Introduction
Hepatocellular carcinoma (HCC) is the most common
malignancy of the liver, the seventh most common type of cancer and
the third leading cause of cancer-related mortality worldwide
(1). The long-term prognosis of
patients with HCC remains unsatisfactory due to tumor recurrence
and a limited response to chemotherapy and radiotherapy (2–5). A
number of treatment options are currently available; however,
mechanisms of liver cancer initiation and progression have yet to
be adequately elucidated. Therefore, an understanding of the
mechanisms of HCC proliferation and metastasis may improve the
efficacy of treatment modalities for CLL.
Toll-like receptor 3 (TLR3) is a pattern-recognition
receptor that is involved in immune signaling and is essential for
survival as it recognizes various viral components, such as
double-stranded (ds)RNA. TLR3 signaling depends solely on the TIR
domain-containing adaptor-inducing interferon-β (TRIF) adaptor
protein. This leads to the activation of the nuclear factor-κB
(NF-κB) and interferon regulatory factor 4 (IRF4) transcription
factors, which induce an antiviral interferon response (6,7).
Furthermore, TRIF exhibits pro-apoptotic activity, suggesting that
TLR3 signaling may lead to cell death (8).
Although TLR3 may directly trigger apoptosis in
certain cancer cells (9,10), dsRNA has been recognized as a TLR3
ligand and was reported to induce apoptosis in several cell types
through multiple pathways. Results of previous studies have
indicated that the neoplastic process may disrupt the TLR signaling
pathways, thereby allowing for the unhindered progression of
cancer. Furthermore, TLRs on tumor cell surfaces facilitate evasion
from immune surveillance via the suppression of T-cell
proliferation and natural killer cell activity. This suggests that
TLR signaling in tumor cells is associated with the progression of
cancer and evasion of host defenses. However, the involvement of
TLR3 signaling pathways in tumor progression, invasion and
metastasis remains unclear.
In the present study, it was hypothesized that dsRNA
acts as a TLR3 ligand and may be capable of inhibiting HCC activity
in vivo by regulating the TLR3 signaling pathways. In the
study, several dsRNA sequences were previously designed and
synthesized based on cell surface TLR3 sensitive viral sequences in
human echovirus. The expression of TLR3 signaling proteins
following stimulation with one particular dsRNA (BM-06) in HCC
tissues was investigated. In addition, the correlation between TLR3
activation and cell proliferation or apoptosis in HCC tissues was
analyzed using an orthotopic Sprague-Dawley (SD) rat HCC model.
Materials and methods
Reagents
Polyinosinic-polycytidylic acid [poly(I:C)], a
dsRNA, TLR3 specific ligand, was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). Several dsRNAs were designed
based on cell surface TLR3 sensitive viral sequences in human
echovirus, human poliovirus, enterovirus 70 and coxsackievirus from
GenBank. Furthermore, the viral sequences were submitted for basic
local alignment search tool (BLAST) analysis (http://www.ncbi.nlm.nih.gov/blast/) to ensure
that the sequence was not homologous to human genes. The target
sequence of BM-06 was CCGGCCCCUGA AUGCGGCUAAUC (23 nt) and was
synthesized by Biomics Biotechnologies Co., Ltd. (Jiangsu,
China).
Orthotopic Sprague-Dawley (SD) rat HCC
model
Thirty male SD rats, (age, 4–6 weeks; weight,
120–160 g), were used. SD rats were purchased from the animal
center of Nantong University (Jiangsu, China) and housed according
to the guidelines for experimental animals approved by the Animal
Care and Use Committee of Nantong University. All 30 rats were fed
with animal feed containing 0.03% 2-acetylaminofluorene (2-AAF;
Sigma-Aldrich, St. Louis, MO, USA) and kept in an air-conditioned
environment for 16 weeks to establish the HCC model. Ten rats were
sacrificed under mild ether anesthesia at the 12th, 14th, 16th and
18th week after the rats were fed with animal feed containing
2-AAF. Livers were collected to confirm malignant transformation.
Fifteen HCC-established rats were randomly selected and divided
into three groups of five rats per cage. The remaining five HCC
rats were used to replace, if required, any rat that died during
the experiment. The study was approved by the Ethics Committee of
Nantong University, Jiangsu, China.
Drug treatment
All procedures were conducted in accordance with the
guidelines for experimental animals approved by the Animal Care and
Use Committee of Nantong University, (Jiangsu, China).
Two of the above animal groups were treated,
respectively, with the drug candidate (BM-06) and poly(I:C) which
served as a positive control. BM-06 and poly(I:C) were prepared in
sterile 0.01M phosphate-buffered saline (PBS) and injected
intraperitoneally at 1.0 mg/kg once a week for 6 weeks. The third
rat group was treated in the same manner; however, the rats were
injected with 1.0 mg/kg PBS to be used as a control group.
Treatments were initiated when the rats had been fed with 2-AAF for
16 weeks. At the end of the treatment period, all 15 treated rats
were sacrificed and the livers were excised and weighed. Specimens
were taken from the liver for pathological examination and
immunohistochemical analysis, and the remaining liver samples were
stored at −80°C for RNA and protein analyses.
Hematoxylin and eosin (H&E)
staining
Rat liver tissues fixed in a 10% formalin solution
were sectioned (4-μm thickness) following dehydration, being made
transparent and paraffin-embedding. The sections were flattened,
pasted and heated on glass slides. Histological evaluations were
performed by H&E staining and pathological examination
(11,12).
Immunohistochemical staining
Rat HCC sections (4-μm thickness) were
deparaffinized and hydrated. Antigen retrieval was conducted in a
140 mM citrate buffer (pH 6.0) in a decloaking chamber (BioCare
Medical, Walnut Creek, CA, USA) for 3 min at 115°C. The slides were
then transferred to boiling de-ionized water and cooled for 20 min
at room temperature (RT) to block endogenous peroxidase, endogenous
biotin and non-specific proteins. The slides were incubated with
the following antibodies: anti-survivin (dilution, 1:100; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), -Bcl-2 (dilution,
1:50; Santa Cruz Biotechnology, Inc.), -proliferating cell nuclear
antigen (PCNA; dilution, 1:100; Santa Cruz Biotechnology, Inc.) and
-caspase-3 (dilution, 1:100; Santa Cruz Biotechnology, Inc.) for 30
min at RT, and maintained overnight at 4°C. The slides were
subjected to a standard avidin-biotin-peroxidase complex technique.
Staining was visualized using a DAB+ substrate chromogen solution
and hematoxylin QS counterstain (Vector Laboratories, Burlingame,
CA, USA). The sections were analyzed by two independent
investigators with knowledge of histopathology. Five fields of view
were captured on each section of the harvested tumor.
Representative images were selected and shown.
qPCR
Total RNA was isolated from liver tissues using
TRIzol (Invitrogen Life Technologies). qPCR was performed for TLR3,
NF-κB, TRIF, caspase-8, interferon (IFN)-γ and vascular endothelial
growth factor (VEGF) using an ABI Prism® 7700 Sequence
Detection system (Applied Biosystems, Carlsbad, CA, USA). The
cycling conditions for amplification were as follows: denaturation
at 95°C for 3 min, 35 cycles of 45 sec at 95°C, 45 sec at 60°C,
annealing at 72°C for 30 sec and a final extension at 72°C for 7
min. The primer sequences are listed in Table I. Gene expression for each rat was
normalized to the β-actin mRNA copies from the same sample.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Primer | Sequence (5′→3′) |
|---|
| R-TLR3-F |
CGGTCAAGGTGTTCAAGA |
| R-TLR3-R |
GGATGGTAGAAGCGTGTT |
| R-NF-κB-F |
TGGCTTCTATTACCTGTA |
| R-NF-κB-R |
TAACGACATATACCATCAG |
| R-caspase-8-F |
TGAACTATGATGTGAGCAATA |
| R-caspase-8-R |
TTCCGTAGTGTGAAGATG |
| R-IFN-γ-F |
TCTTCACATCAAAGGAGTCATC |
| R-IFN-γ-R |
TGCTGCTGGAGGTCATTA |
| R-VEGF-F |
GCAGCATAGCAGATGTGAAT |
| R-VEGF-R |
TTGACCCTTTCCCTTTCCT |
| R-β-actin-F |
TATGGAATCCTGTGGCATC |
| R-β-actin-R |
GTGTTGGCATAGAGGTCTT |
Western blot analysis
The HCC rat liver tissues were homogenized. Equal
quantities of protein were resolved by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto a
polyvinylidene difluoride membrane filter (Immobilon, Millipore,
Billerica, MA, USA). The membrane was blocked for 2 h with 5%
non-fat milk in Tris-buffered saline containing 0.1% Tween-20
(TBST), incubated with anti-phospho-NF-κB p65 antibody (dilution,
1:500; Cell Signaling Technology Inc., Danvers, MA, USA) and
anti-TLR3 antibody (dilution, 1:500; Abcam, Cambridge, UK), and
maintained overnight at 4°C. The membranes were washed 3 times in
TBST for 5 min, incubated with a secondary antibody anti-β-actin
(dilution, 1:2500; Santa Cruz Biotechnology, Inc.) for 2 h at RT
and developed using a chemiluminescence system (Pierce
Biotechnology Inc., Rockford, IL, USA). The film was scanned and
the density of the bands was measured using ImageQuant software
(Molecular Dynamics, Sunnyvale, CA, USA) and expressed as a
percentage of the density of the β-actin band.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software for Windows (SPSS, Inc., Chicago, IL, USA). The data are
presented as the mean ± standard deviation. Differences between
groups were evaluated with analysis of variance (ANOVA) or
factorial design ANOVA. P<0.05 was considered to indicate a
statistically significant difference. The size of the tumor nodules
was quantified using Histolab 5.8 software (Microvision
Instruments, Cedex, France).
Results
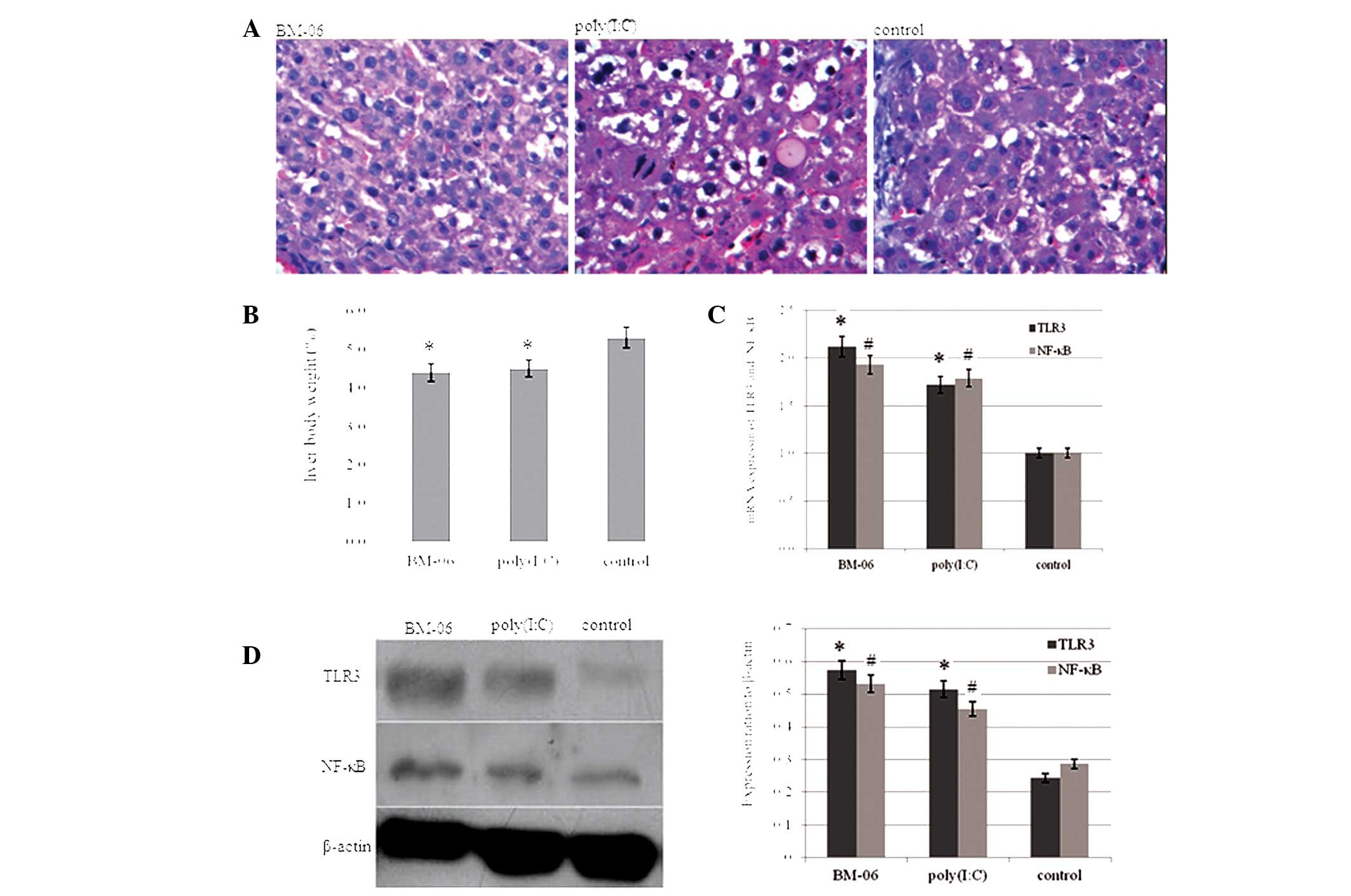
Effects of dsRNA-induced inhibition on
mouse HCC growth
After being fed with 2-AAF for 16 weeks and treated
with BM-06 or poly(I:C) for 6 weeks, the rats were sacrificed. The
morphological malignant changes in the rat livers were observed by
H&E staining analysis (Fig.
1A). No lesions were observed in the other organs. This
suggested that the dsRNA exhibited no toxic side effects on the
other organs of the rats. The liver/body weight ratio, a measure of
tumor progression, of the experimental rats was calculated
(Fig. 1B). As shown, the
liver/body weight ratio in the rats treated with BM-06 and
poly(I:C) was significantly decreased compared with the PBS-treated
group (P<0.05). The expression of TLR3, NF-κB mRNA and protein
in the rat liver was investigated using qPCR and western blot
analysis, respectively. Increased expression of TLR3 and NF-κB was
observed in the rat livers treated with BM-06 and poly(I:C),
compared with the PBS control group (P<0.05; Fig. 1C and 1D).
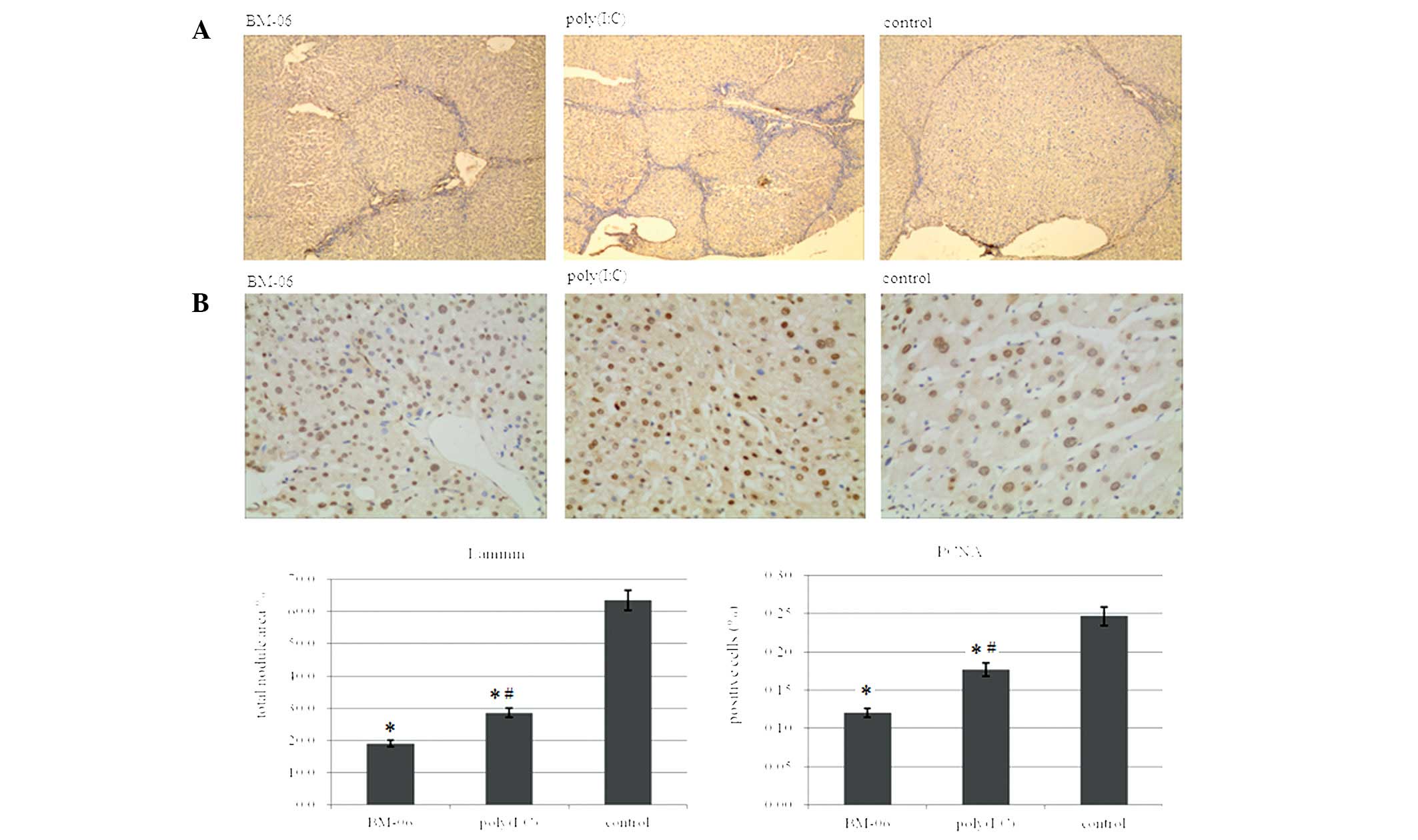
Inhibition of cell proliferation and
induction of cell apoptosis in HCC
Sections of the livers from the treated groups were
immunohistochemically stained for laminin, survivin, Bcl-2 (two
anti-apoptosis markers), caspase-3 (a pro-apoptosis marker) and
PCNA (a proliferation marker). As capillarization of the blood
supply in the mouse HCC was accompanied by the upregulation of
laminin expression within the tumor nodules (13), laminin immunostaining was used to
visualize tumor nodules in the liver sections of the treated rats
and to determine their sizes with Histolab software (Fig. 2A). Treatment with BM-06 and
poly(I:C) was shown to lead to a reduction in the tumor nodule size
compared with that in PBS-treated animals, particularly for the
BM-06 treated group (P<0.05). In addition, immunohistochemical
staining for positive cells was identified in five randomly
selected microscopic fields on sections of five different liver
specimens per treatment group. The number of PCNA-, survivin- and
Bcl-2-positive cells was significantly reduced, while the number of
caspase-3 positive cells was significantly increased in the BM-06
and poly(I:C) groups, compared with that in the PBS control group
(P<0.05). Representative micrographs are shown in Figs. 2 and 3 together with quantification results
expressed as a proportion of positive cells.
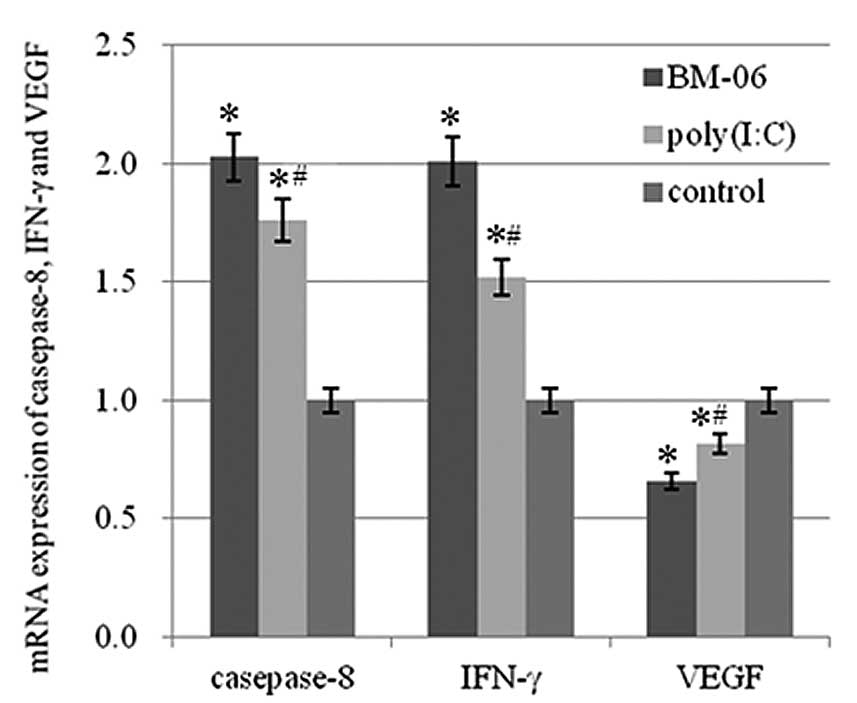
Increase in mRNA levels of caspase-8 and
IFN-γ, and a decrease in VEGF mRNA
mRNA levels of caspase-8, IFN-γ and VEGF in the
liver tissues of the treated rats were determined by qPCR. The
results demonstrated that the mRNA levels of caspase-8 and IFN-γ
were significantly increased in the liver, while VEGF mRNA levels
were significantly decreased in the BM-06 and poly(I:C) group
compared with that of the PBS control group (P<0.05). The effect
of BM-06 was greater than poly(I:C) (P<0.05; Fig. 4). The results suggested that the
inhibitory effects of BM-06 may be due to the activation of an
innate interferon response and via the initiation of the
death-receptor pathway of cell apoptosis in the rat HCC model.
Discussion
HCC is a complex disease with the involvement of
multiple signaling pathways in its pathogenesis and is difficult to
treat, particularly in advanced stages. Inhibition of specific
growth factor receptors and their various signaling pathways via
targeted therapy appears to be a promising approach in the
treatment of HCC. More advanced studies are required to fully
clarify its molecular pathogenesis and to identify potential key
targets for intervention (14).
Recent studies have suggested that dsRNA triggers
TLR signaling via MyD88, leading to the induction of NF-κB and
secretion of inflammatory cytokines that prime liver regeneration.
TLR3 is unique among TLRs, in that it signals through TRIF but not
through MyD88, an event that may lead to activation of either the
inflammatory or apoptotic pathway. The inflammatory pathway leads
to NF-κB activation, while the apoptotic pathway, considered to be
mediated by Rip3 binding to TRIF and the recruitment of
Fas-associated death domain protein (FADD), leads to caspase-8
activation (14–16).
In a study by Bergé et al(15), it was demonstrated that treatment
of HCC mice with poly(I:C) resulted in the suppression of
vasculature remodeling and tumor liver growth. It was also observed
that the levels of INF-γ in the liver were significantly increased
in mice treated with dsRNA. INF-γ was identified as an inhibitor of
endothelial cell proliferation and a potent suppressor of
tumor-associated neovascularization. Furthermore, it was suggested
that INF-γ, detected in mouse HCC liver extracts, was released by
circulating or resident immune cells. Previous studies have shown
that in breast cancer cells, synthetic dsRNA induced apoptosis in a
TLR-dependent manner (17). In
melanoma cells, TLR3 agonists were able to directly inhibit cell
proliferation and induce tumor cell death (9). Zorde-Khvalevsky et al(16) demonstrated that during the initial
phase following partial hepatectomy, TLR3 signaling was induced in
hepatocytes, leading to the activation of NF-κB as well as
increased Rip3 protein levels and caspase-8 activation.
Furthermore, NF-κB was observed to induce pro-IL-1β expression in
hepatocytes, which was then activated by caspase-8, leading to the
inhibition of hepatocyte proliferation.
In the present study, BM-06, a 23-nt dsRNA was
designed prior to the experiments and was used as a drug candidate
in the rat HCC model. The results showed that BM-06, a TLR3
agonist, stimulated TLR3 on the cell surface and activated NF-κB.
To the best of our knowledge, this study demonstrated for the first
time, that the activation of TLR3 inhibited the growth of liver
tumors and induced apoptosis of tumor cells in the rat HCC model.
The levels of INF-γ, caspase-8 and caspase-3-positive cells in the
liver were significantly increased, while the levels of VEGF,
PCNA-, survivin- and Bcl-2-positive cells were significantly
decreased in rat HCCs treated with the dsRNA. It was suggested that
the TLR3-dependent activation of NF-κB in hepatocytes results in an
increase in the expression of caspase-8 and -3, which subsequently
inhibits hepatocyte proliferation and induces HCC cell apoptosis
with the recruitment of FADD via caspase-8 to activity caspase-3.
These results demonstrated that the dsRNA inhibited tumor growth
through the TLR3 pathway. In addition, BM-06 increased the
expression of TLR3. Activation of TLR3 results in the production of
IFN-γ, which may further upregulate the expression of TLR3. In the
present study, TLR3 activation by viruses or dsRNA increased the
inflammatory potential of the HCC cells, including increased IL-6
production, and further increased IFN- and TGF-β-responsive gene
expression. IFNs may then increase the expression of a number of
molecules on the HCC cells, including TLR3. The observations
suggested that IFN-γ increased the inflammatory potential of the
HCC cells by the upregulation of TLR3 and its downstream
responses.
Kleinman et al(18) demonstrated that unlike 21- or
23-nucleotide Luc siRNA, 7-, 13-, 16- and 19-nucleotide versions
did not suppress choroidal neovascularization (CNV). This
observation, coupled with data that longer duplexes including
~1,000-nucleotide dsRNA and poly(I:C) suppressed CNV, suggested
that at least 21 nucleotides are required to activate TLR3. In this
study, the length of the dsRNA BM-06 was 23 nt and it achieved TLR3
activation. BM-06, a 23-nucleotide dsRNA, was determined to be
superior to poly(I:C) in the inhibition of HCC growth and elevated
apoptosis of HCC cells with reduced side effects. These results
suggested that stimulation of the TLR3 pathway by dsRNA may be
sequence-specific.
In conclusion, BM-06 was shown to inhibit HCC
activity in vivo. Stimulation of functional TLR3 expressed
on rat HCCs by BM-06 activated NF-κB cascade responses, which
effectively inhibited the proliferation and growth of HCC in
vivo and promoted the apoptosis of HCC cells by initiating the
death-receptor pathway of cell apoptosis. Such an effect on the
TLR3 by BM-06 was observed to be greater than that of poly(I:C). A
greater understanding of the target specific and immunostimulatory
function of designed dsRNA as a therapeutic candidate is required
for the future development of dsRNA-based treatments for HCC.
Acknowledgements
This study was supported by funding from the
Production-Study-Research Prospective Joint Research Programs of
Jiangsu (BY2013042-06), the Priority Academic Program Development
of Jiangsu Higher Education Institutions, the Foundation of the
Ministry of Health, Jiangsu, P.R. China (grant no. H201052), the
Science Foundation of Nantong City, Jiangsu, P.R. China (grant nos.
K2009060 and S2010018) and the Advanced Project of Nantong
University. The authors would like to thank Biomics Biotechnologies
Co., Ltd., (Jiangsu, China) for aiding with the synthesis of the
dsRNA.
References
|
1
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nerenstone SR, Ihde DC and Friedman MA:
Clinical trials in primary hepatocellular carcinoma: current status
and future directions. Cancer Treat Rev. 15:1–31. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mathurin P, Rixe O, Carbonell N, Bernard
B, Cluzel P, Bellin MF, Khayat D, Opolon P and Poynard T: Review
article: Overview of medical treatments in unresectable
hepatocellular carcinoma - an impossible meta-analysis? (Review)
Aliment Pharmacol Ther. 12:111–126. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
5
|
Kawano Y, Sasaki A, Kai S, Endo Y, Iwaki
K, Uchida H, Shibata K, Ohta M and Kitano S: Prognosis of patients
with intrahepatic recurrence after hepatic resection for
hepatocellular carcinoma: a retrospective study. Eur J Surg Oncol.
35:174–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamoto M, Sato S, Hemmi H, Hoshino K,
Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K and
Akira S: Role of adaptor TRIF in the MyD88-independent toll-like
receptor signaling pathway. Science. 301:640–643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alexopoulou L, Holt AC, Medzhitov R and
Flavell RA: Recognition of double-stranded RNA and activation of
NFkappaB by Toll-like receptor 3. Nature. 413:732–738. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heylbroeck C, Balachandran S, Servant MJ,
DeLuca C, Barber GN, Lin R and Hiscott J: The IRF-3 transcription
factor mediates Sendai virus-induced apoptosis. J Virol.
74:3781–3792. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salaun B, Coste I, Rissoan MC, Lebecque SJ
and Renno T: TLR3 can directly trigger apoptosis in human cancer
cells. J Immunol. 176:4894–4901. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Salaun B, Lebecque S, Matikainen S,
Rimoldi D and Romero P: Toll-like receptor 3 expressed by melanoma
cells as a target for therapy? Clin Cancer Res. 13:4565–4574. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qun E, Li Chen, Jin Guo Hua, et al:
portfolio tool of paraffin tissue microarray[Z]. CN201402229.
Nantong university; 2010
|
|
12
|
Qun E, Li Chen and Qiu Kai E: preparation
methods of paraffin tissue microarray[Z]. CN101319971. Nantong
university; 2008
|
|
13
|
Khvalevsky E, Rivkin L, Rachmilewitz J,
Galun E and Giladi H: TLR3 signaling in a hepatoma cell line is
skewed towards apoptosis. J Cell Biochem. 100:1301–1312. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chua CW and Choo SP: Targeted therapy in
hepatocellular carcinoma. Int J Hepatol. 2011:3482972011.PubMed/NCBI
|
|
15
|
Bergé M, Bonnin P, Sulpice E, Vilar J,
Allanic D, Silvestre JS, Lévy BI, Tucker GC, Tobelem G and
Merkulova-Rainon T: Small interfering RNAs induce
target-independent inhibition of tumor growth and vasculature
remodeling in a mouse model of hepatocellular carcinoma. Am J
Pathol. 177:3192–3201. 2010.PubMed/NCBI
|
|
16
|
Zorde-Khvalevsky E, Abramovitch R, Barash
H, Spivak-Pohis I, Rivkin L, Rachmilewitz J, Galun E and Giladi H:
Toll-like receptor 3 signaling attenuates liver regeneration.
Hepatology. 50:198–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferreon JC, Ferreon AC, Li K and Lemon SM:
Molecular determinants of TRIF proteolysis mediated by the
hepatitis C virus NS3/4A protease. J Biol Chem. 280:20483–20492.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kleinman ME, Yamada K, Takeda A,
Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S,
Itaya M, Pan Y, et al: Sequence- and target-independent
angiogenesis suppression by siRNA via TLR3. Nature. 452:591–597.
2008. View Article : Google Scholar : PubMed/NCBI
|