Introduction
Lacrimal gland injury secondary to the radiotherapy
of a malignant orbital tumour is a significant clinical problem
(1–3). Radiation therapy damages the lacrimal
gland cells, resulting in cell degeneration, necrosis and apoptosis
(4) and thus, impairs tear
secretion and induces xerophthalmia (1,3,5–7).
Satisfactory treatment strategies for this clinical problem are
lacking due to a poor understanding of the complex process.
Exploring the underlying mechanisms may aid in the identification
of novel therapeutic targets.
Lacrimal gland epithelial (LGE) cells are primary
lacrimal gland cells (8) and are
involved in lacrimal gland physiological homeostasis (9) and pathology (10), including radiotherapy-induced
lacrimal gland injury. LGE cells are necessary for tear secretion
and their proliferation and migration are crucial for lacrimal
gland repair (11,12).
Previous studies focusing on the skin epithelium
have found that expression of specific genes are associated with
radiation (13–15) and that these genes may be involved
in the pathogenesis of radiation-induced skin injury (14). Growth arrest and DNA
damage-inducible 45 α (Gadd45a) is one of these genes (14). Previous studies established that
radiation upregulates Gadd45a expression in the skin (14,16,17).
Gadd45a is also involved in inflammation (18) and numerous fundamental cellular
behaviors associated with injury repair, including cell migration
and proliferation (16,19–21).
However, the roles of the Gadd45a gene in lacrimal gland radiation
injury have not yet been studied.
To investigate whether Gadd45a has functional roles
in radiation-induced lacrimal gland injury repair, the expression
level of Gadd45a in radiation-treated mouse lacrimal glands was
observed by quantitative PCR and immunohistochemical analysis.
Gadd45a was overexpressed in isolated mouse LGE cells and the cell
migration and proliferation ability was determined, as well as the
associated signalling pathways.
Materials and methods
Animal preparation and irradiation
Ten healthy female C57BL/6 mice at 8 weeks of age
were used for the study. Experimental procedures were approved by
the Fudan University Animal Care and Use Committee and all animals
were housed under standard conditions according to
institution-approved guidelines. Mice underwent initial lacrimal
gland scintigraphy. After 1 week, the first group (n=5) was
irradiated with a total dose of 15 Gy under general anaesthesia
using a combination of 3 mg/kg (S)-ketamin-hydrochloride
(Ketanest-S®; Hoofddorp, The Netherlands) and 0.1 mg/kg
xylazin-hydrochloride (Rompun®; Bayer, Leverkusen,
Germany). Scintigraphy was performed 3 days following irradiation,
for a second time, with a subsequent excision of the left side
inferior lacrimal gland for histological examination. The same
procedure was performed 7 days later with the removal of the
contralateral lacrimal gland. The second group (n=5) was
sham-treated and were unirradiated for use as the control glandular
tissue.
Lacrimal gland scintigraphy
Following intravenous administration of 3.7 MBq (1
mCi = 37 MBq and 100 μCi = 3.7 MBq) Na99mTcO4
as a tracer, the mice underwent sequential scintigraphy in a prone
position and frontal projection of the head using a four-head
camera (Picker CX 250 compact, LEHR collimator and field-of-view 25
cm Nano SPECT/CT Plus, Bioscan Inc., Washington DC, USA).
Time-activity curves were additionally registered and analyzed. The
lacrimal ejection was also observed.
Surgical harvesting of the inferior
lacrimal gland
The inferior lacrimal gland was surgically exposed
and excised. The harvested glands were divided into two parts. One
part was fixed immediately with neutral phosphate-buffered 4%
formalin and the other was fixed for transmission electron
microscopy.
Transmission electron microscopy
Following embedding in Araldite, semi-thin sections
were stained with methylene blue to visualize the region of
epithelial cells of interest. Ultrathin sections were sliced and
stained with lead citrate and examined using a transmission
electron microscope (Philips, CM 120, Amsterdam, The
Netherlands).
Quantitative PCR
Total RNA was isolated from mouse lacrimal glands
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s instructions. Reverse
transcription was conducted using SuperScript® III
Reverse Transcriptase (Invitrogen Life Technologies) according to
the manufacturer’s instructions. The obtained cDNA was diluted 1:40
for quantitative PCR. The primers used for the amplification were:
mouse Gadd45a: forward, 5′-ggaggaagtgctcagcaaag-3′ and reverse,
5′-gcaggatccttccattgaga-3′ and mouse GAPDH, forward, 5′-tgtt
gccatcaatgacccctt-3′ and reverse, 5′-ctccacgacgtactcagcg-3′. The
reactions were performed using an ABI 7300 Real-Time PCR system
(Applied Biosystems, Foster City, CA, USA) and SYBR-Green PCR
Master Mix (Applied Biosystems). The thermal cycling conditions
were 95°C for 30 sec, 40 cycles of 95°C for 5 sec and 60°C for 31
sec. The relative expression levels of Gadd45a were determined by
normalizing to the expression of the internal control gene
GAPDH.
Immunohistochemistry
Paraformaldehyde-fixed, paraffin- embedded mouse
lacrimal gland sections (4 μm) were incubated with a primary
antibody against mouse Gadd45a (Millipore, Billerica, MA, USA)
overnight at 4°C and incubated with the appropriate secondary
antibodies. The sections were developed with diaminobenzidine and
counterstained with haematoxylin and eosin (H&E).
Quantification of the staining intensity was conducted by two
independent investigators.
Cell isolation and culture
Primary mouse LGE cell isolation and culture were
performed as previously described (22). LGE cells at passages 2–6 were used
for the subsequent experiments.
Cell transfection
Mouse Gadd45a cDNA was cloned into the CD510B-1
lentivector. The CD510B-1, REV, GAG and VSVG plasmids were then
transfected into HEK 293 cells using Lipofectamine 2000™
transfection reagent (Invitrogen Life Technologies). Lentiviral
infection was performed in the presence of polybrene (6 μg/ml).
Then, following 48 h lentiviral infection, primary LGE cells were
treated with puromycin (2 μg/ml) for 10 days. The empty CD510B-1
vector was used as a control. Transfection efficiency was evaluated
by western blot analysis.
Scratch-wound healing assay
LGE cells overexpressing Gadd45a or a vector control
were seeded in 24-well plates (2.5×105 cells/well) and
cultured to confluency. Next, the monolayer was gently scratched
across the centre with a 10 μl pipette tip. The gaps were imaged at
0, 12 and 24 h post-scratch using a live-cell imaging system
(Olympus Corporation, Tokyo, Japan).
Cell proliferation assay
Proliferation was determined using a standard CCK-8
cell counting kit (Dojindo, Kumamoto, Japan) according to the
manufacturer’s instruction. LGE cells overexpressing Gadd45a or a
vector control (4.0×104 cells/ml) were seeded in 96-well
plates (100 μl/well). The optical density values were measured at
days 0, 2, 4 and 6 by a microplate reader (SpectraMax M5, Molecular
Devices, Sunnyvale, CA, USA).
Western blot analysis
Cells were lysed with RIPA lysis buffer (Beyotime
Biotech, Jiangsu, China) supplemented with 1 mM PMSF
(Sigma-Aldrich, St. Louis, MO, USA). The primary antibodies used
were against the following proteins: Gadd45a (Millipore), Akt,
p-Akt, P38, p-P38, JNK, p-JNK, Erk 1/2 and p-Erk 1/2. All
antibodies, with the exception of the antibody against Gadd45a,
were purchased from Cell Signaling Technology, Inc. ( Danvers, MA,
USA). Signals were detected by an Odyssey infrared imaging system
(LI-COR, Lincoln, NE, USA) following incubation with IRDye 800
anti-rabbit (LI-COR) secondary antibodies. Quantification was
conducted using ImageJ software.
Statistical analysis
Statistical analyses were performed with a
two-tailed Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Radiotherapy impairs lacrimal secretion
and induces lacrimal gland injury in vivo
To evaluate the adverse effect of radiation therapy
on the lacrimal gland, a lacrimal gland scintigraphy assay was
performed and the histological and structural changes were observed
by H&E staining and transmission electron microscopy prior to
and following irradiation. The tracer uptake of the lacrimal glands
prior to irradiation was mounted before the administration of
carbachol. Lacrimal ejection was then induced and the amount of
tracer decreased with the passage of time. Thus, the time-activity
curve had a parabolic shape. Three days following irradiation,
primary tracer uptake was reduced and lacrimal ejection was
significantly lower. The time-activity curve showed an ascending
tendency. This reduction in lacrimal ejection remained 7 days later
and primary uptake remained reduced (Fig. 1A).
In normal lacrimal gland tissue, typical histology
shows the tubulo-acinar structure of the inferior lacrimal gland,
with a cubic, regular shape for the acinar cells and basally
located nuclei (Fig. 1B). However,
secretory retention was observed in the majority of acinar and
tubular cells in the irradiated inferior lacrimal gland 3 days
following irradiation. Scattered vacuolopathy and an increase in
the number of aberrant nuclei in apoptotic acinar cells were
observed, along with extracellular oedema and increased congestion
of the interlobular blood vessels (Fig. 1B). The majority of acute changes in
the appearance of the acinar cells remained evident seven days
later.
Transmission electron microscopy revealed the
intracellular retention of secretory granules, with subsequent
displacement of the acinar nuclei in the lacrimal gland 3 days
following irradiation (Fig. 1C).
In addition, 7 days following irradiation, apoptotic acinar
nuclei were observed and partial remission was noted, including a
reduction in secretory retention (Fig.
1C).
These results confirmed that radiation substantially
damages lacrimal gland function and structure.
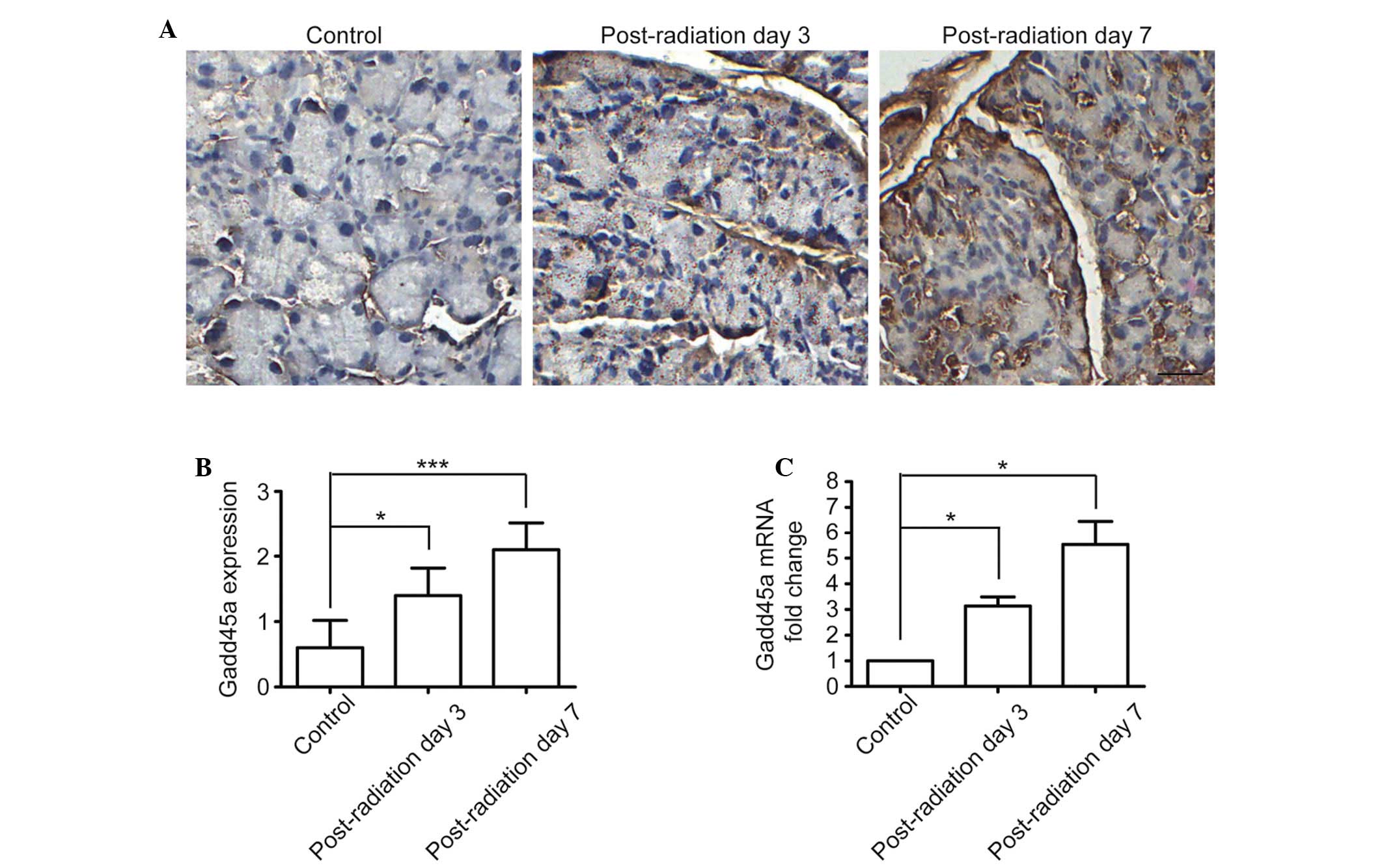
Radiation upregulates Gadd45a expression
in mouse lacrimal glands
To determine the roles of Gadd45a in
radiation-induced lacrimal gland injury, Gadd45a gene expression
was first observed in a radiation-induced lacrimal gland injury
mouse model. At post-irradiation days 3 and 7, lacrimal glands were
collected and the mRNA and protein expression levels of Gadd45a
were detected by quantitative PCR and western blot analysis. The
results showed that Gadd45a was expressed at a low level in normal
lacrimal glands, however, following local radiotherapy, mRNA and
protein expression levels of Gadd45a were significantly upregulated
in vivo (Fig. 2A–C). This
observation indicated that Gadd45a expression may be induced by
radiation, which is consistent with previous reports (14,16,17,23).
Gadd45a overexpression suppresses LGE
cell migration and proliferation
LGE cells are the primary cell type in the lacrimal
gland (8) and normal motility and
proliferation of LGE cells is pivotal for lacrimal gland repair
following local radiotherapy. Studies have demonstrated that
Gadd45a has negative roles in cell proliferation and migration
(20,21,24).
To explore the functional roles of Gadd45a in radiation-induced
lacrimal gland injury, the effects of the overexpression of Gadd45a
on the migration and proliferation of isolated primary LGE cells
were observed. The results revealed that following the
overexpression of Gadd45a (Fig.
3A), LGE cell migration (Fig. 3B
and C) and proliferation (Fig.
3D) were significantly inhibited in vitro. This
observation indicated that the radiation-induced upregulation of
Gadd45a may damage the recovery potentially induced by LGE cell
migration and proliferation.
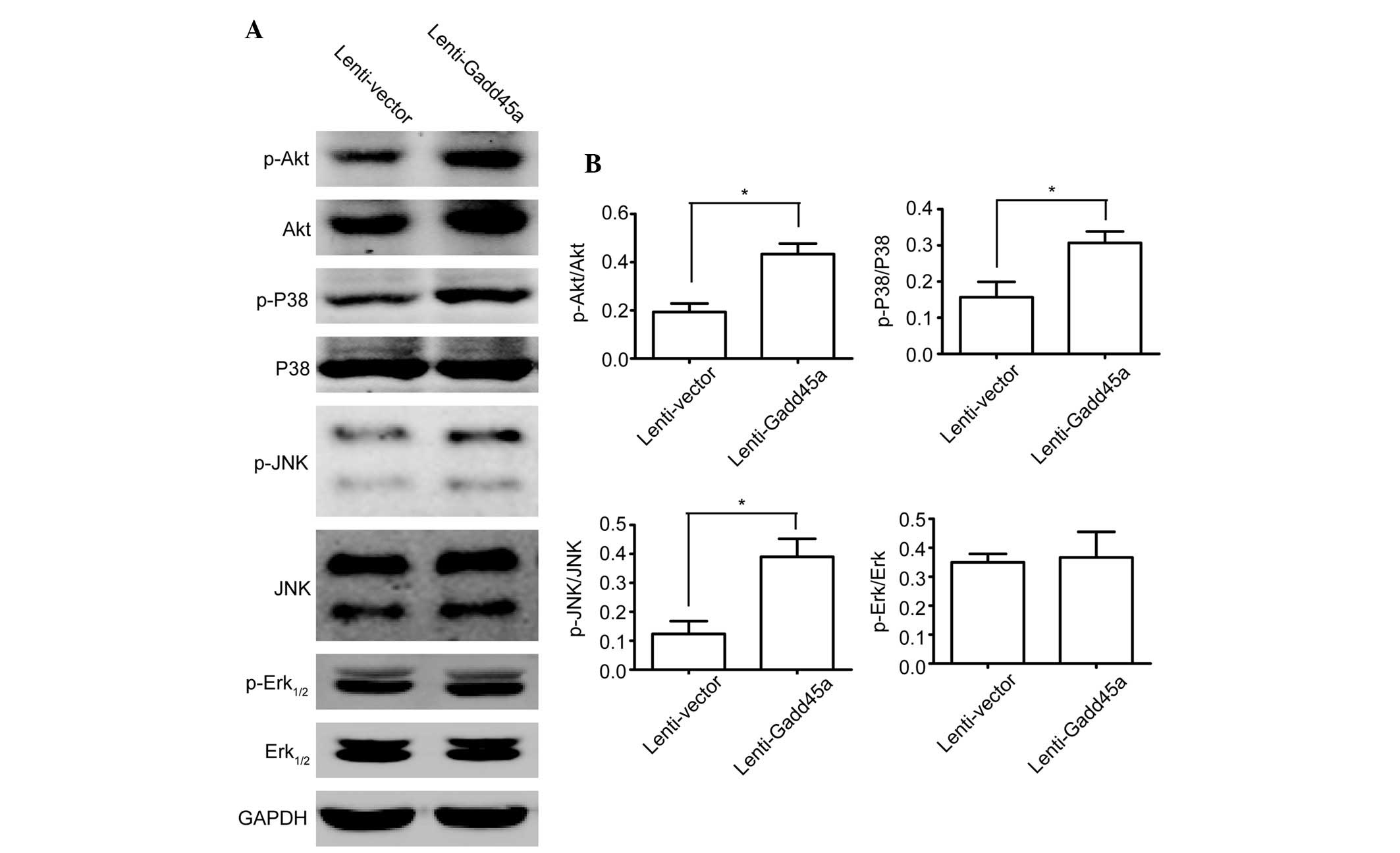
Gadd45a inhibits JNK, P38 and Akt
phosphorylation in LGE cells
Gadd45a has been reported to be associated with Akt
signalling (18) and MAPK stress
signalling (17,24), including P38 and JNK. To determine
whether these kinases are affected by the overexpression of Gadd45a
in LGE cells, western blot analysis of these signalling kinases was
performed. Akt, P38 and JNK phosphorylation were observed to be
significantly promoted by Gadd45a overexpression (Fig. 4A and B), but Erk1/2 was not
markedly affected (Fig. 4A and
B).
Discussion
Findings of the current study revealed that Gadd45a
is upregulated in lacrimal gland tissue by radiotherapy.
Overexpressed Gadd45a impaired two main events in lacrimal gland
repair, LGE cell migration and proliferation. This observation
indicated that Gadd45a prohibits lacrimal gland repair. Thus, this
gene may be used as a therapeutic target in lacrimal gland injury
secondary to orbital radiotherapy.
The present study indicated that beyond directly
damaging the lacrimal gland, radiotherapy also impairs the
reparative ability of the gland by upregulating Gadd45a expression.
Microarray analysis of irradiated skin cells revealed a number of
up- and downregulated genes (13–15,25–27),
however, the roles of the majority of these genes are not well
characterized, particularly in the lacrimal gland, another
superficial organ that is also affected by radiation. Studies
focused on these genes may aid in the understanding of
radiation-induced secondary damage.
The roles and mechanisms of Gadd45a have been
observed in various physiological and pathological situations.
Previous findings have demonstrated that Gadd45a is upregulated
following irradiation (14,16,23)
and in UV radiation-treated skin, Gadd45a may induce damaged
keratinocyte apoptosis via p38 and JNK activation, thus inhibiting
tumorigenesis (17). Gadd45a may
be induced by the stress of DNA damage and regulated by the tumour
suppressor gene p53, through which Gadd45a modulates tumour
angiogenesis (28). The effects of
Gadd45a on tumour cell migration and invasion have also been shown
(20). The roles of Gadd45a in
cell proliferation and cell cycle arrest have been comprehensively
studied (23,24). The current study on
radiation-induced lacrimal gland injury found that Gadd45a may also
significantly affect LGE cell migration and proliferation and
suppress JNK, P38 and Akt phosphorylation, which is consistent with
the observations of previous studies on other cell types (17,18,24).
Radiotherapy-induced lacrimal gland injury is
difficult to treat but has a relatively low incidence rate
(6). However, with the increasing
use of computers, radiation-induced xerophthalmia is becoming
increasingly common clinically (29,30).
Gadd45a is involved in solar UVB-induced cell impairment (19), thus, further studies on Gadd45a are
required to explore the role of Gadd45a on lacrimal gland
dysfunction induced by computer-related radiation.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation for Young Scholars of China
(no. 31100703).
References
|
1
|
Parsons JT, Bova FJ, Fitzgerald CR,
Mendenhall WM and Million RR: Severe dry-eye syndrome following
external beam irradiation. Int J Radiat Oncol Biol Phys.
30:775–780. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parsons JT, Bova FJ, Mendenhall WM,
Million RR and Fitzgerald CR: Response of the normal eye to high
dose radiotherapy. Oncology (Williston Park). 10:837–848. 851–852.
1996.PubMed/NCBI
|
|
3
|
Hempel M and Hinkelbein W: Eye sequelae
following external irradiation. Recent Results Cancer Res.
130:231–236. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gazda MJ, Schultheiss TE, Stephens LC, Ang
KK and Peters LJ: The relationship between apoptosis and atrophy in
the irradiated lacrimal gland. Int J Radiat Oncol Biol Phys.
24:693–697. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Solans R, Bosch JA, Galofré P, et al:
Salivary and lacrimal gland dysfunction (sicca syndrome) after
radioiodine therapy. J Nucl Med. 42:738–743. 2001.PubMed/NCBI
|
|
6
|
Fard-Esfahani A, Mirshekarpour H, Fallahi
B, et al: The effect of high-dose radioiodine treatment on lacrimal
gland function in patients with differentiated thyroid carcinoma.
Clin Nucl Med. 32:696–699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zettinig G, Hanselmayer G, Fueger BJ, et
al: Long-term impairment of the lacrimal glands after radioiodine
therapy: a cross-sectional study. Eur J Nucl Med Mol Imaging.
29:1428–1432. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruskell GL: Nerve terminals and epithelial
cell variety in the human lacrimal gland. Cell Tissue Res.
158:121–136. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vigneswaran N, Wilk CM, Heese A, Hornstein
OP and Naumann GO: Immunohistochemical characterization of
epithelial cells in human lacrimal glands. I. Normal major and
accessory lacrimal glands. Graefes Arch Clin Exp Ophthalmol.
228:58–64. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilk CM, Vigneswaran N, Heese A, Hornstein
OP and Naumann GO: Immunohistochemical characterization of
epithelial cells in human lacrimal glands. II. Inflammatory and
neoplastic lesions of lacrimal glands Graefes. Arch Clin Exp
Ophthalmol. 228:65–72. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zoukhri D, Fix A, Alroy J and Kublin CL:
Mechanisms of murine lacrimal gland repair after experimentally
induced inflammation. Invest Ophthalmol Vis Sci. 49:4399–4406.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
You S, Avidan O, Tariq A, et al: Role of
epithelial-mesenchymal transition in repair of the lacrimal gland
after experimentally induced injury. Invest Ophthalmol Vis Sci.
53:126–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sesto A, Navarro M, Burslem F and Jorcano
JL: Analysis of the ultraviolet B response in primary human
keratinocytes using oligonucleotide microarrays. Proc Natl Acad Sci
USA. 99:2965–2970. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marionnet C, Bernerd F, Dumas A, et al:
Modulation of gene expression induced in human epidermis by
environmental stress in vivo. J Invest Dermatol. 121:1447–1458.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Howell BG, Wang B, Freed I, Mamelak AJ,
Watanabe H and Sauder DN: Microarray analysis of UVB-regulated
genes in keratinocytes: downregulation of angiogenesis inhibitor
thrombospondin-1. J Dermatol Sci. 34:185–194. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thyss R, Virolle V, Imbert V, Peyron JF,
Aberdam D and Virolle T: NF-kappaB/Egr-1/Gadd45 are sequentially
activated upon UVB irradiation to mediate epidermal cell death.
EMBO J. 24:128–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hildesheim J, Bulavin DV, Anver MR, et al:
Gadd45a protects against UV irradiation-induced skin tumors, and
promotes apoptosis and stress signaling via MAPK and p53. Cancer
Res. 62:7305–7315. 2002.PubMed/NCBI
|
|
18
|
Meyer NJ, Huang Y, Singleton PA, et al:
GADD45a is a novel candidate gene in inflammatory lung injury via
influences on Akt signaling. FASEB J. 23:1325–1337. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fayolle C, Pourchet J, Caron de Fromentel
C, Puisieux A, Doré JF and Voeltzel T: Gadd45a activation protects
melanoma cells from ultraviolet B-induced apoptosis. J Invest
Dermatol. 128:196–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shan Z, Li G, Zhan Q and Li D: Gadd45a
inhibits cell migration and invasion by altering the global RNA
expression. Cancer Biol Ther. 13:1112–1122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ji J, Liu R, Tong T, et al: Gadd45a
regulates beta-catenin distribution and maintains cell-cell
adhesion/contact. Oncogene. 26:6396–6405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kobayashi S, Kawakita T, Kawashima M, et
al: Characterization of cultivated murine lacrimal gland epithelial
cells. Mol Vis. 18:1271–1277. 2012.PubMed/NCBI
|
|
23
|
Asuthkar S, Nalla AK, Gondi CS, et al:
Gadd45a sensitizes medulloblastoma cells to irradiation and
suppresses MMP-9-mediated EMT. Neuro Oncol. 13:1059–1073. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liebermann DA and Hoffman B: Gadd45 in
stress signaling. J Mol Signal. 3:152008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee KM, Lee JG, Seo EY, et al: Analysis of
genes responding to ultraviolet B irradiation of HaCaT
keratinocytes using a cDNA microarray. Br J Dermatol. 152:52–59.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takao J, Ariizumi K, Dougherty II and Cruz
PD Jr: Genomic scale analysis of the human keratinocyte response to
broad-band ultraviolet-B irradiation. Photodermatol Photoimmunol
Photomed. 18:5–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li D, Turi TG, Schuck A, Freedberg IM,
Khitrov G and Blumenberg M: Rays and arrays: the transcriptional
program in the response of human epidermal keratinocytes to UVB
illumination. FASEB J. 15:2533–2535. 2001.PubMed/NCBI
|
|
28
|
Yang F, Zhang W, Li D and Zhan Q: Gadd45a
suppresses tumor angiogenesis via inhibition of the mTOR/STAT3
protein pathway. J Biol Chem. 288:6552–6560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakamura S, Kinoshita S, Yokoi N, et al:
Lacrimal hypofunction as a new mechanism of dry eye in visual
display terminal users. PloS One. 5:e111192010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Uchino M, Schaumberg DA, Dogru M, et al:
Prevalence of dry eye disease among Japanese visual display
terminal users. Ophthalmology. 115:1982–1988. 2008. View Article : Google Scholar : PubMed/NCBI
|