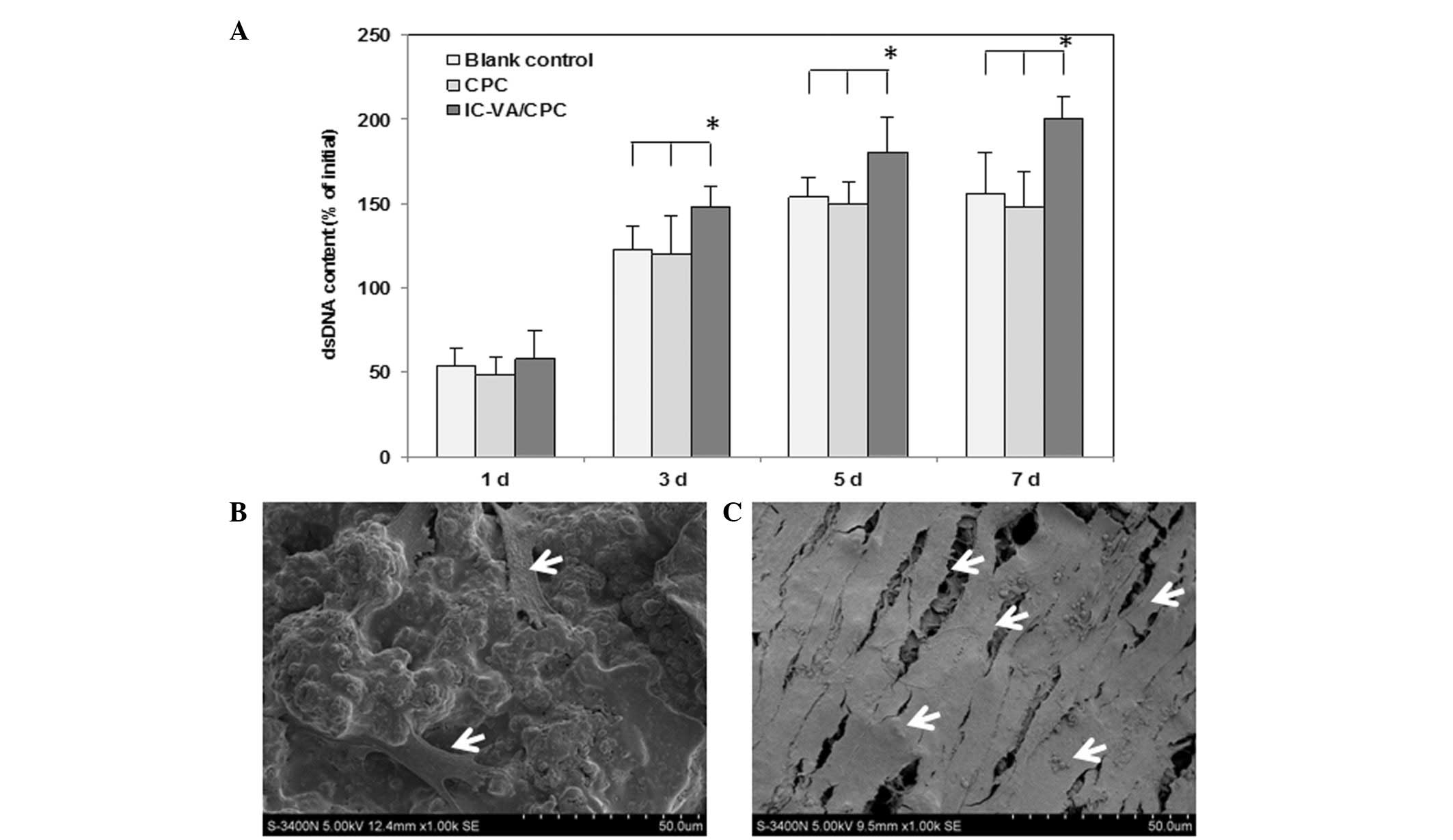
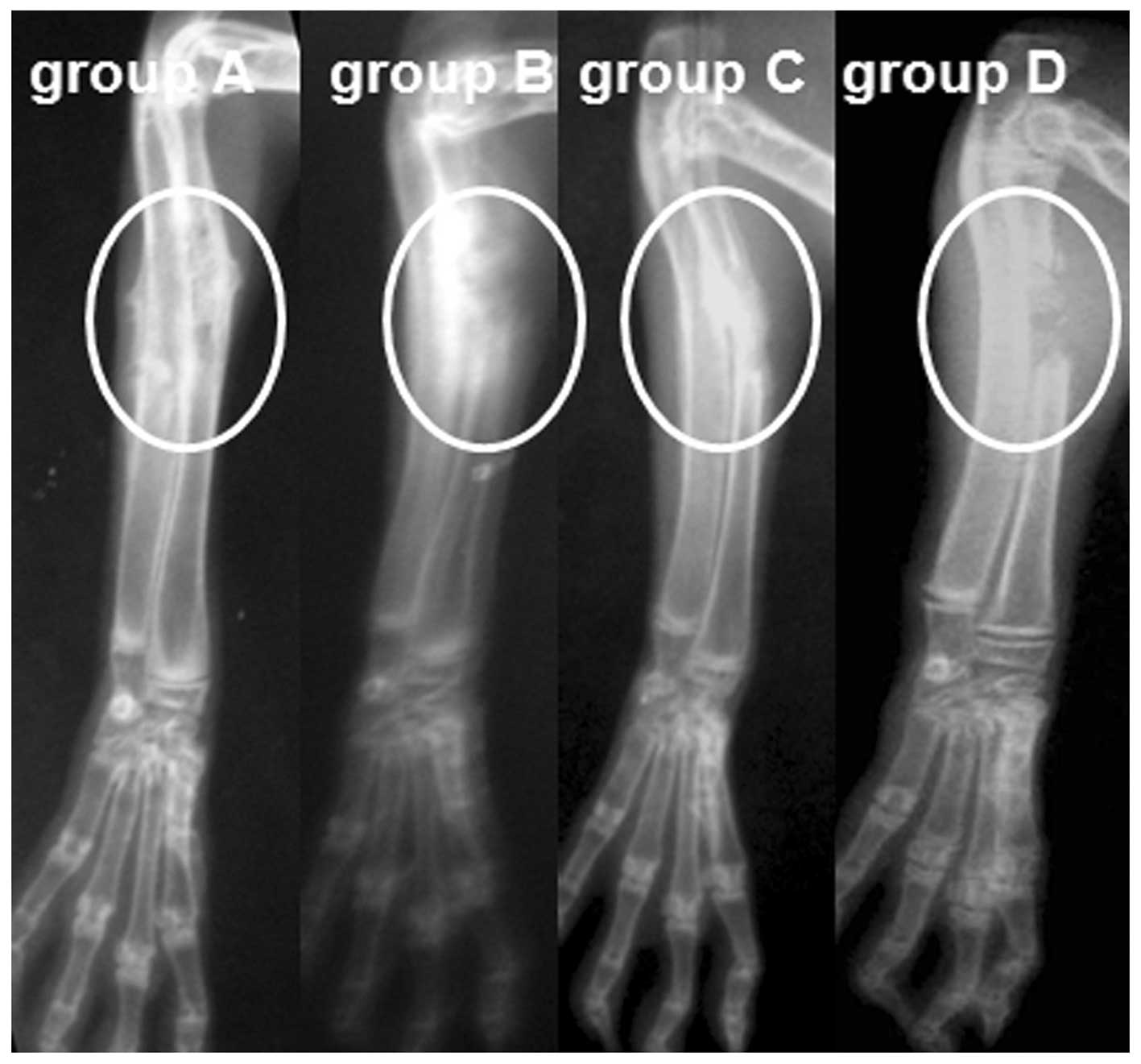
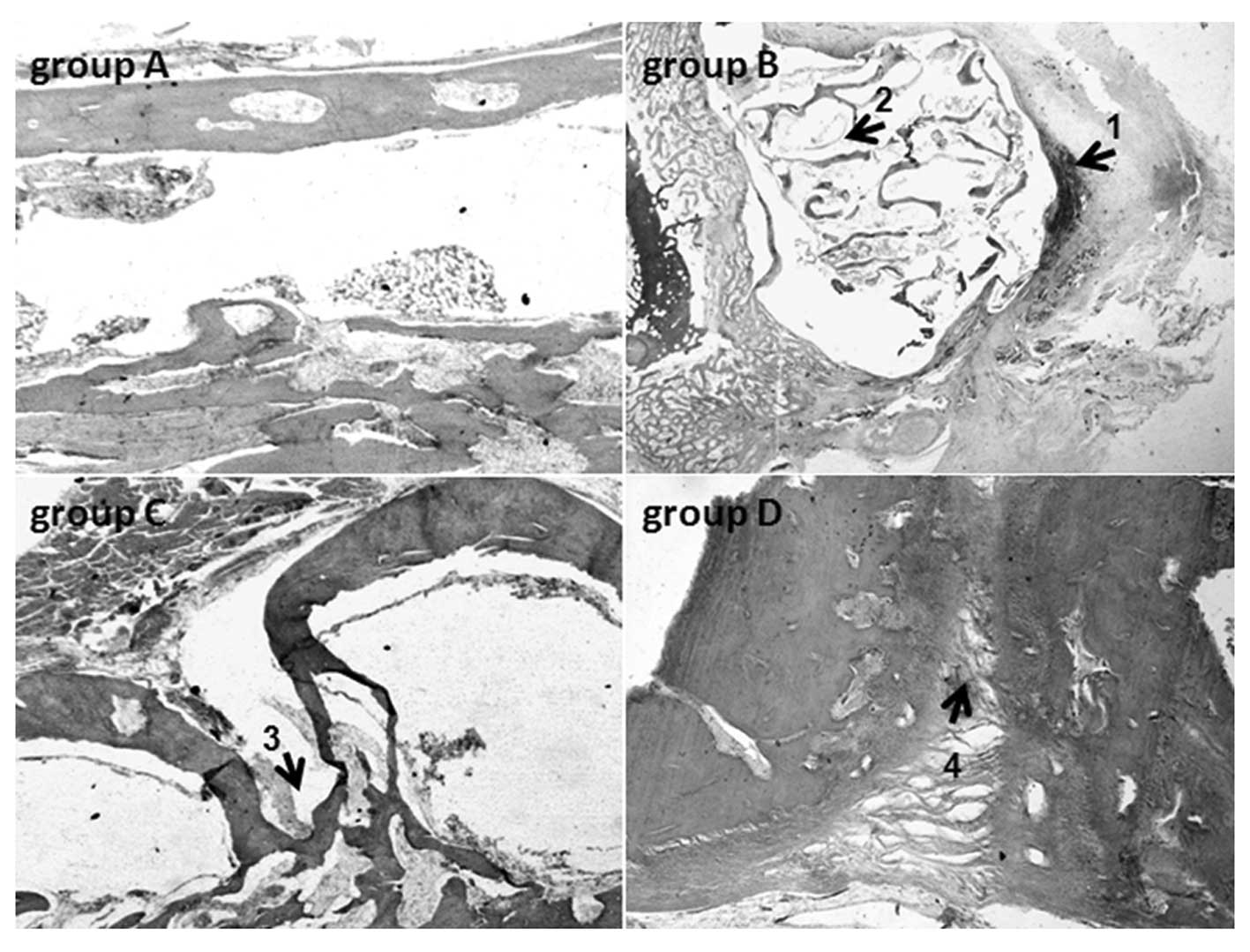
|
1
|
Bi L, Hu Y, Fan H, Meng G, Liu J, Li D and
Lv R: Treatment of contaminated bone defects with
clindamycin-reconstituted bone xenograft-composites. J Biomed Mater
Res B Appl Biomater. 82:418–427. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Korkusuz F, Korkusuz P, Eksioglu F, Gursel
I and Hasirci V: In vivo response to biodegradable controlled
antibiotic release systems. J Biomed Mater Res. 55:217–228. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shinto Y, Uchida A, Korkusuz F, Araki N
and Ono K: Calcium hydroxyapatite ceramic used as a delivery system
for antibiotics. J Bone Joint Surg Br. 74:600–604. 1992.PubMed/NCBI
|
|
4
|
Cornell CN, Tyndall D, Waller S, Lane JM
and Brause BD: Treatment of experimental osteomyelitis with
antibioticim-pregnated bone graft substitute. J Orthop Res.
11:619–626. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moehring HD, Gravel C, Chapman MW and
Olson SA: Comparison of antibiotic beads and intravenous
antibiotics in open fractures. Clin Orthop Relat Res. 372:254–261.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guelcher SA, Brown KV, Li B, Guda T, Lee
BH and Wenke JC: Dual-purpose bone grafts improve healing and
reduce infection. J Orthop Trauma. 25:477–482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bi L, Cheng W, Fan H and Pei G:
Reconstruction of goat tibial defects using an injectable
tricalcium phosphate/chitosan in combination with autologous
platelet-rich plasma. Biomaterials. 31:3201–3211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cui G, Li J, Lei W, Bi L, Tang P, Liang Y,
Tao S and Wang Y: The mechanical and biological properties of an
injectable calcium phosphate cement-fibrin glue composite for bone
regeneration. J Biomed Mater Res B Appl Biomater. 92:377–385.
2010.PubMed/NCBI
|
|
9
|
Urabe K, Naruse K, Hattori H, Hirano M,
Uchida K, Onuma K, Park HJ and Itoman M: In vitro comparison of
elution characteristics of vancomycin from calcium phosphate cement
and polymethylmethacrylate. J Orthop Sci. 14:784–793. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okada M, Sangadala S, Liu Y, Yoshida M,
Reddy BV, Titus L and Boden SD: Development and optimization of a
cell-based assay for the selection of synthetic compounds that
potentiate bone morphogenetic protein-2 activity. Cell Biochem
Funct. 27:526–534. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan JJ, Cao LG, Wu T, Wang DX, Jin D,
Jiang S, Zhang ZY, Bi L and Pei GX: The dose-effect of icariin on
the proliferation and osteogenic differentiation of human bone
mesenchymal stem cells. Molecule. 16:10123–10133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao J, Ohba S, Komiyama Y, Shinkai M,
Chung UI and Nagamune T: Icariin: a potential osteoinductive
compound for bone tissue engineering. Tissue Eng Part A.
16:233–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang F, Wang XL, Wang NL and Yao XS: Two
new flavonol glycosides from Epimedium koreanum Nakai. J
Asian Nat Prod Res. 11:401–409. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nian H, Ma MH, Nian SS and Xu LL:
Antiosteoporotic activity of icariin in ovariectomized rats.
Phytomedicine. 16:320–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao J, Ohba S, Shinkai M, Chung UI and
Nangamue T: Icariin induces osteogenic differentiation in vitro in
a BMP-and Runx2-dependent manner. Biochem Biophys Res Commun.
369:444–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan J, Bi L, Wu T, Cao L, Wang D, Nan K,
Chen J, Jin D, Jiang S and Pei G: A combined chitosan/nano-size
hydroxyapatite system for the controlled release of icariin. J
Mater Sci Mater Med. 23:399–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bitar M, Friederici V, Imgrund P, Brose C
and Bruinink A: In vitro bioactivity of micro metal injection
moulded stainless steel with defined surface features. Eur Cell
Mater. 23:333–347. 2012.PubMed/NCBI
|
|
18
|
Li HB and Chen F: Separation and
purification of epimedin A, B, C, and icariin from the medicinal
herb Epimedium brevicornum maxim by dual-mode HSCCC. J
Chromatogr Sci. 47:337–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Larsson S and Bauer TW: Use of injectable
calcium phosphate cement for fracture fixation: a review. Clin
Orthop Relat Res. 395:23–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
International Organization for
Standardization. ISO 10993-12:2007: Biological evaluation of
medical devices. Part 12: Sample preparation and reference
materials. ISO; Geneva, Switzerland: 2007
|
|
21
|
Yang L, Wang NL and Cai GP: Maohuoside A
promotes osteogenesis of rat mesenchymal stem cells via BMP and
MAPK signaling pathways. Mol Cell Biochem. 358:37–44. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Melicherčík P, Jahoda D, Nyč O, Klapková
E, Barták V, Landor I, Pokorný D, Judl T and Sosna A: Bone grafts
as vancomycin carriers in local therapy of resistant infections.
Folia Microbiol (Praha). 57:459–462. 2012.PubMed/NCBI
|
|
23
|
Bert F, Leflon-Guibout V, Le GJ, Bourdon N
and Nicolas MH: Emergence of vancomycin-dependent enterococci
following glycopeptide therapy: case report and review. Pathol Biol
(Paris). 57:56–60. 2009.(In French).
|