Introduction
Articular cartilage is an integral component of
joint surfaces, however it has limited ability to repair itself and
is difficult to repair once damaged (1). Previous developments in the bone
tissue engineering field have provided hope for clinical repair of
cartilage defects. The primary material used in tissue engineering
is seed cells, which may be isolated and expanded in culture.
Co-culture has been widely used in tissue engineering in previous
years to induce human stem cells to differentiate into the desired
cell type, typically using cells of animal origin, which secrete
the appropriate cytokines (2).
However, determining the optimal ratios of cell types to obtain
specific cells and selecting the best type of human stem cells has
been problematic. In 2005, Broxmeyer (3), first described that umbilical cord
blood is rich in hematopoietic stem cells and subsequent studies
have shown that these cells have the potential to differentiate
into bone and cartilage tissue, similar to bone marrow mesenchymal
stem cells (BMSCs) (4–6). A subsequent study established that
human umbilical cord blood mesenchymal stem cells (HUCB-MSCs) were
more suitable than BMSCs, as they did not trigger the immune
response caused by the graft-versus-host disease following
transplantation (7). Thus,
HUCB-MSCs are considered to be of great value for the research and
development of novel therapies (6). The aim of the current study was to
isolate and co-culture HUCB-MSCs with rabbit chondrocytes in
various proportions and to induce the differentiation of HUCB-MSCs
into human chondrocytes. Notably, this technique provides a new
method for obtaining more suitable seed cells for tissue
engineering purposes and provides a theoretical basis for the
clinical treatment of cartilage defects.
Materials and methods
Experimental materials
HUCB-MSCs (OriCell) were purchased from
Biotechnology Co., Ltd. (Cyagen, Guangzhou, China). Newborn New
Zealand white rabbits (1–3 days old) were provided by the
Experimental Animal Center of Nanjing Medical University. The use
of laboratory animals in these experiments was in accordance with
the guidance and standards of the Ministry of Science and
Technology of the People’s Republic of China (8). Table
I provides a detailed list of the reagents used in these
experiments.
 | Table ISummary of experimental reagents. |
Table I
Summary of experimental reagents.
| Reagents | Company |
|---|
| TIAN script RT
kit | Tiangen, Beijing,
China |
| RNA simple total-RNA
kit | Tiangen, Beijing,
China |
| 2X Taq PCR
Master mix | Tiangen, Beijing,
China |
| Fetal bovine
serum | Sijiqing, Hangzhou,
China |
| DMEM/F12 medium | Gibco-BRL, Carlsbad,
CA, USA |
| Aggrecan IgG | Abcam, Cambridge, MA,
USA |
| Collagen type II
protein IgG | Abcam, Cambridge, MA,
USA |
| PVDF | Millipore, Billerica,
MA, USA |
Culture and identification of
HUCB-MSCs
HUCB-MSCs were cultured in DMEM (F-12) containing
15% fetal bovine serum, and 100 U/ml penicillin and streptomycin,
respectively. Culture medium was changed on average every three
days, depending on the rate of cell growth. The third generation of
HUCB-MSCs was used to confirm their identity. The cells were washed
twice with trypsin and analyzed for the expression of CD29 and CD34
by flow cytometry.
Isolation and culture of rabbit
chondrocytes
Newborn New Zealand white rabbits (1–3 days old)
were sacrificed and cartilage from the limb joints was removed
under aseptic conditions. Cartilage was sliced into
1–3-mm3 sections, placed in sterile tubes, washed three
times with PBS and centrifuged at 80 × g for 3 min. Chondrocytes
were isolated by incubation with 2 g/l trypsin for 30 min, followed
by digestion with 2 g/l type II collagenase for 6–8 h. Digested
cartilage was centrifuged at 80 × g for 3 min and washed twice with
culture medium. Cells were counted and transferred into a 25 ml
culture flask containing DMEM, at a seeding density of
5×109 cells/l. Isolated cells were cultured in an
incubator at 37°C with 5% CO2. Media was changed every
two days. Isolated chondrocytes and HUCB-MSCs were randomly
assigned to experimental groups for further analysis (Table II).
 | Table IISummary of experimental groups. |
Table II
Summary of experimental groups.
| Group | Stem cell suspension
(5×109/l) | Chondrocyte
suspension (5×109/l) | IGF-1 |
|---|
| A, simple stem
cell-induced | 6.0 ml | None | 100 μg/l |
| B, 2:1
co-culture | 4.0 ml | 2.0 ml | None |
| C, 2:1
co-culture+IGF-1 | 4.5 ml | 1.5 ml | 100 μg/l |
| D, 3:1
co-culture | 4.0 ml | 2.0 ml | None |
| E, 3:1
co-culture+IGF-1 | 4.5 ml | 1.5 ml | 100 μg/l |
Analysis of chondrocyte-specific
markers
Aggrecan (ACAN) and collagen type II (COL2A) were
used as chondrocyte-specific markers. TRIzol was used to extract
RNA from cells at days 7 and 14 of the experiment. Isolated RNA was
reserve transcribed into cDNA which was used for quantitative
polymerase chain reaction (qPCR). PCR products (5 μl) were
separated on agarose gels (20 g/l) and visualized with ethidium
bromide under ultraviolet light. Primer sequences are listed in
Table III. Protein was also
extracted from cells at days 7 and 14 of the experiment. Extracts
of total cellular protein were used for western blotting to detect
the expression of ACAN and COL2A and relative expression levels
were assessed by grayscale analysis of digital images.
 | Table IIIOligonucleotide primer sets for
reverse transcription-polymerase chain reaction. |
Table III
Oligonucleotide primer sets for
reverse transcription-polymerase chain reaction.
| Gene | Sequence (5′-3′) | Length, nt | Tm, °C | Size, bp |
|---|
|
Aggrecan-F |
CACCTACAAACGCAGACTACAGA | 23 | 57.6 | 167 |
|
Aggrecan-R |
AAAGCGACAAGAAGAGGACACC | 22 | 60.2 | |
| COL2A-F |
CAGCAAGAGCAAGGAGAAG | 19 | 53.1 | 126 |
| COL2A-R |
AGGCGTAGGAAGGTCATC | 18 | 51.7 | |
| β-actin-F |
CTTAGTTGCGTTACACCCTTTCTTG | 25 | 62.0 | 156 |
| β-actin-R |
CTGTCACCTTCACCGTTCCAGTTT | 24 | 64.4 | |
Statistical analysis
Data are presented as mean ± SD. Differences among
groups were assessed with SPSS version 11.0 statistical software
(SPSS Inc, Chicago, IL), using an independent-samples t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
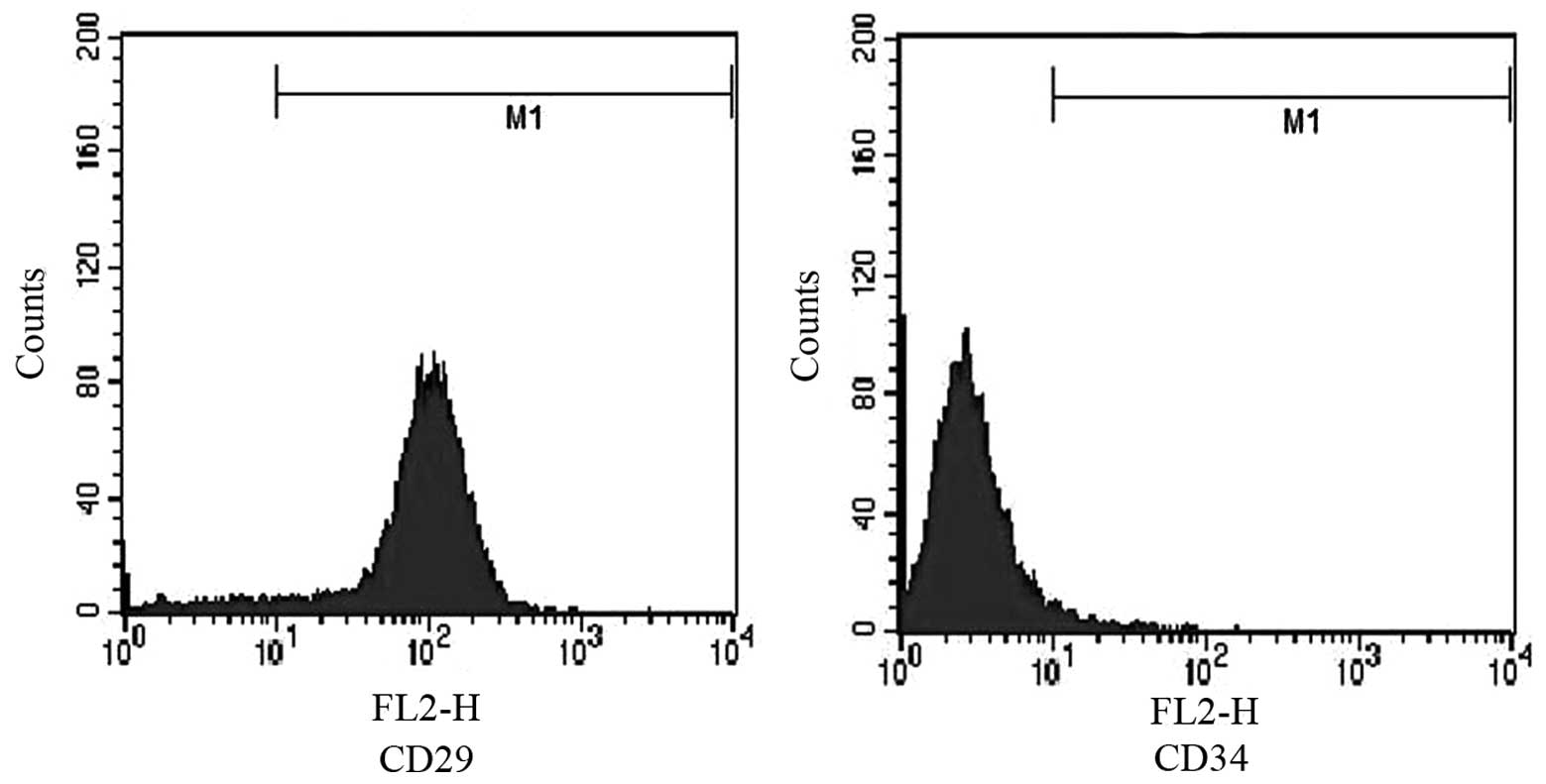
Identification of HUCB-MSCs
Third generation HUCB-MSCs were analyzed to confirm
their identity (Fig. 1).
Expression of CD29 (95.87%) and CD34 (3.1%) were confirmed by flow
cytometry.
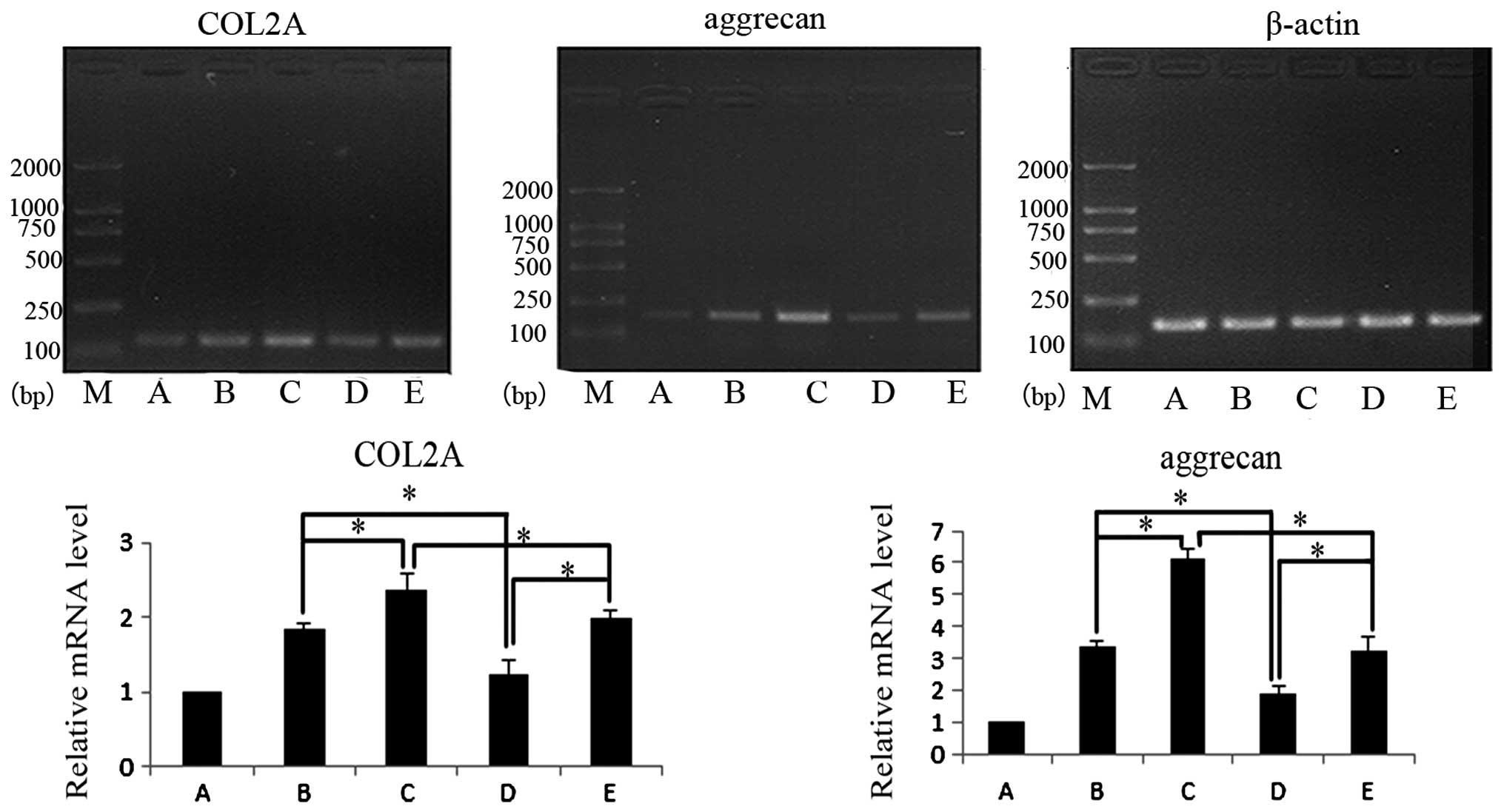
Quantification of chondrocyte-specific
marker mRNA
Expression levels of ACAN and COL2A mRNA were
assessed following 7 days in culture (Fig. 2). Following 7 days, co-cultured
cells in group D exhibited a slight increase in the expression
levels of ACAN and COL2A mRNA with the addition of insulin-like
growth factor (IGF-1), however, all other groups exhibited a higher
expression level of ACAN and COL2A mRNA compared with the control
group (P<0.05). In co-cultures of the two cell ratios tested,
ACAN and COL2A mRNA expression increased following the addition of
IGF-1 (P<0.05). Under the same culture conditions, the effects
in groups with different ratios of cells were compared. ACAN and
COL2A mRNA expression were observed to be higher in group B
compared with group D (P<0.05) and ACAN and COL2A mRNA
expression were higher in group C compared with group E
(P<0.05).
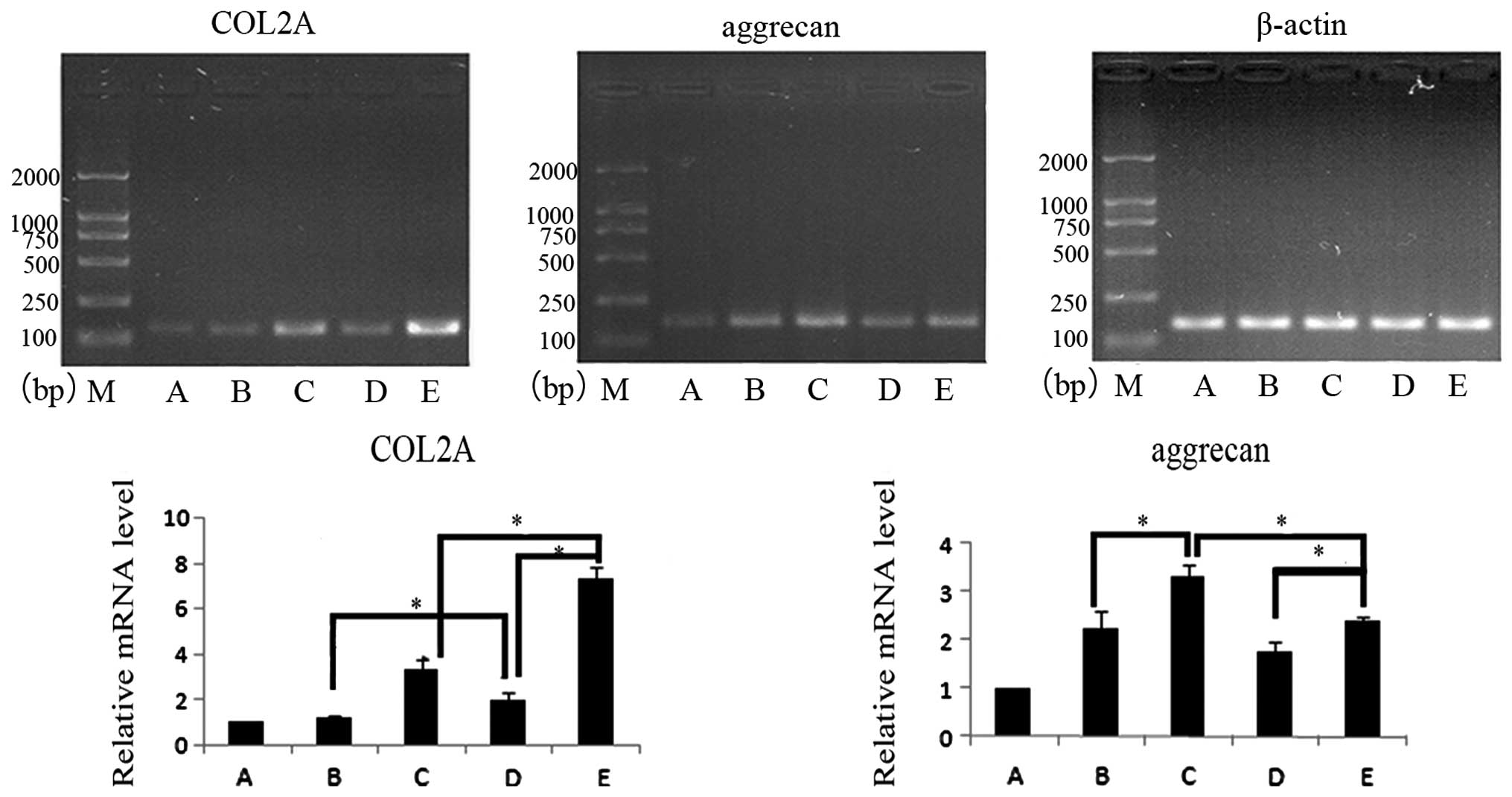
Expression levels of ACAN and COL2A mRNA were also
assessed following 14 days in culture (Fig. 3). Following 14 days, the groups
containing co-cultured cells exhibited a higher expression level of
ACAN and COL2A mRNA compared with the control group (P<0.05).
However, the expression of ACAN and COL2A only increased slightly
in group B, with no statistically significant difference observed
(P>0.05), indicating that co-culture induced slightly improved
chondrocyte differentiation compared with the reduced effect of
IGF-1. In co-cultures of the two cell ratios, ACAN and COL2A mRNA
expression increased following the addition of IGF-1 (P<0.05).
Under the same culture conditions, the effects in the groups with
different ratios of cells were compared. COL2A mRNA expression was
higher in group D compared with group B (P<0.05) and ACAN
expression was higher in group B compared with group D, however,
this difference was not statistically significant (P>0.05). ACAN
expression was higher in group B compared with D, however, this
difference was also not statistically significant (P>0.05). When
groups C and E were compared, COL2A mRNA expression was higher in
group E (P<0.05) and ACAN mRNA expression was higher in group C
(P<0.05). Comparions of days 7 and 14 for groups C and E showed
that ACAN mRNA expression was higher at day 14, similar to the
level observed in group B.
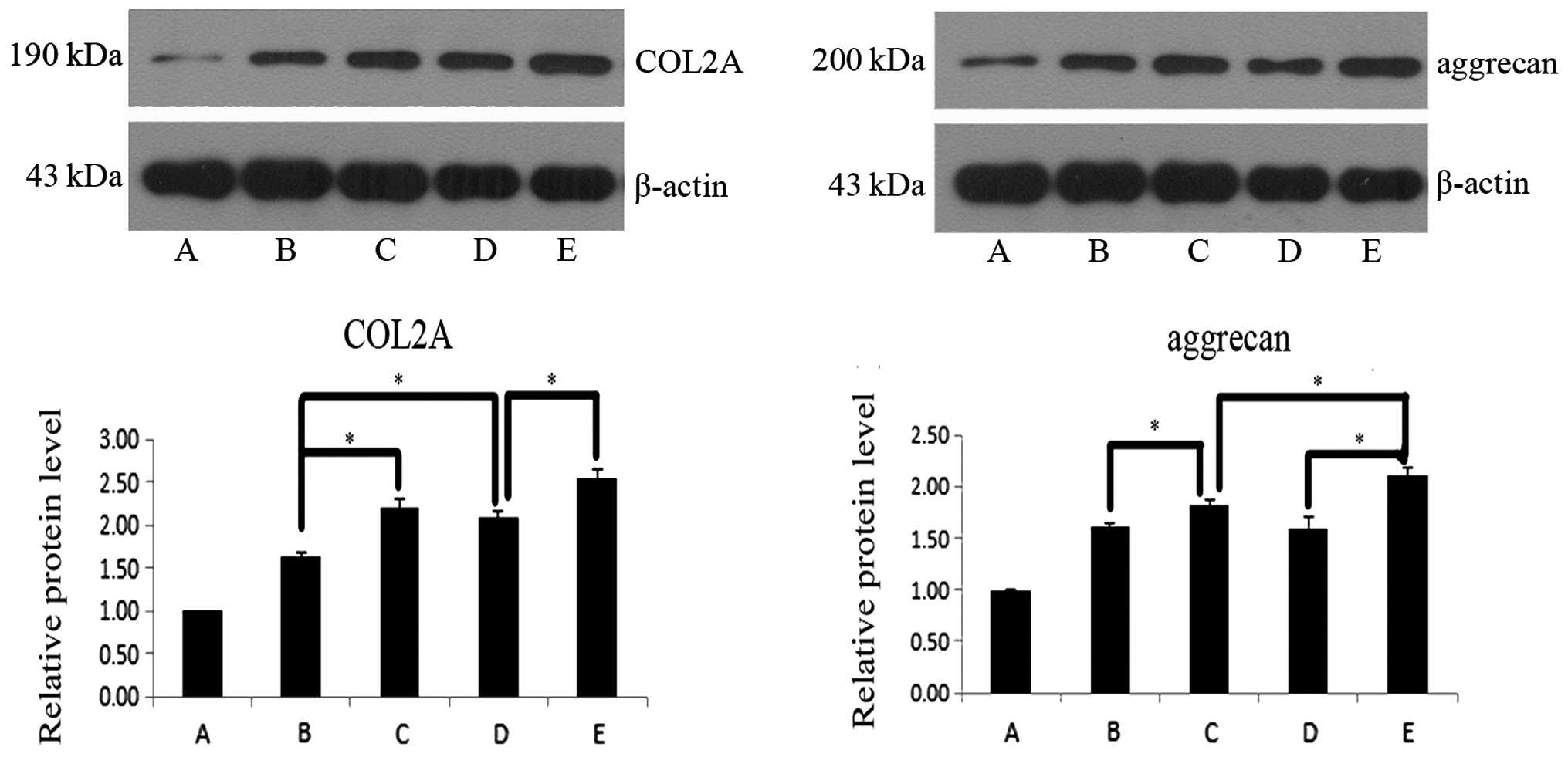
Protein expression levels of
chondrocyte-specific markers
The protein expression levels of ACAN and COL2A were
assessed following 7 days in culture (Fig. 4). Following 7 days, groups
containing co-cultured cells exhibited higher protein expression
levels of ACAN and COL2A compared with the control group
(P<0.05), with the exception of group D, which indicated that
IGF-1 induced improved differentiation in co-culture compared with
IGF-1 alone. In co-cultures of the two cell ratios, ACAN and COL2A
protein expression increased following the addition of IGF-1
(P<0.05). Under the same culture conditions, the effects in
groups with different ratios of cells were compared. ACAN and COL2A
protein expression was found to be higher in group B compared with
D (P<0.05) and ACAN and COL2A protein expression were higher in
group C compared with E (P<0.05).
The protein expression levels of ACAN and COL2A were
also assessed following 14 days in culture (Fig. 5). Following 14 days, the groups
containing co-cultured cells exhibited a higher expression level of
ACAN and COL2A protein compared with the control group (P<0.05).
In co-cultures with the two cell ratios tested, ACAN and COL2A
protein expression increased following the addition of IGF-1
(P<0.05). Under the same culture conditions, the effects in
groups with different ratios of cells were compared. COL2A protein
expression was found to be higher in group D compared with B
(P<0.05) and ACAN expression was higher in group B compared with
D, however, this difference was not statistically significant
(P>0.05). ACAN expression was higher in group B compared with
group D, however, this difference was not statistically significant
(P>0.05). When groups C and E were compared, COL2A protein
expression was observed to be higher in group E, but this
difference was not statistically significant (P>0.05). However,
ACAN protein expression levels were significantly higher in group E
compared with group C (P<0.05).
Discussion
In previous years, bone tissue engineering has been
applied for the repair of articular cartilage defects in basic and
clinical research and has gained increasingly wider attention from
investigators and clinicians. Studies have shown that co-culturing
chondrocytes with BMSCs and the use of chondrocyte paracrine
secretions, may help to regulate the differentiation of mesenchymal
stem cells into chondrocytes and may significantly reduce the
requirements of chondrocytes. This has provided a practical method
to solve the insufficient source of chondrocytes for tissue
engineering (2). Results of recent
studies have shown that animal-derived cartilage cells secrete the
same cytokines and induce human stem cells to differentiate into
chondrocytes (7,9). This observation was a major
breakthrough in tissue engineering and allow the possibility of
using animal cells in co-culture with human stem cells to promote
chondrogenic differentiation.
HUCB-MSCs have numerous advantages compared with
BMSCs, including convenient collection, easy separation and
preservation, reduced immunogenicity, the absence of tumor cell
contamination, a lower risk of latent virus and pathogenic
microorganism infection and transmission and less ethical
controversy (10). IGF-1,
transforming growth factor-β (TGF-β), bone morphogenetic protein
and fibroblast growth factor 2 have all been shown to induce bone
and cartilage differentiation in HUCB-MSCs, similar to BMSCs
(11–13). The ability of co-culture of
HUCB-MSCs and chondrocytes to obtain large numbers of chondrocytes
has not been reported in previous literature and the majority of
stem cells and chondrocytes are co-cultured at a ratio of 1:1 or
2:1. As HUCB-MSCs may be obtained more readily than chondrocytes,
inducing more chondrogenic differentiation of stem cells with fewer
chondrocytes is likely to be the focus of future studies. In the
current study, HUCB-MSCs and rabbit chondrocytes were co-cultured
at a ratio of 2:1 and 3:1, in the presence or absence of IGF-1, to
assess the feasibility of using fewer chondrocytes to induce
HUCB-MSC differentiation and thus, provide an improved source of
cells for tissue engineering.
To determine the relative levels of HUCB-MSC
differentiation into chondrocytes, the expression of the markers of
chondrocyte differentiation, ACAN and COL2A, were compared at the
protein and mRNA levels. Primers specific to human transcripts and
human-specific antibodies were used to exclude the possibility of
interference from endogenous rabbit chondrocytes. The results
showed that co-culture with rabbit chondrocytes induced greater
differentiation of HUCB-MSCs into chondrocytes compared with a
reduced effect of IGF-1. This observation was confirmed by higher
mRNA and protein levels of ACAN and COL2A. The addition of IGF-1 to
the culture medium increased chondrocyte differentiation. Although
cells co-cultured at a ratio of 2:1 exhibited higher rates of
differentiation compared with cells co-cultured at a 3:1 ratio, the
addition of IGF-1 yielded superior results. This indicates that the
addition of cytokines, specifically IGF-1, induces HUCB-MSC
differentiation in the presence of fewer chondrocytes.
In conclusion, the co-culture of HUCB-MSCs and
rabbit chondrocytes induces the differentiation of HUCB-MSCs into
human chondrocytes. The current study provides evidence of a
principle for a theoretical source of multi-potent stem cells with
low immunogenicity, yielding a rich source of seed cells for tissue
engineering. Although seed cells are an important consideration in
tissue engineering, other factors, including the tissue scaffold
and micronutrient environment are also significant. Accordingly, to
obtain the desired tissue with the maximum efficiency, exploration
of total culture time (14), the
use of growth factors, including IGF-1 and TGF-β (15), hierarchical co-culture, conditioned
medium and joint specific scaffolds (16), requires further study.
Acknowledgements
This study was supported by a grant from the Health
Bureau of Nanjing (no. QYK10163). The authors would like to thank
Yue Lou, for her guidance in the writing of this manuscript.
References
|
1
|
Ochi M: Clinical results of
transplantation of tissue-engineered and future direction of
cartilage repair-novel approach with minimally invasive procedure.
Yonsei Med J. 45:74. 2004. View Article : Google Scholar
|
|
2
|
Tsuchiya K, Chen G, Ushida T, Matsuno T
and Tateishi T: The effect of coculture of chondrocytes with
mesenchymal stem cells on their cartilaginous phenotype in vitro.
Mater Sci Eng C Mater Biol Appl. 24:391–396. 2004. View Article : Google Scholar
|
|
3
|
Broxmeyer HE: Biology of cord blood cells
and future prospects for enhanced clinical benefit. Cytotherapy.
7:209–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang X, Hirai M, Cantero S, Ciubotariu R,
Dobrila L, Hirsh A, Igura K, Satoh H, Yokomi I, Nishimura T, et al:
Isolation and characterization of mesenchymal stem cells from human
umbilical cord blood: reevaluation of critical factors for
successful isolation and high ability to proliferate and
differentiate to chondrocytes as compared to mesenchymal stem cells
from bone marrow and adipose tissue. J Cell Biochem. 112:1206–1218.
2011.
|
|
5
|
Feng X, Tian S, Sun K, Zhang J, Zhang C,
Liu S, Zhou M and Lü J: Effect of platelet lysate on chondrogenic
differentiation of human umbilical cord derived mesenchymal stem
cells in vitro. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.
25:1250–1255. 2011.(In Chinese).
|
|
6
|
Ghen MJ, Roshan R, Roshan RO, et al:
Potential clinical applications using sten cells derived from human
umbilical cord blood. Reprod Biomed Online. 13:562–572. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meretoja VV, Dahlin RL, Kasper FK and
Mikos AG: Enhanced chondrogenesis in co-cultures with articular
chondrocytes and mesenchymal stem cells. Biomaterials.
33:6362–6369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
The Ministry of Science and Technology of
the People’s Republic of China. Guidance suggestion of caring
laboratory animals. http://www.most.gov.cn/fggw/zfwj/zfwj2006/200609/t20060930_54389.htm.
Accessed September 30, 2006(In Chinese).
|
|
9
|
Wu L, Prins HJ, Helder MN, van
Blitterswijk CA and Karperien M: Trophic effects of mesenchymal
stem cells in chondrocyte co-cultures are independent of culture
conditions and cell sources. Tissue Eng Part A. 18:1542–1551.
2012.PubMed/NCBI
|
|
10
|
Gennery AR and Cant AJ: Cord blood stem
cell tansplantation in primary immune deficiencies. Curr Opin
Allergy Clin Immunol. 7:528–534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Puetzer JL, Petitte JN and Loboa EG:
Comparative review of growth factors for induction of
three-dimensional in vitro chondrogenesis in human mesenchymal stem
cells isolated from bone marrow and adipose tissue. Tissue Eng Part
B Rev. 16:435–444. 2010. View Article : Google Scholar
|
|
12
|
Weiss S, Hennig T, Bock R, Steck E and
Richter W: Impact of growth factors and PTHrP on early and late
chondrogenic differentiation of human mesenchymal stem cells. J
Cell Physiol. 223:84–93. 2010.PubMed/NCBI
|
|
13
|
Djouad F, Bony C, Canovas F, Fromigué O,
Rème T, Jorgensen C and Noël D: Transcriptomic analysis identifies
Foxo3A as a novel transcription factor regulating mesenchymal stem
cell chrondrogenic differentiation. Cloning Stem Cells. 11:407–416.
2009. View Article : Google Scholar
|
|
14
|
Chang Q, Cui W-D and Fan W-M: Co-culture
of chondrocytes and bone marrow mesenchymal stem cells in vitro
enhances the expression of cartilaginous extracellular matrix
components. Braz J Med Biol Res. 44:303–310. 2011. View Article : Google Scholar
|
|
15
|
Ertan AB, Yılgor P, Bayyurt B, et al:
Effect of double growth factor release on cartilage tissue
engineering. J Tissue Eng Regen Med. 7:149–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang N, Liu X, Guan Y, Wang J, Gong F,
Yang X, Yan L, Wang Q, Fu X, Cao Y and Xiao R: Effects of
co-culturing BMSCs and auricular chondrocytes on the elastic
modulus and hypertrophy of tissue engineered cartilage.
Biomaterials. 33:4535–4544. 2012. View Article : Google Scholar : PubMed/NCBI
|