Introduction
Glutamate, the primary rapid excitatory
neurotransmitter, elicits postsynaptic effects by activating three
main classes of ionotropic glutamate receptor, termed according to
the selective agonists, N-methyl-D-aspartate (NMDA),
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) and
kainate (KA) (1). The NMDA
receptor contributes to excitatory synaptic transmission within the
spinal cord and is an important target in inflammatory pain
(2,3). The AMPA receptor also plays a
critical role in the dorsal horn of the spinal cord in the
processing of nociceptive information that is involved in
persistent inflammatory pain (4–6).
The spinal AMPA receptor is a heterotetrameric
cation channel composed of four GluR1-R4 subunits consisting of
homo- and heteromultimers (1,4,6). All
four subunits are expressed within the spinal dorsal horn, with the
GluR1 and GluR2 subunits being the most abundant in the superficial
dorsal horn (7,8). Peripheral inflammation induced by
complete Freund’s adjuvant (CFA) leads to GluR1 membrane insertion
and GluR2 internalization in dorsal horn neurons (9). The phosphorylation of serine residues
of the AMPA receptor is one of the key mechanisms in regulating
channel properties (2,8).
Electroacupuncture (EA) is widely used in clinical
and basic research in Korea. The AMPA receptor may be involved in
the induction of EA analgesia in the spinal dorsal horn of healthy
animals (10); thus, any changes
in the constituent subunits may affect EA analgesia. However, it is
unclear whether EA analgesia is associated with selective changes
in AMPA receptor subunits at the transcriptional or the
translational level. The present study investigated the possible
involvement of the AMPA receptor and the phosphorylation of its
constituent serine residues on EA analgesia in chronic inflammatory
pain.
Subjects and methods
Animals
Male Sprague-Dawley rats, (mean weight, ~120 g),
were obtained from Dooyeol Biotech Co. (Seoul, Korea). The rats
were housed at 22°C with an alternating 12-h dark-light cycle, were
fed a commercial diet and water was provided ad libitum from
2 weeks prior to and throughout the study. All experiments
conformed to guidelines approved by the Animal Care and Use
Committee at the Pusan National University (Yangsan, Korea).
CFA injection and EA simulation
Rats were injected subcutaneously with 100 μl CFA
(Sigma-Aldrich, St. Louis, MO, USA) in the plantar surface of the
left hindpaw. For the EA stimulation, two 0.25 mm-diameter needles
were inserted in each hind leg at acupoints corresponding to
Zusanli (ST36) and Sanyinjiao (SP6) as described in a previous
study (10). The needles were
connected to a model SM-60 electric stimulator (Saechang, Seoul,
Korea). EA stimulation (2 Hz, 1.0 mA) was initiated immediately
following injection with CFA and lasted for 30 min. This process
was repeated daily for the remainder of the experiment. Rats in the
control group were injected with 100 μl phosphate-buffered saline
(PBS, pH 7.4) only and did not receive EA stimulation.
Measurements of thermal hyperalgesia
Rats were immediately placed individually on a
heated (56°C) platform and the latency of the first sign of paw
licking or jumping to avoid heat pain was recorded by an automatic
electronic timer (Harvard Apparatus, Holliston, MA, USA). A cut-off
period of 20 s was used. The latency response was monitored for 5
days following CFA injection, with or without EA stimulation.
qPCR
SYBR-Green PCR amplification of the whole L4-5
segments of the spinal cord was performed using an ABI PRISM 7900
sequence detection system (Applied Biosystems, Foster City, CA,
USA). Reaction mixtures contained 10 pmol/μl of each primer and 2X
SYBR® Green PCR Master mix (Applied Biosystems), which
included HotStarTaqt DNA-polymerase in an optimized buffer, dNTP
mix (with added dUTP), SYBR® Green I fluorescent dye,
and ROX dye as a passive reference. Each of the 384-well, qPCR
plates included serial dilutions (1, 1/2 and 1/4, 1/8, and 1/16) of
cDNA, which were used to generate relative standard curves for the
genes. Primers were amplified under the same conditions. The
thermal cycling conditions were as follows: 50°C for 2 min and 95°C
for 10 min, followed by 40 cycles of 95°C for 30 sec, 60°C for 30
sec and 72°C for 30 sec. The qPCR cycle numbers were converted to
gene amounts (ng). The primer sequences used were: Forward:
5′-GACAACTCAAGCGTCCAGAA-3′ and reverse: 5′-CGTCGCTGACAATCTCAAGT-3′
for GluR1; forward: 5′-GAGGACTACCGCAGAAGGAG-3′ and reverse:
5′-GATCCTTTAGGTGTGGCGAT-3′ for GluR2; forward:
5′-GTCTCCTGTGACTTCAACAG-3′ and reverse: 5′-AGTTGTCATTGAGAGCAATGC-3′
for glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Western blot analysis
To examine the changes in the AMPA subunits, the
L4-5 segments of the spinal cord were removed following the
termination of EA stimulation on day 5 of the experiment, under 10%
chloral hydrate anesthesia (350 mg/kg injected intraperitoneally).
The dorsal region of the spinal cord was excised from the ventral
region and eventually the ipsilateral and contralateral regions of
the injected side were obtained. The spinal cords were washed in
cold HEPES buffer, and homogenized in nine volumes of lysis buffer.
Equal quantities of protein were separated by 8–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The resolved
proteins were transferred to a nitrocellulose membrane (Whatman,
Dassel, Germany), and the membrane was blocked with 5% non-fat milk
in Tris-buffered saline containing 0.4% Tween-20.
The membranes were incubated with anti-GluR1 (Abcam,
Cambridge, UK), anti-phospho-GluR1 (pGluR1, ser845; Millipore,
Billerica, MA, USA), anti-GluR2 (Abcam) and anti-phosho-GluR2
(pGluR2, ser880; Abcam) for 1–2 h at room temperature. The blots
were incubated with horseradish peroxidase-conjugated secondary
antibody and the antibody-specific proteins were visualized by the
enhanced chemiluminescence detection system (Pierce, Rockford, IL,
USA). β-actin was used as a loading control for all experiments.
Quantification of the immunoreactivity corresponding to the total
and phosphorylated bands was performed by densitometric analysis
using Multi Gauge software, version 3.0 (Fujifilm, Tokyo,
Japan).
Immunohistochemistry
Rats anesthetized with 10% chloral hydrate were
intracardially perfused with saline and 4% paraformaldehyde in PBS.
The L4-5 segments of the spinal cord were removed, post-fixed in 4%
paraformaldehyde for 4 h at 4°C, and immersed in 30% sucrose for 48
h at 4°C for cryoprotection. Frozen 30 μm sections were prepared
and collected in 0.1 M phosphate buffer (PB, pH 7.4) to be
processed immunohistochemically as free-floating sections. The
sections were pre-incubated in 0.3% hydrogen peroxide for 15 min,
rinsed thoroughly, and then incubated in a blocking solution
containing 3% normal goat serum and 0.3% Triton X-100 in PBS for 30
min at room temperature. Sections were incubated for 16 h at 4°C
with the same primary antibody used in the western blot analysis
diluted in PBS containing 0.3% Triton X-100. Sections were washed
with PBS, incubated with biotinylated anti-rabbit IgG secondary
antibody (Vector, Burlingame, CA, USA) for 30 min and then washed
again with PBS.
Sections were further incubated with an
avidin-biotin-peroxidase complex kit (Vector) for 60 min at room
temperature and peroxidase activity was detected using the DAB
Peroxidase Substrate kit (Vector). To quantify the laminar
expression of AMPA, the ipsilateral dorsal horn of the spinal cord
was divided into three regions: The superficial dorsal horn (SDH,
laminae I and II), the nucleus proprius (NP, laminae III and IV),
and the neck of the dorsal horn (NECK, laminae V and VI). To
visualize the expression of the AMPA receptor subunits, images of
the dorsal horns were captured at ×100 magnification using an
AxioCam digital CCD camera (Zeiss, Jena, Germany). The integrated
optical density (IOD) of each region of the dorsal horn was
measured automatically using Visus Image Analysis software
(Foresthill Products, Foresthill, CA, USA).
Statistical analysis
Data are expressed as the mean ± SEM, and a
Student’s t-test was performed using the SigmaPlot software (SyStat
Software, San Jose, CA, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Thermal thresholds
Hyperalgesia was examined using the measurements of
hot plate latency at 1-day intervals following CFA injection. Among
the 10 rats in each group, only five rats demonstrating significant
EA analgesia were selected and included in the behavioral analysis
(Fig. 1). The hot plate latency of
the ipsilateral hindpaw of CFA-treated rats was significantly
decreased compared with that of the control rats. There was no
significant difference in latency in pain resulting from noxious
thermal stimuli, 1 and 2 days following CFA injection, between the
CFA-treated rats with and without EA stimulation. However, EA
stimulation significantly inhibited hyperalgesia induced by CFA
from day 3 onwards.
qPCR analysis of AMPA receptor
subunits
To determine whether the effects of EA on
CFA-induced hyperalgesia were associated with AMPA receptor subunit
mRNA, the expression of AMPA receptor GluR1 and GluR2 subunits was
determined by qPCR in the L4-5 segments. Five rats presenting with
significant EA analgesia 5 days following CFA injection were used
for the mRNA analysis. There were no significant changes in the
GluR1 mRNA in response to CFA injection with or without EA
stimulation. However, GluR2 mRNA was significantly decreased in
these groups (CFA treated with or without EA stimulation) (Fig. 2).
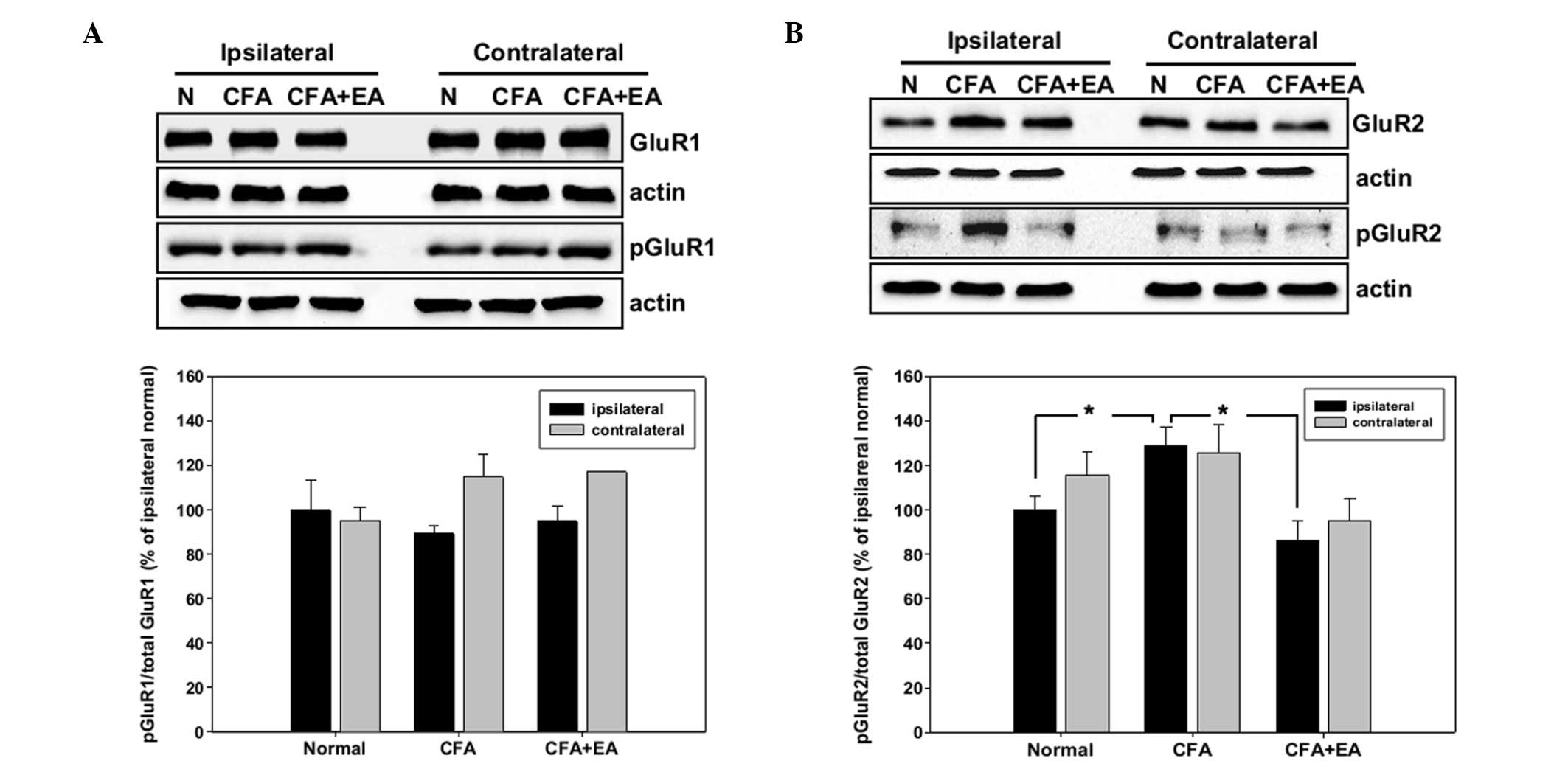
Western blot analysis of the AMPA
receptor subunits
For the western blot analysis, the ipsilateral and
contralateral dorsal region of the L4-5 segments of the spinal cord
were removed at day 5 of the experiment, and the expression of the
total and the phosphorylated GluR1 and GluR2 subunits was
determined. The protein levels of each subunit were normalized to
that of β-actin in the same sample. The increase of activity in the
AMPA subunits was calculated as the ratio of phosphorylated
subunits to total subunits and compared with that in the
ipsilateral region of control rats. Phosphorylation of the GluR1
subunit was only marginally changed and not significantly different
in CFA-injected rats with and without EA stimulation, compared with
the control rats (Fig. 3A).
However, the phosphorylation of GluR2 was significantly increased
in CFA-injected rats in the ipsilateral dorsal horn of the spinal
cord. The increased pGluR2 level was blocked by EA treatment
(Fig. 3B).
Immunohistochemical analysis of the AMPA
receptor subunits
The ipsilateral GluR1 and GluR2 subunits are widely
distributed in the SDH region of the spinal cord. The expression of
the GluR1 and GluR2 subunits did not exhibit significant changes
following CFA injection with or without EA stimulation (data not
shown). The expression of the phosphorylated GluR1 and GluR2
subunits was increased in the SDH region of CFA-injected rats
compared with the control rats, and was significantly inhibited by
EA stimulation (Fig. 4).
Discussion
EA is modified from traditional acupuncture, as the
manual stimulations of the needle are replaced by electric pulse
generation. EA treatment is widely used in the clinic to relieve
acute or chronic pain in human patients, and may contribute to
prolonged analgesia lasting for hours or days (11). The long-term synaptic alterations
between primary afferent fibers and neurons in the spinal dorsal
horn involve the activation of ionotropic glutamate receptors
(12). The AMPA receptor in the
spinal dorsal horn may be involved in EA analgesia (10). However, the exact involvement of
this receptor in chronic pain remains to be elucidated.
The AMPA receptor is composed of the GluR1, GluR2,
GluR3 and GluR4 subunits. GluR1 and GluR2 are predominant in the
superficial laminae of the spinal dorsal horn (5). Therefore, it was hypothesized that
these AMPA receptor subunits may be involved in the control of
inflammatory nociception by EA stimulation through their expression
and phosphorylation. Thus, EA stimulation at 2 Hz with 1 mA
intensity in the CFA-induced pain rat model was used to select rats
demonstrating significant EA analgesia.
CFA treatment induced thermal hyperalgesia lasting
up to 5 days following injection and the significant inhibition of
hyperalgesia by EA stimulation was evident from day 3 following
injection (Fig. 1). The GluR1 and
GluR2 subunit mRNAs of the AMPA receptor were examined by qPCR at
day 5 following CFA injection. A previous study demonstrated a
significant upregulation of the mRNAs following CFA injection and a
return to the control levels by day 7 (GluR1-flop) and day 3
(GluR2-flip) (5). Furthermore,
another study demonstrated a 25% bilateral decrease in GluR1
expression in the substantia gelatinosa at the level of the lumbar
cord 1 day following injection with lipopolysaccharide, with no
significant change in the GluR2 levels (13).
These up- and downregulations correlate with the
early development of hyperalgesia following inflammation. A
previous study demonstrated specific regulation of the AMPAR
subunits in response to the development of inflammatory
hyperalgesia (5,13) and during the sampling period after
inflammation (14). In the present
study, the level of GluR2 subunit mRNA in CFA-treated rats was
significantly decreased compared with that in the control rats
(Fig. 2), suggesting that the
selective expression of the GluR2 subunit is related to the
maintenance of chronic pain or the sampling period.
Increased GluR1 and GluR2 expression has been
detected early after inflammation (5), but CFA injection does not alter the
expression of GluR1 and GluR2 proteins in rats (8,9).
GluR1 expression is marginally increased after CFA injection, but
the change is not significant and the GluR2 levels are not affected
(15). The expression of the GluR1
and GluR2 subunits predominantly occurs in the superficial laminae
of the spinal dorsal horn (13,16).
AMPA receptor phosphorylation of serine residues is
a mechanism for modulating channel properties in the central
nervous system (2,8). Upregulation of the phosphorylated
GluR1 serine residues has been demonstrated in the dorsal horn
following capsaicin or CFA treatment (8,17).
These results indicate that the phosphorylation of the GluR1
subunit participates in the induction of inflammatory pain.
The expression of each protein in the CFA-injected
rats as a percentage of such expression in the control rats in the
western blot analyses demonstrated a change in the phosphorylation
of the GluR1 subunit between the control rats and the CFA-injected
rats, with or without EA stimulation. However, these changes were
not statistically significant (Fig.
3A). By contrast, the phosphorylation of GluR2 was
significantly increased by CFA injection and this induction was
inhibited by EA stimulation (Fig.
3B). Furthermore, the regional distribution of the subunits was
examined by immunohistochemistry. The expression of the GluR2
subunit was not significantly altered at day 5 following CFA
injection (data not shown). The phosphorylated form of GluR2 was
significantly increased by the injection of CFA and inhibited by EA
stimulation in the SDH region (Fig.
4B).
CFA-induced inflammation activated spinal protein
kinase C and induced the phosphorylation of the serine 880 residue
of GluR2 in the dorsal horn, which promotes the internalization of
GluR2. The internalization of the GluR2 subunit of the AMPA
receptor leads to the maintenance of CFA-induced pain
hypersensitivity by increasing the permeability of the membrane to
calcium (6).
The present data suggest that the phosphorylation of
serine 880 of the GluR2 subunit is increased in the spinal cord
following CFA injection. This increase in phosphorylated GluR2 may
be significantly blocked by peripheral EA stimulation. A previous
study demonstrated that CFA-induced peripheral inflammation leads
to GluR2 internalization and GluR1 membrane insertion in the dorsal
horn neurons, without altering the expression and distribution of
the total GluR1 and GluR2 proteins in the dorsal horn (9).
The involvement of the spinal AMPA receptor in EA
analgesia remains to be elucidated. The anti-pGluR2 used in the
present study detected phosphorylation at serine 880 and greater
changes in the GluR2 subunit upon EA stimulation. The majority of
AMPA receptors of principal neurons contain the GluR2 subunit,
rendering them impermeable to calcium. However, certain AMPA
receptors lacking this subunit are expressed under pathological
conditions (3). In addition, AMPA
receptors undergo selective transcriptional and translational
modulation following prolonged inflammation (14).
In summary, the present results suggest that the
AMPA receptor may be involved in EA analgesia for chronic
inflammatory pain by regulating the expression and phosphorylation
state of its subunits. The GluR2 subunit of the AMPA receptor may
be important in EA analgesia through its modulation of the
phosphorylation state.
References
|
1
|
Collingridge GL, Isaac JT and Wang YT:
Receptor trafficking and synaptic plasticity. Nat Rev Neurosci.
5:952–962. 2004. View
Article : Google Scholar
|
|
2
|
Gao X, Kim HK, Chung JM and Chung K:
Enhancement of NMDA receptor phosphorylation of the spinal dorsal
horn and nucleus gracilis neurons in neuropathic rats. Pain.
116:62–72. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katano T, Furue H, Okuda-Ashitaka E, et
al: N-ethylmaleimide- sensitive fusion protein (NSF) is involved in
central sensitization in the spinal cord through GluR2 subunit
composition switch after inflammation. Eur J Neurosci.
27:3161–3170. 2008. View Article : Google Scholar
|
|
4
|
Burnashev N, Khodorova A, Jonas P, et al:
Calcium-permeable AMPA-kainate receptors in fusiform cerebellar
glial cells. Science. 256:1566–1570. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou QQ, Imbe H, Zou S, Dubner R and Ren
K: Selective upregulation of the flip-flop splice variants of AMPA
receptor subunits in the rat spinal cord after hindpaw
inflammation. Brain Res Mol Brain Res. 88:186–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park JS, Voitenko N, Petralia RS, et al:
Persistent inflammation induces GluR2 internalization via NMDA
receptor-triggered PKC activation in dorsal horn neurons. J
Neurosci. 29:3206–3219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kerr RC, Maxwell DJ and Todd AJ: GluR1 and
GluR2/3 subunits of the AMPA-type glutamate receptor are associated
with particular types of neurone in laminae I–III of the spinal
dorsal horn of the rat. Eur J Neurosci. 10:324–333. 1998.PubMed/NCBI
|
|
8
|
Lu Y, Sun YN, Wu X, et al: Role of
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA)
receptor subunit GluR1 in spinal dorsal horn in inflammatory
nociception and neuropathic nociception in rat. Brain Res.
1200:19–26. 2008. View Article : Google Scholar
|
|
9
|
Park JS, Yaster M, Guan X, et al: Role of
spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic
acid receptors in complete Freund’s adjuvant-induced inflammatory
pain. Mol Pain. 4:672008.PubMed/NCBI
|
|
10
|
Choi BT, Lee JH, Wan Y and Han JS:
Involvement of ionotropic glutamate receptors in low frequency
electroacupuncture analgesia in rats. Neurosci Lett. 377:185–188.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Melzack R, Vetere P and Finch L:
Transcutaneous electrical nerve stimulation for low back pain. A
comparison of TENS and massage for pain and range of motion. Phys
Ther. 63:489–493. 1983.PubMed/NCBI
|
|
12
|
Chen J and Sandkühler J: Induction of
homosynaptic long-term depression at spinal synapses of sensory a
delta-fibres requires activation of metabotropic glutamate
receptors. Neuroscience. 98:141–148. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pellegrini-Giampietro DE, Fan S, Ault B,
Miller BE and Zukin RS: Glutamate receptor gene expression in
spinal cord of arthritic rats. J Neurosci. 14:1576–1583.
1994.PubMed/NCBI
|
|
14
|
Guan Y, Guo W, Zou SP, Dubner R and Ren K:
Inflammation- induced upregulation of AMPA receptor subunit
expression in brain stem pain modulatory circuitry. Pain.
104:401–413. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee J and Ro JY: Differential regulation
of glutamate receptors in trigeminal ganglia following masseter
inflammation. Neurosci Lett. 421:91–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garry EM and Fleetwood-Walker SM: A new
view on how AMPA receptors and their interacting proteins mediate
neuropathic pain. Pain. 109:210–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagy GG, Al-Ayyan M, Andrew D, Fukaya M,
Watanabe M and Todd AJ: Widespread expression of the AMPA receptor
GluR2 subunit at glutamatergic synapses in the rat spinal cord and
phosphorylation of GluR1 in response to noxious stimulation
revealed with an antigen- unmasking method. J Neurosci.
24:5766–5777. 2004. View Article : Google Scholar
|