Introduction
Sterigmatocystin is a mycotoxin derived from the
Aspergillus fungus, and is a known carcinogen (1–4). The
development of esophageal cancer has been linked to
sterigmatocystin consumption (1,3);
however, a variety of factors, including esophageal mucosal injury
and immune function, are likely to be involved in disease onset
(5,6). Epidemiological studies have
demonstrated that the eating habits of residents in China
contribute to the relatively high incidence of esophageal cancer
and the prevalence of reflux esophagitis (6,7). The
presence of contaminating mycotoxins in the Chinese food supply is
a serious and widely recognized issue (1,8).
Previous studies of the sterigmatocystin mycotoxin have
demonstrated its ability to negatively impact immune function.
Sterigmatocystin treatment of human peripheral blood mononuclear
cells resulted in a significant downregulation of the gene
expression of transporter associated with antigen processing 1
(TAP1) and low molecular weight protein 2 (LMP2), two key
regulators of the immune response. Moreover, sterigmatocystin was
observed to inhibit the expression of human leukocyte antigen
(HLA)-I in human esophageal squamous cells in a dose-dependent
manner (9,10). Considering the extent of
sterigmatocystin mycotoxin contamination in the general food supply
and its suggested link to esophageal cancer and decreased immune
function, this study was conducted to investigate the effects of
sterigmatocystin exposure in rats with reflux esophagitis.
Proliferating cell nuclear antigen (PCNA), LMP2 and TAP1 protein
expression was determined in these rats in the absence and presence
of sterigmatocystin.
Materials and methods
Materials
Thirty healthy male Wistar rats (age, 4 weeks;
weight, 40–50 g) were purchased from the Experimental Animal Center
of Hebei Medical University in China (Hebei, China). This study was
conducted with the approval of the Local Ethics Committee of Hebei
Medical University (no. DK051207). Sterigmatocystin was purchased
from Sigma-Aldrich (St. Louis, MO, USA). Goat anti-human LMP2
polyclonal antibody and goat anti-human TAP1 polyclonal antibody
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The PCNA polyclonal antibody was purchased from Wuhan Boster
Biological Technology, Ltd. (Wuhan, China). The immunohistochemical
staining kit (model no. 1386323) was purchased from Beijing Zhong
Shan Golden Bridge Biotechnology Co., Ltd. (Beijing, China).
Grouping and modeling
Rats (n=30) were randomly divided into the following
three groups (n=10): i) Control group, treated with placebo and
surgery; ii) esophagitis model group, experimental reflux
esophagitis achieved by cardiectomy and ligation of half of the
pylorus without sterigmatocystin; and iii)
sterigmatocystin-treatment group, the reflux esophagitis model with
sterigmatocystin treatment. Surgery was performed when the rats
acclimated to their environment for 1–2 days.
In the control group (placebo treatment), esophageal
diameter was small (~3 mm), thus the surgery required precise
technical procedures. While under 10% chloral hydrate anesthesia (3
ml/kg, administered intraperitoneally), the abdominal cavity was
opened to expose the stomach and esophagus, the anterior branch of
the left gastric artery was ligated to reduce bleeding and the
abdomen was closed. Prior to and following surgery, rats were
allowed liquid glucose only for 24 h. Additionally, a liquid diet
was provided for three days following surgery, subsequent to which
a normal diet was resumed. After 7 days, placebo treatment
(intraperitoneal injection with saline) was administered once a day
for seven days.
In the esophagitis model group, the abdominal cavity
was opened to expose the stomach and esophagus while mice were
under anesthesia (10% chloral hydrate administered
intraperitoneally). The anterior branch of the left gastric artery
was ligated and the sphincter was cut off by the removal of the
cardiac smooth muscle; simultaneously, one-third of the
circumference of the lower esophageal smooth muscle was removed.
The abdomen was closed and the rats were provided with the same
diet and saline injections as described for the control group.
In the sterigmatocystin treatment group, anesthesia,
surgical procedures and food intake were identical to those
described for the experimental esophagitis group. Seven days after
the normal diet was resumed, an intraperitoneal injection of
sterigmatocystin (30 μg/kg) was administered, followed by
consecutive daily sterigmatocystin injections for seven days. A
parenteral route of sterigmatocystin administration was performed
rather than peroral administration, as this route failed to induce
esophageal cancer in our previous study (1).
A number of rats from all groups were sacrificed 5
or 11 weeks after the seventh day of treatment (n=6 in the control
group, n=8 in the esophagitis model group, n=10 in the
sterigmatocystin treatment group). The groups were split evenly
into two groups for the two sacrifice time points.
Pathological analysis
The esophagus was excised en bloc and examined for
general morphological changes. Tissue samples were then placed in
formalin solution to be longitudinally fixed. Conventional
hematoxylin and eosin (H&E) staining was performed on the
sections and histological changes were evaluated by light
microscopy.
Immunohistochemical detection of PCNA,
TAP1 and LMP2 expression
Immunohistochemical staining was performed according
to the manufacturer’s instructions (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA). Phosphate-buffered saline was used in lieu of
primary antibody as a negative control.
Criteria for pathological analysis
Two blinded experts performed the pathology-based
diagnosis. The esophagitis classification of specimens was
conducted according to the following Tokyo standards (11,12):
0, no esophagitis observed; 1, redness or white turbidity; 2,
erosion, ulcer esophageal length of ≤1/3 of the total oesophageal
length (units) and non-integration; 3, erosion, esophageal ulcers
account for the full length (1/3–2/3 units) and with integration;
and 4, erosion, esophageal ulcers account for the full length
(>2/3 units). Inflammation scores were allocated based on the
following criteria (per high power field): 0, no inflammatory cell
infiltration; 1, a small number of inflammatory cells; 2, moderate
inflammatory cell infiltration; and 3, a large quantity of
inflammatory cell infiltration. Hyperplasia scores were allocated
according to the following classifications: 0, normal esophageal
stratified squamous epithelium basal cell layer; 1, basal cell
hyperplasia was 1/3 of the total thickness; and 2, basal cell
hyperplasia was 1/3–2/3 of the total thickness.
Criteria for immunohistochemical
analysis
This was performed by two common film reading
statistical procedures. For PCNA, the number of positive cells per
high power field (×400) for 10 fields was counted. The average rate
of positive cells for every 100 epithelial cells was used as the
PCNA labeling index (PCNA LI). For TAP1 and LMP2, the number of
positive cells per high power field (×400) for 10 fields was
counted. The average rate of positive cells for every 100
epithelial cells was used as the rate of expression of TAP1 and
LMP2.
Statistical analysis
SPSS 11.0 (SPSS Inc., Chicago, IL, USA) statistical
software was used for single-factor analysis of variance (ANOVA)
and Student-Newman-Keuls test was used for pairwise comparisons.
P<0.05 was considered to indicate statistically significant
differences.
Results
Condition of rats
One rat died of an anesthesia overdose during the
surgical procedure, while a second rat died due to an esophageal
perforation. Following surgery, two rats died as a result of
intestinal obstruction and an additional two rats died from unknown
causes. A total of 24 rats survived and completed the study. Reflux
behavior via the mouth was observed in the esophagitis model and
sterigmatocystin-treated animals after 1 week, and suggested that
the reflux surgery had been successful. Compared with the control
and esophagitis model groups, the sterigmatocystin-treated animals
exhibited a reduced intake of food and water, reduced locomotor
activity and a less glossy coat.
Esophageal specimens from rats with
reflux esophagitis
Smooth and normal esophageal mucosa were identified
in the control group (Fig. 1). By
contrast, the esophagitis model and sterigmatocystin-treated groups
exhibited mucosal congestion with edema, erosion and ulcers
(Fig. 2).
H&E staining of the esophagus in rats
with reflux esophagitis
At 5 weeks following treatment, the esophageal
tissues in the esophagitis model group exhibited epithelial
hyperplasia, acanthosis of the basal layer in association with
general congestion and edema, vein dilation, angiogenesis,
submucosal infiltration of inflammatory cells and the presence of
eosinophils, neutrophils and lymphocytes. In severe cases, the
esophagus presented mucosal cell degeneration and necrosis with
epithelial shedding and granulation tissue formation. An ulcer
structure was identified in one case. The esophagus in
sterigmatocystin-treated rats showed esophageal epithelial
hyperplasia, acanthosis of the basal layer and the majority of the
nuclei exhibited loss of polarity, mild to moderate atypia,
congestive edema, vein dilation and angiogenesis. In addition,
mucous membranes exhibited inflammatory cell infiltration
consisting predominantly of neutrophils and lymphocytes, severe
mucosal cell degeneration and necrosis and a typical ulcer detached
structure; however, no atypical cells were observed in the
groups.
At 11 weeks, the esophagus in the esophagitis model
and sterigmatocystin-treated groups exhibited no ulcers, but did
show esophageal mucosal epithelial hyperplasia, mucosal congestion
and edema and cystic fibrosis in the submucosa. In addition, no
atypical cells were observed in the groups.
At weeks 5 and 11, the sterigmatocystin-treated
group had marginally higher inflammation scores compared with the
esophagitis model group; however, the difference was not
statistically significant (Table
I; P>0.05).
 | Table IEsophageal squamous cell score in
reflux esophagitis rats. |
Table I
Esophageal squamous cell score in
reflux esophagitis rats.
| Score | Control group
(n=6) | Esophagitis model
group (n=8) | ST-treated group
(n=10) |
|---|
| Inflammation score at
week 5 | | 1.47±0.46 | 1.89±0.10 |
| Inflammation score at
week 11 | | 1.70±0.28 | 1.87±0.447 |
| Hyperplasia severity
score at week 5 | | 1.43±0.06 | 1.68±0.08a |
| Hyperplasia severity
score at week 11 | | 1.40±0.15 | 1.62±0.14a |
| PCNA LI | 21.50±9.79 | 49.17±18.67 | 70.10±12.55a |
The hyperplasia severity score (Table I) for the sterigmatocystin-treated
group was higher than that of the model group at weeks 5 and 11.
Pairwise analysis between the sterigmatocystin-treated and
esophagitis model groups indicated that the difference was
statistically significant (P<0.05).
PCNA immunohistochemical analysis
PCNA-positive esophageal squamous cell nuclei were
observed in rats with reflux esophagitis. This is depicted by
brown-yellow granules in Fig. 3,
which also represent cell proliferation. The proliferation index of
PCNA expression was 21.50±9.79 in the control group (n=6),
49.17±18.67 in the esophagitis model group (n=8) and 70.10±12.55 in
the sterigmatocystin-treated group (n=10) (Table I). According to statistical
analysis using one-way ANOVA, the PCNA proliferation index was
statistically different among the three groups (P<0.05). Further
pairwise analysis of the control and model groups indicated that
there was a significant difference between these two groups
(P<0.05), and there was also a significant difference
(P<0.05) between the model and sterigmatocystin-treated groups
(Table I).
Thus, sterigmatocystin exacerbated reflux
esophagitis with marked hyperplasia of the squamous epithelium. In
addition, epithelial cells in the sterigmatocystin-treated group
demonstrated significantly higher PCNA expression compared with
that of the control and esophagitis model groups. It is suggested
that gastric reflux increased the esophageal mucosal injury and
promoted epithelial cell proliferation. Sterigmatocystin exposure
resulted in more evident esophageal squamous cell hyperplasia.
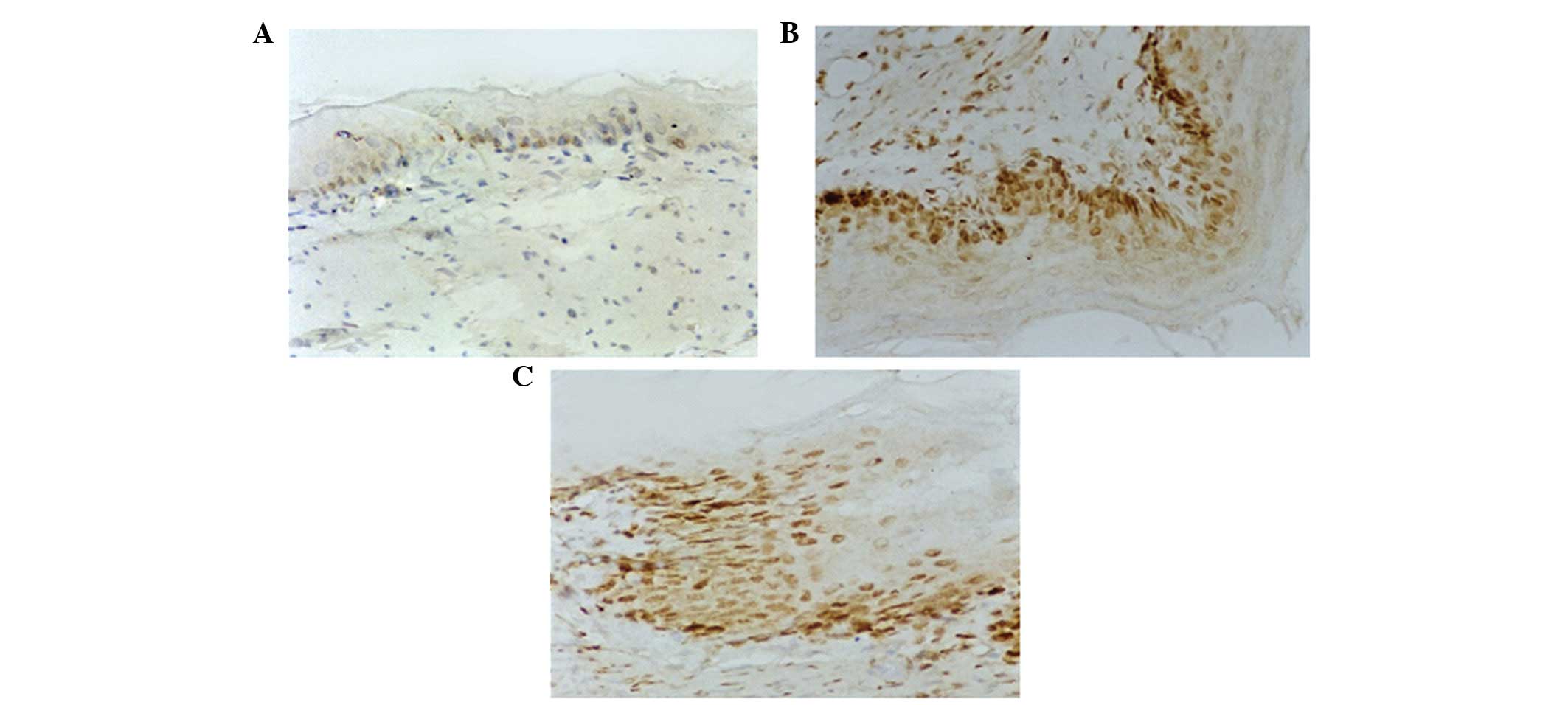
TAP1 immunohistochemical analysis
TAP1 expression in the esophageal squamous cell
cytoplasm was indicated by brown-yellow granules (Fig. 4). The rate of TAP1-positive cells
in the esophagitis model (n=8) and sterigmatocystin-treated groups
(n=10) was reduced compared with that of the control group (n=6)
(36.17±5.31 and 20.83±7.08% vs. 71.17±6.62%, respectively;
P<0.05l; Table II). TAP1 in
the sterigmatocystin-treated group was significantly lower than
that in the model group (P<0.05; Table II, Fig. 4).
 | Table IIThe rate of TAP1- and LMP2-positive
cells identified by immunohistochemical staining. |
Table II
The rate of TAP1- and LMP2-positive
cells identified by immunohistochemical staining.
| Group | TAP1 (%) | LMP2 (%) |
|---|
| Control | 71.17±6.62 | 53.83±5.81 |
| Esophagitis
model | 36.17±5.31a | 34.50±6.44a |
|
Sterigmatocystin-treated | 20.83±7.08a | 22.50±6.72a |
LMP2 immunohistochemical analysis
LMP2 expression in the cytoplasm was indicated by
brown-yellow granules (Fig. 4).
The number of positive cells in the esophagitis model group (n=8)
and sterigmatocystin-treated group (n=10) was lower than that in
the control group (n=6) (34.50±6.44 and 22.50±6.72% versus
53.83±5.81%; P<0.05, Table
II). LMP2 expression in the sterigmatocystin-treated group was
significantly lower than that in the model group (P<0.05;
Table II, Fig. 4).
Discussion
The fungus, sterigmatocystin, is a carcinogenic
mycotoxin. Sterigmatocystin contamination of human food sources and
animal feed has been confirmed in areas of China with a high
incidence of esophageal cancer (1). Epidemiological studies have
demonstrated that this high incidence of esophageal cancer in
certain areas of China is also associated with a higher prevalence
of reflux esophagitis (6,7); thus, mycotoxin contamination is a
serious public health concern. Zhang et al(1) demonstrated that sauerkraut soup
samples and cornmeal bought from the Chinese Hebei Taihang Mountain
region were significantly contaminated with sterigmatocystin. This
study was extended by oral administration of purified
sterigmatocystin to experimental animals, and it was determined
that sterigmatocystin induced lung gland cancer and gastric
epithelial dysplasia; however, not esophageal cancer. Thus,
sterigmatocystin may cause esophageal cancer in conjunction with
other underlying, yet unidentified, physiological and/or
environmental factors.
Esophageal cancer is presumed to be the result of a
multi-factorial process. The normal physiological function of the
esophagus is a muscular tube through which food passes and
esophageal squamous cells have the ability to repair themselves.
However, esophageal mucosal injury appears to be an important
factor involved in esophageal cancer onset in response to
sterigmatocystin. Esophageal sphincter-relaxation may result in
gastric reflux and esophageal inflammation. The rat
gastro-esophageal junction area is similar to that in humans.
Esophageal smooth muscle and gastric sling fibers of the
gastroesophageal junction area are important anatomical structures
controlling the anti-reflux mechanism (13,14).
Cardiac sphincter resection was successfully conducted in a rabbit
model of reflux esophagitis by Xu et al(15). The esophagitis model and
sterigmatocystin-treated groups presented with significant changes
in esophagitis; however, the degree of inflammation was not
significantly different between the two groups, suggesting a
successful reflux esophagitis model in which there may be cardiac
relaxation, reflux of gastric juice and injury to the esophagus
(14,16). There was noticeable esophageal
squamous epithelium cell hyperplasia in sterigmatocystin-treated
groups. Hyperplasia may also translate DNA damage into harmful
genetic mutations, and thus result in oncogene activation or
inactivation of tumor suppressor genes, eventually leading to
cancer (17).
In Barrett’s esophagus, the normal squamous
epithelial lining of the esophagus is replaced by columnar cells,
thus Barrett’s esophagus is presumed to be associated with
esophageal adenocarcinoma. Inflammation-associated esophageal
tumorigenesis in Barrett’s carcinoma (6) was investigated in the reflux
esophagitis rat model; however, only hyperplasia of the squamous
cells was induced by sterigmatocystin. The involvement of
sterigmatocystin in reflux esophagitis and carcinoma thus remains
to be determined.
Esophageal squamous epithelial hyperplasia with
sterigmatocystin treatment was marginally more evident in the fifth
week than in the eleventh week. It is conceivable that the
repairing capacity of esophageal squamous cells may be high in rats
or the metabolism of sterigmatocystin in rats may be fast.
Therefore, sterigmatocystin-induced esophageal cancer genesis may
be associated with the amount and duration of exposure to toxic
stimuli and esophageal mucosal injury. The doses and duration of
treatment with sterigmatocystin that are associated with the
development of esophageal cancer have not yet been elucidated.
Furthermore, the results of the present study
support the hypothesis that sterigmatocystin negatively impacts the
immune function and monocytes (9,10).
Sterigmatocystin downregulated the gene expression of TAP1 and LMP2
in human peripheral blood mononuclear cells and inhibited human
esophageal squamous cell expression of HLA-I in a dose-dependent
manner (9,10). Endogenous antigen processing,
presentation and the resulting immune disorder are considered to be
principal mechanisms of tumor immune escape. The LMP and TAP genes
were identified to be involved in the HLA-II gene regions and are
considered to be closely associated with the handling of endogenous
antigen presentation. Their normal expression is the molecular
basis of endogenous antigen-presention (18,19).
Endogenous antigens are combined with LMP in the cytoplasm to
further cleave peptide fragments. These are transported into the
endoplasmic reticulum by TAP, for presentation at the cell surface
and identification by circulating T lymphocytes. Thus, LMP and TAP
molecular changes may directly affect tumor immunity (20,21),
strengthening the ability of a tumor to escape the body’s immune
surveillance.
Sterigmatocystin exposure resulted in esophageal
squamous epithelial cells downregulating the expression of TAP1 and
LMP2 in reflux esophagitis rats. Thus, it was suggested that
peptide transport is blocked and degradation of endogenous peptides
is limited, thus affecting the MHC class I molecules in the
endoplasmic reticulum assembly and harming the cell-mediated immune
response (19–23). One of the underlying molecular
mechanisms of sterigmatocystin-induced esophageal cancer may
involve sterigmatocystin functionally altering gene expression and
signaling in transformed cells to facilitate their escape from host
immune surveillance, thereby promoting cancer onset.
References
|
1
|
Zhang X, Wang F, Wang J, et al:
Experimental lung carcinoma induced by fungi and mycotoxins - a
review. Beijing Da Xue Xue Bao. 35:4–6. 2003.(In Chinese).
|
|
2
|
Versilovskis A and De Saeger S:
Sterigmatocystin: occurrence in foodstuffs and analytical methods -
an overview. Mol Nutr Food Res. 54:136–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang XH and Xue LY: Carcinogenicity and
biological effectiveness of sterigmatocystin. Zhonghua Bing Li Xue
Za Zhi. 38:136–138. 2009.(In Chinese).
|
|
4
|
Xing LX, Zhang XH, Shen HT, et al:
Experimental study on the carcinogenic effects of sterigmatocystin
in new born BALB/c mice. Zhonghua Bing Li Xue Za Zhi. 36:265–266.
2007.(In Chinese).
|
|
5
|
Nakajima M, Kato H, Miyazaki T, et al:
Tumor immune systems in esophageal cancer with special reference to
heat-shock protein 70 and humoral immunity. Anticancer Res.
29:1595–1606. 2009.PubMed/NCBI
|
|
6
|
Abdel-Latif MM, Duggan S, Reynolds JV and
Kelleher D: Inflammation and esophageal carcinogenesis. Curr Opin
Pharmacol. 9:396–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu JY, Zhang JK and Wang RM: Control
observation on esophageal carcinoma and esophagitis detected by
endoscopy between high and low incidence areas of esophageal
carcinoma. Chin J Clin Gastroenterol. 7:101–103. 1995.(In
Chinese).
|
|
8
|
Ho JA and Durst RA: Detection of fumonisin
B1: comparison of flow-injection liposome immunoanalysis with
high-performance liquid chromatography. Anal Biochem. 312:7–13.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang X, Zhang X, Yan X and Yin G: Effects
of sterigmatocystin on interleukin-2 secretion of human peripheral
blood mononuclear cells in vitro. Wei Sheng Yan Jiu. 31:112–114.
2002.(In Chinese).
|
|
10
|
Tong P, Zhang X, Yan X, Wang JL and Zhang
GJ: Effects of sterigmatocystin on the expression of HLA class I in
esophageal squamous cells in vitro. Chin J Exp Surg. 23:583–584.
2006.(In Chinese).
|
|
11
|
Yu ZL: Reflux esophagitis diagnostic
criteria and the existing problems. J Chin Med. 39:151–152.
2000.(In Chinese).
|
|
12
|
Fiocca R, Mastracci L, Riddell R, et al:
Development of consensus guidelines for the histologic recognition
of microscopic esophagitis in patients with gastroesophageal reflux
disease: the Esohisto project. Hum Pathol. 41:223–231. 2010.
View Article : Google Scholar
|
|
13
|
Yuan S and Brookes SJ: Neuronal control of
the gastric sling muscle of the guinea pig. J Comp Neurol.
412:669–680. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miller L, Dai Q, Vegesna A, et al: A
missing sphincteric component of the gastro-oesophageal junction in
patients with GORD. Neurogastroenterol Motil. 21:e813–e852. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu Z, Hu T and Liu W: A new procedure in
making reliable experimental models of gastroesophageal reflux.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 18:288–290. 2004.(In
Chinese).
|
|
16
|
Rohof WO, Hirsch DP and Boeckxstaens GE:
Pathophysiology and management of gastroesophageal reflux disease.
Minerva Gastroenterol Dietol. 55:289–300. 2009.PubMed/NCBI
|
|
17
|
Zhu SC, Li R, Wang YX, Feng W, Li J and
Qiu R: Impact of simultaneous assay, the PCNA, cyclinD1, and DNA
content with specimens before and after preoperative radiotherapy
on prognosis of esophageal cancer-possible incorporation into
clinical TNM staging system. World J Gastroenterol. 11:3823–3829.
2005.
|
|
18
|
Bandoh N, Ogino T, Katayama A, et al: HLA
class I antigen and transporter associated with antigen processing
downregulation in metastatic lesions of head and neck squamous cell
carcinoma as a marker of poor prognosis. Oncol Rep. 23:933–939.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Belicha-Villanueva A, Blickwedehl J,
McEvoy S, Golding M, Gollnick SO and Bangia N: What is the role of
alternate splicing in antigen presentation by major
histocompatibility complex class I molecules? Immunol Res.
46:32–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Medina F, Ramos M, Iborra S, de Leon P,
Rodriguez-Castro M and Del Val M: Furin-processed antigens targeted
to the secretory route elicit functional TAP1−/−CD8+ T lymphocytes
in vivo. J Immunol. 183:4639–4647. 2009.PubMed/NCBI
|
|
21
|
Herget M, Kreissig N, Kolbe C, Schölz C,
Tampé R and Abele R: Purification and reconstitution of the antigen
transport complex TAP: a prerequisite for determination of peptide
stoichiometry and ATP hydrolysis. J Biol Chem. 284:33740–33749.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Atkins D, Ferrone S, Schmahl GE, Störkel S
and Seliger B: Down-regulation of HLA class I antigen processing
molecules: an immune escape mechanism of renal cell carcinoma? J
Urol. 171:885–889. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seliger B, Atkins D, Bock M, et al:
Characterization of human lymphocyte antigen class I
antigen-processing machinery defects in renal cell carcinoma
lesions with special emphasis on transporter-associated with
antigen-processing down-regulation. Clin Cancer Res. 9:1721–1727.
2003.
|