Introduction
Disuse osteoporosis is defined as localized or
generalized bone loss induced by a reduction of mechanical loading
on the skeleton (1). Since
physical activity is one of the important factors required to
maintain an adequate bone remodeling rate and bone mass (2,3),
prolonged removal of mechanical stimuli on bone cells may lead to
osteoporosis (4,5).
Microgravity is one of the main causes of disuse
osteoporosis. The development of disuse osteoporosis among
astronauts during spaceflight is well known (6,7). One
study showed that astronauts experienced 1–2% loss of bone mass
each month in the microgravity environment and this resulted in
osteopenia (8). Another study also
reported 6.8 and 7.8% of bone mineral density (BMD) loss at the
trochanter and femoral neck regions, respectively, in cosmonauts
and astronauts following flights (9).
Another possible factor related to osteoporosis is
low body weight. Mechanical loading on the skeleton is reduced when
body weight decreases. Low body weight has been found to be
associated with a high prevalence of osteoporosis (10,11).
A recent publication reported that men who were underweight or
normal weight have a higher chance of osteoporosis and a higher
fracture risk than those who were overweight or obese (12).
The tail-suspension (TS) rat is a new animal model
for studying the biological mechanisms, treatment or prevention of
disuse osteoporosis. This model has been used in studies relating
to microgravity, since it mimics certain aspects of exposure to
microgravity by inducing a state of weightlessness on the
hindquarters of the animal, thereby producing a cephalic fluid
shift (13). Effects of disuse on
the unloaded hindlimb bones have also been well observed (14,15).
Therefore, this animal model may be utilized to study bone changes
associated with disuse, weightlessness or low body weight. This new
model provides a good alternative for the study of osteoporosis,
which uses ovariectomized female rats as the standard in
vivo model.
Estrogen is important in the adaptive response of
bones to load. It may lower the threshold of bone sensitivity to
mechanical loads (16) and was
suggested to have an amplifying effect on the osteogenic response
to strain (17). Estrogen receptor
α has also been shown to be involved in the response of bone cells
to mechanical strain (18). To
avoid the adverse effects of estrogen, synthetic pharmaceutical
agents resembling estrogen, selected estrogen receptor modulators
(SERMs), were created to prevent bone loss due to disuse.
Raloxifene is one of the SERMs commonly used to tackle
postmenopausal osteoporosis (19,20).
In this study, we investigated whether raloxifene
exhibited an osteoprotective effect on osteoporotic bone induced by
the state of weightlessness in the tail-suspension rat model. At
the same time, bone strength, which is dependent on both the
material and micro-architectural properties of bone, was
studied.
Materials and methods
Animals and grouping
In total, 46 male and 18 female 3-month-old
Sprague-Dawley rats (weighing 446.0±38.4 g and 225.2±15.4 g,
respectively) were obtained from the Laboratory of Animal Services
Centre of the Chinese University of Hong Kong. The rats were housed
in a temperature- (25°C) and light-controlled (12-h light/dark
cycle) environment. After seven days of acclimatization, the male
rats were divided into six groups: tail-suspension control (TS)
group, non-tail suspension control (Non-TS) group, tail-suspension
with high-, medium- or low-dose raloxifene hydrochloride (Evista,
Eli Lilly, Indianapolis, IN, USA) (TS-RH, TS-RM and TS-RL,
respectively) groups or the alendronate (Fosamax, Merck Sharp &
Dohme, Whitehouse Station, NJ, USA) (TS-A) group as the positive
control. The female rats were divided into two groups: ovariectomy
(OVX) and sham control (Sham). At least six rats were included in
each group.
In the Non-TS group, rats were allowed to move
freely (without hindlimb unloading). In the TS, TS-RH, TS-RM and
TS-RL groups, the tails of the rats were suspended in order that
their hind limbs were unloaded. Using a gastric tube, rats in the
TS-RH group received raloxifene at a dose of 2.5 mg/kg/day orally
while those in the TS-RM and TS-RL groups received raloxifene at a
dose of 1.25 and 0.625 mg/kg/day, respectively. Rats in the TS-A
group received alendronate at a dose of 1.0 mg/kg/day. The dose of
the TS-RM and TS-A groups was designed based on the human clinical
dose, which is ~1.0 and 0.17 mg/kg/day, respectively. The drugs
were dissolved in distilled water. Rats in the TS and Non-TS groups
received distilled water vehicle (2.0 ml/day). Rats in the OVX
group were ovariectomized bilaterally, while those in the Sham
group underwent sham surgery. Sham and OVX groups were non-tail
suspended.
The animals were allowed access to standard rodent
chow and water ad libitum throughout the study. At the end
of the study, rats were euthanized by an overdose of ketamine and
xylazine cocktail intravenously. Body weights of the animals were
recorded at the beginning and end of the study. Animal ethics
approval was obtained from the Animal Experimental Ethics Committee
of the Chinese University of Hong Kong (ref no. 07/052/MIS) for
this study.
Tail-suspension model
The protocol of the tail-suspension was modified
from that of Morey-Holton and Globus (21). The tail of each rat was suspended
by applying an adhesive tape on its lateral surfaces and then
secured by three surgical tapes applied circularly. The loop of the
adhesive tape at the tip of the tail passed through a metallic
hollow column, which was then connected to a free rotating hook.
The hook was hung on an overhead bar. As a result, the rat was
maintained in an ~35° head-down tilt with its hind limbs unloaded,
while its forelimbs could be used for locomotion. The overall
suspension period was 28 days. This new model of hindlimb bone
osteoporosis was to be compared with the conventional ovariectomy
model. Ovariectomy was performed under general anesthesia in the
usual manner. A sham-operation group was created as a control.
Study of bone structure
Peripheral quantitative computed
tomography (pQCT)
Changes in BMD at the distal femoral metaphysis and
proximal tibial metaphysis of the rat were studied using pQCT
(XCT2000, Stratec Medizintechnik GmbH, Pforzheim, Germany). Quality
assurance of measurements was confirmed by using the hydroxyapatite
cone and standard phantoms prior to the scanning of the rats each
time. The rat was fixed on a custom-made translucent plastic holder
to ensure repeatable positioning. The right distal femur and
proximal tibia were scanned at a voxel resolution of 0.2 mm. The
analytical parameters for trabecular BMD were set as threshold 280
mg/cm3, contour mode 1 and peel mode 20. The parameters
for cortical BMD were set as threshold 551 mg/cm3 and
peel mode 2. The trabecular bone region was defended by setting an
inner area to 35% of the total cross-sectional area.
Micro-computed tomography
(micro-CT)
The micro-architecture of the left distal femur was
analyzed using a micro-CT (MicroCT 40, Scanco Medical, Bassersdorf,
Switzerland). The scanning was conducted at 55 kVp and 144 μA with
a resolution of 16 μm per voxel. Segmentation parameters were:
Sigma, 0.5; support, 1.0; and threshold, 245. The
micro-architectural parameters, including bone volume density
(BV/TV), connectivity density (Conn.D.), structure model index
(SMI), trabecular number (Tb.N), trabecular thickness (Tb.Th) and
trabecular plate separation (Tb.Sp), were obtained.
Biomechanical test
The right femur of the rat was harvested for a
three-point bending test using a Hounsfield material testing
machine (KM25, Redhill, United Kingdom) with a 250 N load-cell. The
span of the lower supports was 20 mm. The mid-shaft of the bone was
loaded at a constant speed of 5 mm/min in a medial-lateral approach
until failure. Loads at yield, maximum (ultimate) and break
(failure) were recorded for analysis. Young’s modulus (stiffness)
was calculated as the steepest slope of the elastic region of the
strength-displacement curve of the test.
Statistical analysis
The differences between the TS and Non-TS groups,
and the OVX and Sham groups were analyzed by the Student’s t-test.
Percentage differences of BMD between different time intervals in
each group and comparisons of BMD, micro-architectural and
biomechanical parameters between drug treatment groups and the TS
group were tested using one-way ANOVA, followed by the post-hoc
Dunnett’s test. There was no direct statistical comparison between
the TS and OVX groups on micro-architectural and biomechanical
parameters, since the two groups consisted of different genders and
had different bone sizes. Statistical analyses were performed by
using the Statistical Package of Social Science (SPSS) version 16.0
for Windows and were carried out at the 5% level of significance
(P<0.05). Data are expressed as the means ± standard error of
mean (SEM).
Results
Body weight
There was no difference in body weight among the
various tail suspension groups. All the animals were observed
without any adverse effects throughout the study.
Validation of the tail-suspension rat
model
Changes of BMD
Femoral and tibial BMD was significantly reduced in
total and in the trabecular regions in both the OVX and TS groups
at day 14 and 28 (P<0.05 for femoral total and tibial trabecular
of the TS group at day 14, P<0.01 for others) (Table I). The mean percentage difference
of total and trabecular BMD of the two groups was similar at each
time point. Moreover, the region with the most BMD loss of the two
groups was the trabecular region. For the cortical BMD, there was
no significant difference from baseline in both the OVX and TS
groups at the proximal tibia. The cross-sectional comparison showed
that the mean percentage difference of BMD at all three regions of
the OVX and TS groups was significantly lower than that in the Sham
and Non-TS groups, respectively, at days 14 and 28 (Table I).
 | Table IMeans of percentage difference from
baseline ± standard error of mean of the bone mineral density at
different regions of interest (ROI) in rats assessed using
peripheral quantitative computed tomography. |
Table I
Means of percentage difference from
baseline ± standard error of mean of the bone mineral density at
different regions of interest (ROI) in rats assessed using
peripheral quantitative computed tomography.
| Body part | ROI | Days | Sham | OVX | Non-TS | TS |
|---|
| Distal femur | Total | 14 | −0.72±0.55 | −10.04±1.04b,d | 0.67±1.07 | −8.08±0.85a,f |
| 28 | 1.17±0.84 | −10.90±1.50b,d | 3.02±1.46 | −13.19±1.46b,f |
| Trabecular | 14 | −0.35±0.71 | −13.32±1.51b,d | 0.16±1.66 | −15.74±1.46b,f |
| 28 | 1.16±1.48 | −15.38±2.38b,d | 2.47±2.22 | −24.46±1.84b,f |
| Cortical | 14 | −0.51±0.38 | −4.66±0.45b,d | 1.54±0.47 | −0.98±0.52f |
| 28 | 0.92±0.56 | −5.24±0.78b,d | 3.30±0.63 | −1.82±0.51f |
| Proximal tibia | Total | 14 | 1.65±0.80 | −9.77±1.29b,d | 2.42±1.72 | −11.64±1.23b,f |
| 28 | 2.87±1.02 | −16.40±1.54b,d | 5.08±2.01 | −13.57±1.59b,f |
| Trabecular | 14 | 0.49±2.14 | −21.35±2.11b,d | 1.39±3.37 | −16.28±3.57a,e |
| 28 | 0.45±1.83 | −36.21±2.35b,d | −2.76±5.38 | −26.72±3.44b,e |
| Cortical | 14 | 1.60±0.54 | −0.45±0.72c | 3.21±0.69 | −3.15±0.74f |
| 28 | 3.24±0.41 | −1.26±0.86d | 6.07±0.85 | −1.80±1.40f |
Differences in micro-architecture
BV/TV was reduced significantly in the OVX (−18.0%)
and TS (−26.7%) groups when compared with the Sham and Non-TS
groups, respectively (P<0.05 for both) (Table II). A significant increase in SMI
was also observed when the OVX group was compared with the Sham
group, as well as when the TS group was compared with the Non-TS
group. Tb.N and Tb.Th were reduced in the OVX and TS groups,
although a significant difference was found in Tb.Th of the TS
group only. Tb.Sp was found to be increased slightly in the OVX and
TS groups, but a significant difference was observed in the TS
group only.
 | Table IIMeans ± standard error of mean and
percentage difference of mean (%) of the structural indices of
different groups of rats assessed using micro-computed
tomography. |
Table II
Means ± standard error of mean and
percentage difference of mean (%) of the structural indices of
different groups of rats assessed using micro-computed
tomography.
| Structural
index | Sham | OVX | % | Non-TS | TS | % |
|---|
| BV/TV (1) | 0.32±0.01 | 0.26±0.02a | −18.0 | 0.33±0.01 | 0.24±0.01b | −26.7 |
| Conn.D.
(1/mm3) | 87.68±5.17 | 80.34±6.06 | −8.4 | 57.82±2.89 | 62.78±2.65 | 8.6 |
| SMI (1) | 0.28±0.15 | 0.91±0.16a | 220.8 | 0.38±0.08 | 1.00±0.13b | 160.3 |
| Tb.N (1/mm) | 4.49±0.06 | 3.76±0.34 | −16.2 | 3.76±0.05 | 3.54±0.07 | −5.8 |
| Tb.Th (mm) | 0.08±0.002 | 0.08±0.004 | −5.9 | 0.10±0.003 | 0.08±0.002b | −17.4 |
| Tb.Sp (mm) | 0.22±0.004 | 0.24±0.01 | 6.0 | 0.25±0.005 | 0.28±0.01b | 12.0 |
Effects of raloxifene or alendronate on
tail-suspension-induced disuse osteoporosis
Changes of BMD
Distal femur
Total BMD was lost significantly only in the TS-RL
group at days 14 and 28 (P<0.05 and P<0.01, respectively)
(Fig. 1A). When compared with the
TS group horizontally, the mean percentage difference of total BMD
was significantly lower in other drug-treated groups at day 28
(P<0.001 for the TS-RH and TS-RM groups; P<0.05 for the TS-RL
group; P<0.01 for the TS-A group).
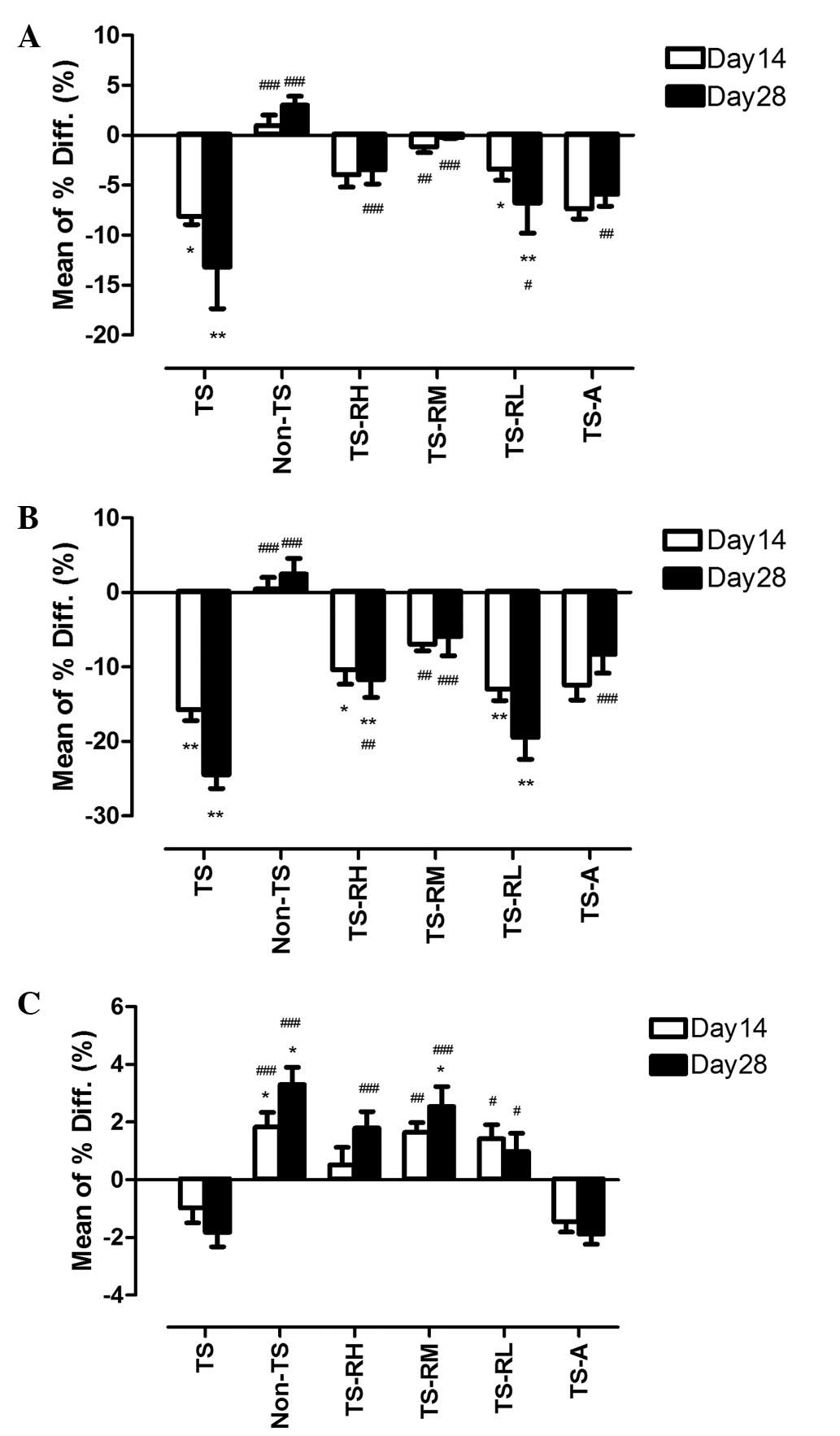 | Figure 1Mean percentage differences of bone
mineral density (BMD) in the distal femur between the baseline and
days 14 and 28, respectively. The error bar presents the standard
error of the mean. (A) Total BMD. (B) Trabecular BMD. (C) Cortical
BMD. TS, tail-suspension; Non-TS, non-tail suspension; TS-RH,
tail-suspension with high-dose raloxifene; TS-RM, tail-suspension
with medium-dose raloxifene; TS-RL, tail-suspension with low-dose
raloxifene; TS-A, tail-suspension with alendronate.
*P<0.05, **P<0.01 (day 14 and 28 vs.
baseline of the same group). #P<0.05,
##P<0.01, ###P<0.001 (vs. TS group at
the same time point). |
In the trabecular region, there was no significant
reduction of BMD in the TS-RM and TS-A groups throughout the study
(Fig. 1B). However, the BMD was
reduced significantly in the TS-RH (P<0.05 and P<0.01 for
days 14 and 28, respectively) and TS-RL (P<0.01 for both time
points) groups compared with baseline. A cross-sectional comparison
with the TS group showed that the mean percentage difference of the
TS-RH, TS-RM and TS-A groups was significantly lower than the TS
group at day 28 (P<0.01 for TS-RH group, P<0.001 for both
TS-RM and TS-A groups). The same observation was made at day 14 in
the TS-RM group (P<0.01).
The cortical BMD of drug-treated groups was either
unchanged or increased slightly when compared with baseline
(Fig. 1C). The mean percentage
difference of all raloxifene-treated groups, with the exception of
the TS-RH group at day 14, was significantly higher than the TS
group.
The cross-sectional comparison showed that there was
no significant difference in BMD in the three regions when the
raloxifene-treated groups were compared with the TS-A group.
Proximal tibia
Total tibial BMD was reduced significantly in the TS
group (P<0.01 for both time points), but not in the TS-RM and
TS-A groups, throughout the experimental period (Fig. 2A). No significant decrease was
observed in the TS-RH group at day 28 and in the TS-RL group at day
14. At day 28, the mean percentage difference of the TS-RH and
TS-RM groups was lower than that of the TS group (P<0.05 and
P<0.001, respectively).
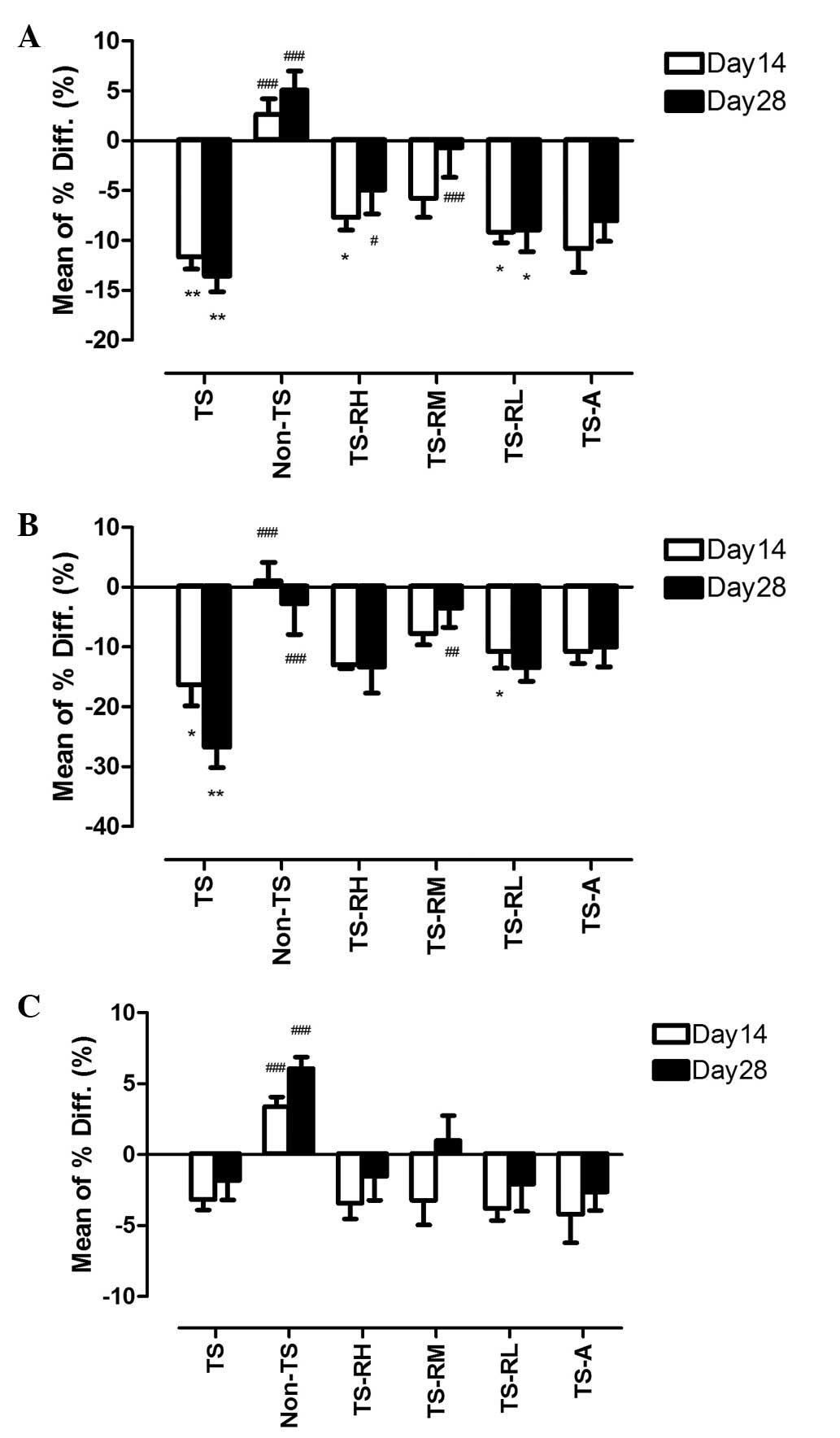 | Figure 2Mean percentage differences of bone
mineral density (BMD) in the proximal tibia between the baseline
and days 14 and 28, respectively. The error bar presents the
standard error of mean. (A) Total BMD. (B) Trabecular BMD. (C)
Cortical BMD. TS, tail-suspension; Non-TS, non-tail suspension;
TS-RH, tail-suspension with high-dose raloxifene; TS-RM,
tail-suspension with medium-dose raloxifene; TS-RL, tail-suspension
with low-dose raloxifene; TS-A, tail-suspension with alendronate.
*P<0.05, **P<0.01, (day 14 and 28 vs.
baseline of the same group). #P<0.05,
##P<0.01, ###P<0.001 (vs. TS group at
the same time point). |
For the trabecular region, no significant BMD loss
was observed in TS-RH, TS-RM and TS-A groups throughout the
tail-suspension period (Fig. 2B).
The mean percentage difference of the TS-RM group was significantly
lower than the TS group at day 28. No significant change of tibial
cortical BMD from baseline was found throughout the study in all
the drug-treated groups (Fig.
2C).
Similar to the distal femur, no significant
difference was observed in BMD in the three regions when
raloxifene-treated groups were compared with the TS-A group
horizontally in the proximal tibia.
Differences in micro-architecture
Loss of BV/TV and Tb.Th was mitigated significantly
when the tail-suspended rats were treated with high- or medium-dose
raloxifene. These two parameters in the TS-RH and TS-RM groups were
significantly higher than those of the TS groups (Table III). The reduction of Tb.N was
also retarded when raloxifene was administered. Tb.N was
significantly higher in the TS-RM and TS-RL groups than that in the
TS group (Table III). Tb.Sp was
found to be similar to normal levels (0.25 mm as in the Non-TS
group, Table II) with raloxifene
administration. A significant difference of Tb.Sp was found when
the TS-RH group was compared with the TS group (p<0.05)
(Table III). The differences in
Tb.Sp between the TS-RM and TS groups, as well as the S-RL and TS
groups, were marginally significant (p=0.097 and 0.083,
respectively).
 | Table IIIMeans ± standard error of mean
(percentage difference of mean from TS group) of the structural
indices of different groups of rats assessed using micro-computed
tomography. |
Table III
Means ± standard error of mean
(percentage difference of mean from TS group) of the structural
indices of different groups of rats assessed using micro-computed
tomography.
| Group |
|---|
|
|
|---|
| Structural
index | TS-RH | TS-RM | TS-RL | TS-A |
|---|
| BV/TV (1) | 0.30±0.02a (22.7%) | 0.30±0.01a (24.3%) | 0.29±0.01
(18.8%) | 0.31±0.01b (25.1%) |
| Conn.D.
(1/mm3) | 62.28±4.29
(−0.8%) | 78.07±6.38
(24.4%) | 70.17±8.74
(11.8%) | 54.66±2.06
(−12.9%) |
| SMI (1) | 0.74±0.14
(−26.1%) | 0.76±0.13
(−24.5%) | 0.83±0.07
(−16.8%) | 0.39±0.13b (−60.7%) |
| Tb.N (1/mm) | 3.73±0.10
(5.2%) | 4.17±0.12b (17.7%) | 3.99±0.19a (12.7%) | 3.60±0.07
(1.7%) |
| Tb.Th (mm) | 0.10±0.005a (13.6%) | 0.10±0.001b (15.6%) | 0.09±0.003
(8.9%) | 0.09±0.001a (11.5%) |
| Tb.Sp (mm) | 0.25±0.01a (−10.2%) | 0.25±0.01
(−8.9%) | 0.25±0.01
(−9.3%) | 0.26±0.01
(−5.8%) |
In the TS-A group, significant differences in BV/TV,
SMI and Tb.Th were observed when compared with the TS group
(Table III). Nonetheless, there
was no statistical difference in any of the micro-architectural
parameters when the TS-A group was compared with the three
raloxifene-treated groups.
Biomechanical properties of bone assessed by the
three-point bending test
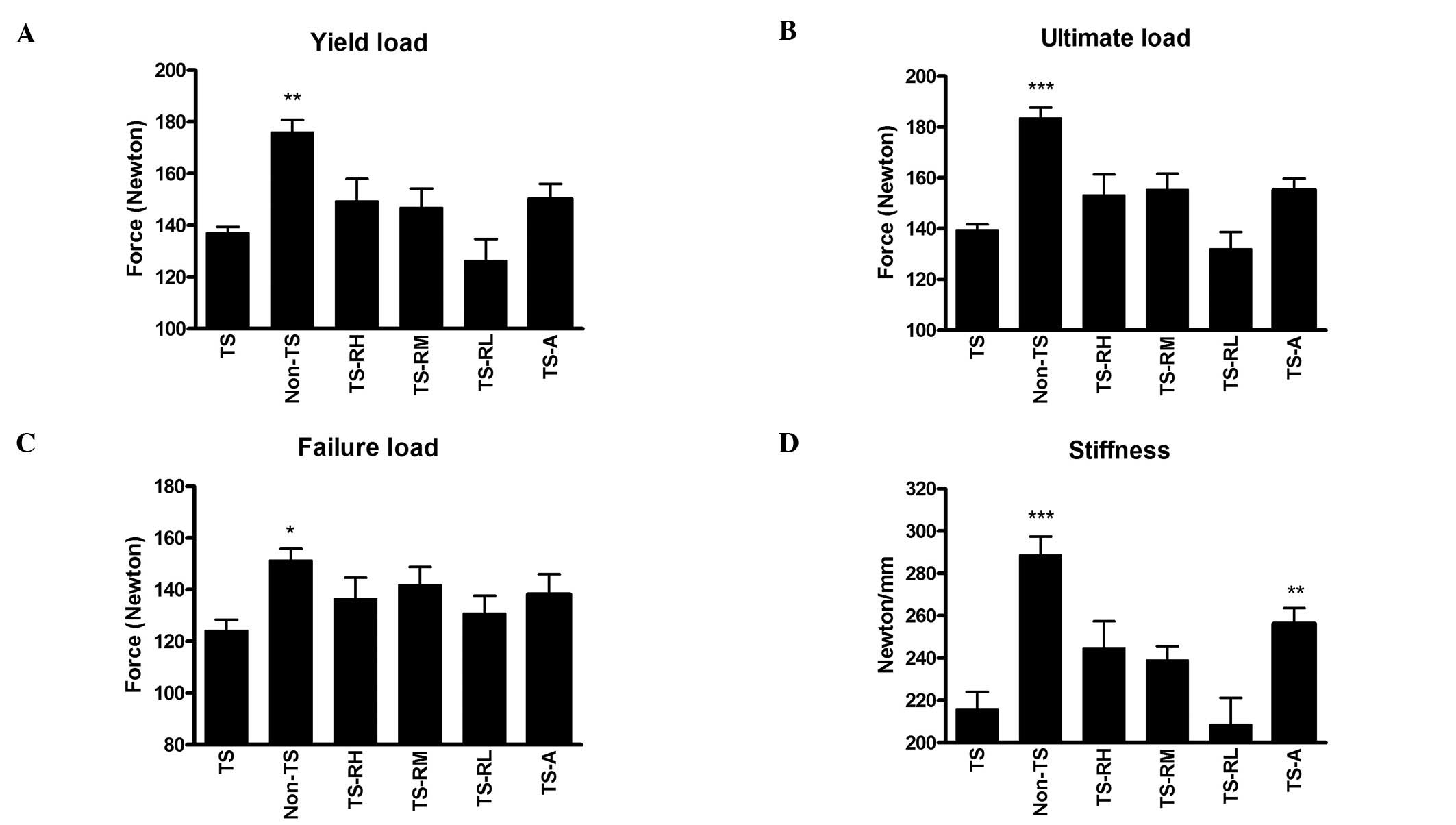
Losses of biomechanical properties were observed in
the femoral diaphysis of the TS group. Yield load, ultimate load
and failure load in the Non-TS group were significantly higher than
those in the TS group (Fig. 3A–C).
Raloxifene treatment at high- and medium-dose appeared to retard
the loss of these biomechanical properties; however, this was not
statistically significant. Stiffness of the femur was also reduced
significantly after tail-suspension (−24.8% when the TS group was
compared with the Non-TS group, P<0.001) (Fig. 3D). However, treatment with
alendronate prevented the reduction of stiffness and a significant
18.1% higher stiffness was observed when the TS-A group was
compared with the TS group (P<0.01). Similarly, raloxifene at
high- and medium-doses showed, although insignificantly, higher
stiffness values than those found in the TS group. There was no
significant difference in the four biomechanical parameters when
the raloxifene-treated groups were compared with TS-A group.
Discussion
In order to confirm that the TS rat is a valid
animal model for the study of osteoporosis and is comparable to the
OVX rat model, a group of OVX rats was also included in this study.
The bone material, micro-architectural and biomechanical properties
were compared. The changes of BMD in the OVX and TS groups were
found to be comparable. The percentage loss of total or trabecular
BMD in the femur and tibia of the TS group was similar to that of
the OVX group in the 28-day experiment. The region where the
highest reduction of BMD occurred was the trabecular region. Higher
percentage bone loss was observed in the trabecular region than in
the cortical region in the femur and tibia of the two groups.
Similar findings were observed by Bloomfield et al(14). The authors reported that cancellous
BMD at the proximal tibia and femoral neck of their rats was
significantly lowered by 21 and 20%, respectively, after 28 days of
hindlimb unloading. Our results were also consistent with the
observation that cosmonauts experience selective loss of cancellous
bone mineral density after 6 months of spaceflight (8). In the micro-architectural analysis,
almost all parameters of the two groups were similar at the end of
the study. This observation was similar to those obtained in a
reported study, in which a micro-CT was used to evaluate the bone
architecture of immobilized osteoporotic rats (22). From the biomechanical point of
view, the bone strengths, including yield, ultimate and failure
load of the TS and OVX groups were lower than those of the other
groups. These findings revealed that, from the bone material,
micro-architectural and biomechanical perspectives, the effects of
weightlessness on weight-bearing bones are comparable to those of
ovariectomy in experimental rats. This new model is particularly
suitable for the study of bone changes in male animals, although
orchidectomy has been used as a valid model in previous studies
(23,24).
The most frequently tested drugs in pharmaceutical
studies using TS rats are bisphosphonates. An 80% decrease in bone
mass was fully prevented by high-dose tiludronate after 23 days of
treatment in hindlimb unloaded rats (25). However, concerns have been raised
about potential over-suppression of bone turnover during the
long-term use of bisphosphonates. There is increasing evidence
showing that subtrochanteric or proximal diaphyseal femoral
fracture is related to long-term bisphosphonate use (26,27).
Jaw osteonecrosis has also been found to be associated with
bisphosphonate therapy in recent years (28,29).
Risedronate was also reported as being unable to overcome the
intense stimulus for osteoclast recruitment in long-term disuse
osteoporosis (30).
The effect of raloxifene on disuse osteoporosis has
been demonstrated in the present study. Reduction of BMD after
tail-suspension in the raloxifene-treated groups was lower than
that in the TS group. Raloxifene at 1.25 mg/kg/day (TS-RM) was the
most effective among the three doses tested. It significantly
protected the femur and tibia of the rats against bone
deterioration within 28 days of tail-suspension. The protection was
found not only in the trabecular bone, but also in the cortical
bone at the metaphysis. Results showed that there was no
significant reduction in total, trabecular and cortical BMD in both
the distal femur and proximal tibia of the TS-RM group. The
micro-architectural properties of the trabecular bone on the distal
femur were also improved by raloxifene at this dosage. Bone volume
density, trabecular number and trabecular thickness in the TS-RM
group were significantly higher than those in the TS group. These
results indicated that raloxifene at 1.25 mg/kg/day protected the
trabeculae against degradation in an environment lacking in
biomechanical stimulation. Improvements in material (BMD) and
micro-architectural properties of bone by raloxifene resulted in a
better bone quality, which was revealed by the biomechanical test.
The TS-RM group sustained a higher yield, ultimate and fracture
load than the TS group, although such differences were not
statistically significant. Raloxifene at 2.5 mg/kg/day (TS-RH) also
showed a preventive effect against bone loss. There was no
significant BMD reduction observed at the whole proximal tibia and
at the total and cortical regions of the femur in this group,
although a significant reduction of trabecular BMD was observed at
the distal femur.
These results suggested that raloxifene, although it
is a SERM, showed an osteoprotective effect in male animals with
disuse osteoporosis. Its pharmaceutical effects on the prevention
of bone loss in the state of weightlessness were comparable to
those of alendronate. However, the molecular mechanism by which
alendronate tackles osteoporosis development is different from that
of raloxifene (31,32). This aspect should be confirmed by
histomorphometry or gene expression in future studies.
The Food and Drug Administration (FDA) has approved
three SERMs for clinical use thus far, and only raloxifene has been
approved for the prevention and treatment of postmenopausal
osteoporosis (33). However, there
remains a lack of studies revealing the efficacy of raloxifene on
disuse osteoporosis. Using this tail-suspension rat model, the
in-depth in vivo mechanisms by which raloxifene tackle
disuse osteoporosis are due to be further investigated by means of,
for instance, analyses of serum bone markers, histomorphometry,
immunohistochemistry and observation of gene expression.
To the best of our knowledge, the present study has
for the first time, demonstrated the preventive effects of
raloxifene against bone loss in disuse osteoporosis using the
tail-suspension rat model. With the administration of raloxifene,
deterioration of both material and micro-architectural properties
of unloaded trabecular bone were reduced significantly. The
reduction of bone strength, however, is alleviated out after 28
days of treatment of disuse osteoporosis. This new animal model,
suitable for male and female animals, could be conveniently used in
future studies of therapeutic agents against osteoporosis.
Acknowledgements
The authors would like to acknowledge Ming Lai
Foundation and The International Association of Lions Clubs
District 303 - Hong Kong and Macau Tam Wah Ching Chinese Medicine
Resource Centre for their support to our Institute.
References
|
1
|
Takata S and Yasui N: Disuse osteoporosis.
J Med Invest. 48:147–156. 2001.
|
|
2
|
Karlsson MK, Nordqvist A and Karlsson C:
Physical activity increases bone mass during growth. Food Nutr Res.
52:2008. View Article : Google Scholar
|
|
3
|
Wilsgaard T, Emaus N, Ahmed LA, Grimnes G,
Joakimsen RM, Omsland TK and Berntsen GR: Lifestyle impact on
lifetime bone loss in women and men: the Tromso Study. Am J
Epidemiol. 169:877–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ehrlich PJ and Lanyon LE: Mechanical
strain and bone cell function: a review. Osteoporos Int.
13:688–700. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bikle DD, Sakata T and Halloran BP: The
impact of skeletal unloading on bone formation. Gravit Space Biol
Bull. 6:45–54. 2003.
|
|
6
|
Tilton FE, Degioanni JJ and Schneider VS:
Long-term follow-up of Skylab bone demineralization. Aviat Space
Environ Med. 51:1209–1213. 1980.PubMed/NCBI
|
|
7
|
Smith SM, Wastney ME, O’Brien KO, Morukov
BV, Larina IM, Abrams SA, Davis-Street JE, Oganov V and Shackelford
LC: Bone markers, calcium metabolism, and calcium kinetics during
extended-duration space flight on the mir space station. J Bone
Miner Res. 20:208–218. 2005. View Article : Google Scholar
|
|
8
|
Vico L, Collet P, Guignandon A,
Lafage-Proust MH, Thomas T, Rehaillia M and Alexandre C: Effects of
long-term microgravity exposure on cancellous and cortical
weight-bearing bones of cosmonauts. Lancet. 355:1607–1611. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sibonga JD, Evans HJ, Sung HG, Spector ER,
Lang TF, Oganov VS, Bakulin AV, Shackelford LC and LeBlanc AD:
Recovery of spaceflight-induced bone loss: bone mineral density
after long-duration missions as fitted with an exponential
function. Bone. 41:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Michaëlsson K, Bergström R, Mallmin H,
Holmberg L, Wolk A and Ljunghall S: Screening for osteopenia and
osteoporosis: selection by body composition. Osteoporos Int.
6:120–126. 1996.PubMed/NCBI
|
|
11
|
Nayak S, Roberts MS and Greenspan SL:
Factors associated with diagnosis and treatment of osteoporosis in
older adults. Osteoporos Int. 20:1963–1967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nelson RE, Nebeker JR, Sauer BC and
LaFleur J: Factors associated with screening or treatment
initiation among male United States veterans at risk for
osteoporosis fracture. Bone. 50:983–988. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morey-Holton ER and Globus RK: Hindlimb
unloading of growing rats: a model for predicting skeletal changes
during space flight. Bone. 22:S83–S88. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bloomfield SA, Allen MR, Hogan HA and Delp
MD: Site- and compartment-specific changes in bone with hindlimb
unloading in mature adult rats. Bone. 31:149–157. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimano MM and Volpon JB: Biomechanics and
structural adaptations of the rat femur after hindlimb suspension
and treadmill running. Braz J Med Biol Res. 42:330–338. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saxon LK, Robling AG, Castillo AB, Mohan S
and Turner CH: The skeletal responsiveness to mechanical loading is
enhanced in mice with a null mutation in estrogen receptor-beta. Am
J Physiol Endocrinol Metab. 293:E484–E491. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lanyon LE: Using functional loading to
influence bone mass and architecture: objectives, mechanisms, and
relationship with estrogen of the mechanically adaptive process in
bone. Bone. 18:37S–43S. 1996. View Article : Google Scholar
|
|
18
|
Ehrlich PJ, Noble BS, Jessop HL, Stevens
HY, Mosley JR and Lanyon LE: The effect of in vivo mechanical
loading on estrogen receptor alpha expression in rat ulnar
osteocytes. J Bone Miner Res. 17:1646–1655. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Licata AA, Ciaccia AV, Wong M and Draper
MW: Raloxifene: a new choice for treating and preventing
osteoporosis. Cleve Clin J Med. 67:273–280. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morii H, Ohashi Y, Taketani Y, Fukunaga M,
Nakamura T, Itabashi A, Sarkar S and Harper K: Effect of raloxifene
on bone mineral density and biochemical markers of bone turnover in
Japanese postmenopausal women with osteoporosis: results from a
randomized placebo-controlled trial. Osteoporos Int. 14:793–800.
2003. View Article : Google Scholar
|
|
21
|
Morey-Holton ER and Globus RK: Hindlimb
unloading rodent model: technical aspects. J Appl Physiol.
92:1367–1377. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Laib A, Barou O, Vico L, Lafage-Proust MH,
Alexandre C and Rügsegger P: 3D micro-computed tomography of
trabecular and cortical bone architecture with application to a rat
model of immobilisation osteoporosis. Med Biol Eng Comput.
38:326–332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fitts JM, Klein RM and Powers CA:
Comparison of tamoxifen and testosterone propionate in male rats:
differential prevention of orchidectomy effects on sex organs, bone
mass, growth, and the growth hormone-IGF-I axis. J Androl.
25:523–534. 2004.PubMed/NCBI
|
|
24
|
Urasopon N, Hamada Y, Asaoka K,
Cherdshewasart W and Malaivijitnond S: Pueraria mirifica, a
phytoestrogen-rich herb, prevents bone loss in orchidectomized
rats. Maturitas. 56:322–331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barou O, Lafage-Proust MH, Martel C,
Thomas T, Tirode F, Laroche N, Barbier A, Alexandre C and Vico L:
Bisphosphonate effects in rat unloaded hindlimb bone loss model:
three-dimensional microcomputed tomographic, histomorphometric, and
densitometric analyses. J Pharmacol Exp Ther. 291:321–328.
1999.
|
|
26
|
Neviaser AS, Lane JM, Lenart BA,
Edobor-Osula F and Lorich DG: Low-energy femoral shaft fractures
associated with alendronate use. J Orthop Trauma. 22:346–350. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abrahamsen B, Eiken P and Eastell R:
Subtrochanteric and diaphyseal femur fractures in patients treated
with alendronate: A register-based national cohort study. J Bone
Miner Res. 24:1095–1102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Migliorati CA, Siegel MA and Elting LS:
Bisphosphonate-associated osteonecrosis: a long-term complication
of bisphosphonate treatment. Lancet Oncol. 7:508–514. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vieillard MH, Maes JM, Penel G, Facon T,
Magro L, Bonneterre J and Cortet B: Thirteen cases of jaw
osteonecrosis in patients on bisphosphonate therapy. Joint Bone
Spine. 75:34–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li CY, Price C, Delisser K, Nasser P,
Laudier D, Clement M, Jepsen KJ and Schaffler MB: Long-term disuse
osteoporosis seems less sensitive to bisphosphonate treatment than
other osteoporosis. J Bone Miner Res. 20:117–124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heringa M: Review on raloxifene: profile
of a selective estrogen receptor modulator. Int J Clin Pharmacol
Ther. 41:331–345. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Russell RG, Watts NB, Ebetino FH and
Rogers MJ: Mechanisms of action of bisphosphonates: similarities
and differences and their potential influence on clinical efficacy.
Osteoporos Int. 19:733–759. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Riggs BL and Hartmann LC: Selective
estrogen-receptor modulators – mechanisms of action and application
to clinical practice. N Engl J Med. 348:618–629. 2003.
|