Introduction
Common myeloid progenitors (CMPs), which derive from
hematopoietic stem cells, are capable of differentiating into
immature myeloid cells (IMCs). In pathological conditions,
including cancer, various infectious diseases, sepsis, trauma, bone
marrow (BM) transplantation and some autoimmune disorders, a
partial inhibition of the differentiation of IMCs into mature
myeloid cells results in the expansion of this population.
Importantly, the activation of these cells in a pathological
context results in the upregulated expression of several immune
suppressive factors, including arginase, inducible nitric oxide
synthase (iNOS), nitric oxide (NO) and reactive oxygen species
(ROS). Together, these factors lead to the expansion of the IMC
population which possesses immunosuppressive activity. These cells
are collectively known as myeloid-derived suppressor cells (MDSCs)
(1).
Dendritic cells (DCs) and MDSCs perform different
functions in the immune response. DCs are important in initiating
the innate and adaptive immune responses (2,3). DCs
are also capable of inducing a T-cell immune response by presenting
the foreign antigen, upregulating costimulatory molecules and
releasing proinflammatory cytokines following microbial or
inflammatory activation (4,5). The
immune response is regulated by various cell types, including
Foxp3+ and IL-10-producing regulatory T cells (6), regulatory DCs (7,8) and
MDSCs. MDSCs have been demonstrated to regulate the immune response
through arginase, iNOS (9,10), ROS (11) and the induction of
Foxp3+ regulatory cells (12,13).
The development and expansion of MDSCs is associated with cancer,
infection and autoimmunity (1).
Lipopolysaccharide (LPS), the ligand of Toll-like
receptor 4, is able to either induce the maturation of DCs
(4) or expand the
Gr1+CD11b+ cell population in the spleen
during polymicrobial sepsis (14).
Also, our previous study demonstrated that BM precursor cells are
capable of differentiating into MDSCs when co-cultured with poly
(I:C) (15).
However, whether other conserved structural patterns
of microbial components, including CpG oligodeoxynucleotide (CpG
ODN) possess a similar ability to induce MDSCs has yet to be
reported. In the present study, we used CpG ODN to investigate the
balance between the development of MDSCs and DCs from the same BM
precursor cells. We observed that sustained stimulation with CpG
ODN leads to the development and expansion of MDSCs and inhibition
of DC development.
Materials and methods
Mice
Male and female wild-type C57BL/6 mice, 5–6 weeks of
age, were purchased from the Chinese Academy of Sciences (Shanghai,
China). DO11.10 OVA323–339-specific TCR-transgenic mice
with a C57BL/6 background were obtained from The Jackson Laboratory
(Bar Harbor, ME, USA). Mice were housed in a specific pathogen-free
facility for all experiments. All animal experiments were
undertaken in accordance with the National Institutes of Health
‘Guide for the Care and Use of Laboratory Animals’ (NIH Publication
no. 85-23, National Academy Press, Washington, DC, revised 1996),
with approval by the Laboratory Animal Center and Ethics Committee
of the Second Military Medical University (Shanghai, China).
Reagents
Recombinant mouse granulocyte-monocyte
colony-stimulating factor (GM-CSF), interleukin 4 (IL-4) and ELISA
kits for murine interleukin 1 (IL-1), interleukin 6 (IL-6),
interleukin 12p40 (IL-12p40), matrix metalloproteinase 9 (MMP-9),
tumor necrosis factor α(TNF-α), IFN-γ and transforming growth
factor β (TGF-β) were purchased from R&D Systems (Minneapolis,
MN, USA). Fluorescein-conjugated monoclonal antibodies (mAbs) to
CD4, CD11b, Iab, CD40, CD80, CD86 and isotype control were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Fluorescein-conjugated mAbs to Gr1 were obtained from
eBioscience (San Diego, CA, USA). 7-Aminoactinomycin D (7-AAD), LPS
and BSA were purchased from Sigma-Aldrich (St. Louis, MO, USA).
CpG-A DNA (ODN 2216) was purchased from HyCult Biotech (Uden, The
Netherlands).
Preparation of DCs from mouse BM
precursor cells and pre-treated DCs
BM mononuclear cells were prepared from mouse (5–6
weeks old) femur BM suspensions by depletion of red blood cells.
The cells were cultured at a density of 2×106 cells/ml
in 6-well plates in RPMI-1640 medium supplemented with 10% fetal
calf serum (FCS), 10 ng/ml of recombinant mouse GM-CSF and 1 ng/ml
of recombinant mouse IL-4. Nonadherent cells were gently washed out
on day 4 of culture. On day 5, the dendritic-proliferating clusters
were collected and purified by anti-CD11c microbeads as immature
DCs (imDCs). ImDCs were stimulated with LPS (100 ng/ml) for a
further 2 days and then collected as mature DCs (mDCs). CpG ODN (6
μg/ml) was added to the BM-DC culture system (1×106) on
day 0 (long stimulation) or on day 5 (short stimulation) (16).
Analysis of phagocytic ability
Cells from different groups were incubated at 37°C
for 4 h with FITC-conjugated OVA to a final concentration of 100
μg/ml in RMPI-1640 medium containing 10% FCS, and were washed twice
with ice-cold PBS (pH 7.2) containing 0.1% NaN3 and 0.5%
BSA. The cells were resuspended in chilled PBS for immediate
analysis by flow cytometry.
Assay for cytokines and NO
The aforementioned cytokines in the supernatant of
the DC system were assayed with the corresponding ELISA kits. NO
production was assayed by measurement of the nitrite concentration
using the Griess assay.
Assay for antigen (Ag)-specific
CD4+ T-cell response
In order to assay the Ag-specific CD4+
T-cell response, splenic CD4+ T cells from DO11.10
OVA323–339-specific TCR-transgenic mice were obtained.
Cells were positively selected with anti-CD4-coated microbeads
(Miltenyi Biotech, Minneapolis, MN, USA) by magnetic-activated cell
sorting (MACS) and then co-cultured with treated DCs as indicated
in the presence of OVA323–339 peptide at a ratio of 1:10
(DC:T) in round-bottom 96-well plates (1×105 T
cells/200μl/well) for 5 days. The proliferation of T cells was
analyzed by double staining with anti-CD4+ and 7-AAD
cells were counted by fluorescence-activated cell sorting
(FACS).
Listeria monocytogenes (L. monocytogenes)
and experimental infection of mice
L. monocytogenes strain 10403s was purchased
from American Type Culture Collection (Manassas, VA, USA) and the
bacterial stocks were stored in aliquots that were maintained at
−70°C. The titers were checked for each experiment to confirm the
number of viable injected bacteria. The level of infection in the
various experimental groups was determined by enumerating the L.
monocytogenes CFU in the liver and spleen as described
previously (17). In order to
confirm whether sustained L. monocytogenes infection was
capable of causing the accumulation of MDSCs, 10 wild-type C57BL/6
mice were challenged i.v. with a dose of 103 CFU of L.
monocytogenes strain 10403s. Mice were monitored daily and
euthanized 1 or 5 days later. Following isolation of the spleen and
depletion of red blood cells, cells were labeled with anti-CD11c,
anti-CD11b and anti-Gr1 mAbs for flow cytometry analysis.
Statistical analysis
Comparisons between the experimental groups and
relevant control were performed by ANOVA. P<0.05 was considered
to indicate a statistically significant difference.
Results
BM precursor cells demonstrate a distinct
phenotype and cytokine profile following long stimulation with CpG
ODN
Mouse BM-derived DCs were generated by culturing
cells in GM-CSF and IL-4 (7,18–20).
In the standard DC-induced protocol, on day 0, BM precursor cells
were washed from the femurs of mice and then were co-cultured with
GM-CSF and IL-4 for 5 days. On day 5, the dendritic-proliferating
clusters were collected and purified by anti-CD11c microbeads as
imDCs. In this study, CpG ODN was added to the BM-DC culture system
on day 0 (long stimulation) or on day 5 (short stimulation). On day
6, the phenotype and function of cells were analyzed. We observed
that cells in the long stimulation group (L-group) demonstrated a
distinct morphology with fewer cell colonies (Fig. 1A). We additionally observed that
cells in the L-group exhibited a distinct phenotype with lower
expression levels of Iab, CD40, CD80 and CD86, and cells in the
short stimulation group (S-group) exhibited higher expression
levels of these costimulated molecules (Fig. 1B). We then harvested the day 6
cells from each group separately. Cells were washed in PBS 3 times
and were then seeded in a 24-well plate. Supernatants from each
well were collected for cytokine and NO analysis 24 h later (day
7). Compared with the cytokine profile of the S-group and control,
cells in the L-group secreted lower levels of IL-1β, IL-6,
IL-12p40, MMP-9 and TNF-α, and higher levels of IFN-γ, IL-10, TGF-β
and NO (Fig. 1C). Altogether,
these data demonstrate that long stimulation with CpG ODN may exert
a strong effect on the development of DCs from BM cells.
 | Figure 1CpG ODN induced the differentiation of
bone marrow precursor cells into a number of different cell types.
(A) Distinct morphology of cells from each group. Bone marrow
mononuclear cells were prepared from mouse (5–6 weeks old) femur
bone marrow suspensions by depletion of red blood cells, and cells
from the same mouse were then divided equally into 3 groups for
further treatment. The long stimulation group was cultured with
GM-CSF, IL-4 and CpG ODN (6 μg/ml) on day 0; the short stimulation
group was cultured with GM-CSF and IL-4 on day 0, and CpG ODN (6
μg/ml) was added on day 5; the control group was cultured with
GM-CSF and IL-4 on day 0, without CpG ODN. The cells were then
observed under a microscope (magnification ×100 or ×400) on day 6.
Following long stimulation, the cells demonstrated a distinct
morphology with fewer cell colonies. (B) The phenotype of cells
from each group. Cells from the control, short stimulation and long
stimulation groups were labeled with Ab to Iab, CD40, CD80 and CD86
for phenotypic analysis by flow cytometry. Numbers in histograms
indicate the geometric mean fluorescence intensity. (C) Different
cytokine profiles and NO expression levels of each group. On day 6,
cells from each group were collected and washed in PBS 3 times, and
then were seeded in a 24-well plate at a density of
1.0×106 ml/well. Supernatants from each well were
collected 24 h later (day 7) for analysis of cytokine and NO
production. IL-1β, IL-6, IL-12p40, MMP-9, TNF-α, INF-γ, IL-10,
TGF-β and NO produced by cells from each group were measured by
ELISA or Griess assay for 24 h. Results are presented as the mean ±
SD of triplicate wells. *P<0.05. CpG ODN, CpG
oligodeoxynucleotide; GM-CSF, granulocyte-macrophage
colony-stimulating factor; NO, nitric oxide; MMP, matrix
metalloproteinase. |
Phagocytic ability and stimulation of
T-cell proliferation
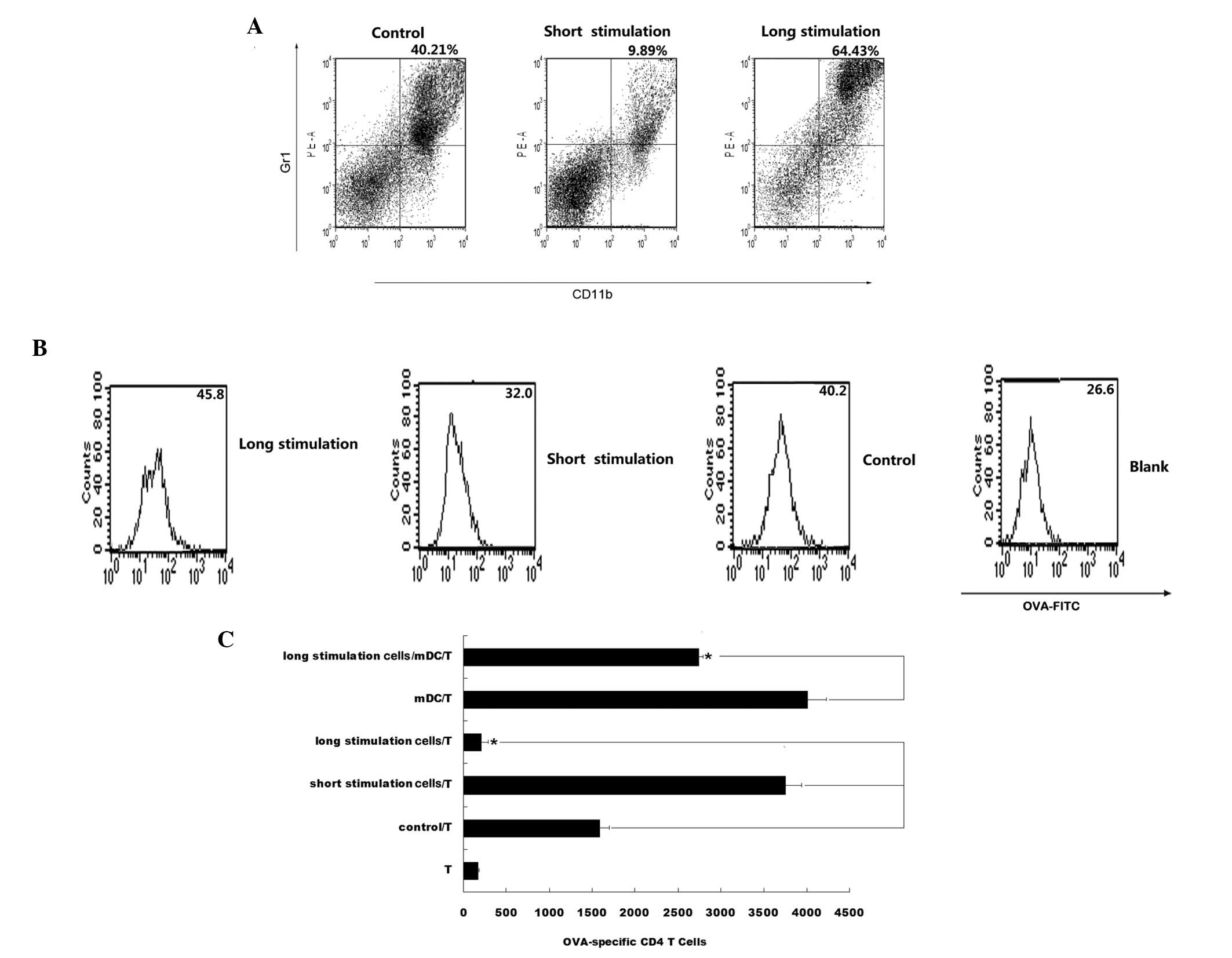
Since cells in the CpG ODN L-group demonstrated
higher expression levels of CD11b, and secreted higher levels of
IL-10, TGF-β and NO (Fig. 1C), the
phenotypes of cells from all groups were analyzed. An increase in
the number of Gr1+CD11b+ cells in the CpG ODN
L-group was observed (Fig. 2A).
Therefore, we hypothesize that cells in the L-group may have
transformed into MDSCs as a result of interaction with CpG ODN. The
phagocytic ability of cells in each group was additionally analyzed
and it was observed that cells in the L-group exhibited an enhanced
phagocytic ability (Fig. 2B). The
ability of DCs to stimulate an antigen-specific T-cell response was
also examined. Cells in the L-group demonstrated a reduced ability
to stimulate the proliferation of OVA-specific CD4+ T
cells compared with the S-group and control. Significantly, when
cells of the L-group were added to the mDCs/CD4+ T-cell
co-culture system, in vitro T-cell proliferation was
partially suppressed (Fig. 2C). As
the long stimulation cells exhibited a
Gr1+CD11b+ phenotype and the ability to
suppress the proliferation of T cells, we hypothesize that the
Gr1+CD11b+ cells that accumulate in the
L-group may be MDSCs.
Sustained L. monocytogenes infection
causes the accumulation of MDSCs
CpG ODN is a short single-stranded synthetic DNA and
CpG motif present in bacterial DNA and an example of a
pathogen-associated molecular pattern (PAMP) (21–24).
We examined whether sustained bacterial infection in vivo is
capable of leading to the accumulation of MDSCs. Previous studies
revealed that Listeria infection elicits a similar spectrum of
cytokine production to CpG ODN (25,26).
Thus, we infected C57BL/6 mice with L. monocytogenes for 1
or 5 days. We observed that L. monocytogenes (Listeria)
infection may upregulate the percentage of DCs and downregulate the
percentage of MDSCs in the spleen due to a 1-day infection, and may
downregulate the percentage of DCs and upregulate the percentage of
MDSCs in the spleen due to a 5-day infection (Fig. 3). These results indicate that a
prolonged infection may lead to an increase in the number of MDSCs
and a decrease in the number of DCs, which is similar to the result
induced by CpG ODN.
 | Figure 3Sustained L. monocytogenes
infection induced the proliferation of MDSCs and inhibited the
expansion of DCs in vivo. Ten C57BL/6 mice, 8–10 weeks of
age, were challenged i.v. with a dose of 103 CFU of L.
monocytogenes strain 10403s. Mice were monitored daily. After 1
or 5 days, the spleen was isolated for DC and MDSC phenotype
analysis. Following isolation of the spleen and depletion of red
blood cells, cells were labeled with anti-CD11c, anti-CD11b and
anti-Gr1 mAbs for flow cytometry analysis. CD11c-positive cells
were counted as DCs; Gr1 and CD11b double-positive cells were
counted as MDSCs. The percentage of DCs and MDSCs were calculated
by FACS software. (A) Percentage of DCs in the spleen on day 1 and
5. (B) Percentage of MDSCs in the spleen on day 1 and 5. Results
were presented as the mean ± SD of 10 mice. *P<0.05.
MDSCs, myeloid-derived suppressor cells; DCs, dendritic cells;
L. monocytogenes, Listeria monocytogenes; mAbs,
monoclonal antibodies; FACS, fluorescence-activated cell sorting;
VSV, vesicular stomatitis virus. |
Discussion
The accumulation of MDSCs is observed in numerous
pathological conditions, including cancer, bacterial infection,
acute and chronic inflammation, traumatic stress, surgical sepsis
and transplantation (1). In these
diverse types of pathological conditions, various factors are
involved, including cyclooxygenase-2 (COX2), prostaglandins
(27–29), stem-cell factor (SCF) (27), macrophage colony-stimulating factor
(M-CSF), IL-6 (30), GM-CSF
(29) and vascular endothelial
growth factor (VEGF) (31).
In our previous study, we added poly (I:C) into the
standard DC-generated system whereby DCs were generated by
co-culturing with GM-CSF and IL-4 (7,18–20).
Following 5 days of stimulation, we observed the accumulation of
MDSCs (15). In the present study,
we observed similar phenomena following long stimulation with CpG
ODN. Previous studies (14) and
our data (not shown) also indicated that LPS is capable of
expanding the Gr1+CD11b+ cell population and
inhibiting DC development. It is useful to compare each of these
three PAMPs, as these three TLR ligands are capable of expanding
the Gr1+CD11b+ cell population and inhibiting
DC development. Treatment of myeloid precursor cells with LPS led
to a partial activation as indicated by NO release that allows a
transient suppressive activity. It has been demonstrated that
combined LPS/IFN-γ signaling, as opposed to its components alone,
is capable of activating myeloid precursor cells to differentiate
into functionally suppressive MDSCs, which impairs their
developmental potential into DCs (32). Our data suggests that poly (I:C)
and CpG ODN induce myeloid precursor cells to differentiate into
MDSCs, and the difference between poly (I:C) and CpG ODN is that a
short and long stimulation of CpG ODN induces the production of
high levels of IFN-γ. In the present study, CpG ODN class A was
used, which exhibits potent immunostimulatory effects on
plasmacytoid DCs (pDCs), and IFN-γ is its key cytokine. In adult
mice, pDCs are produced constantly in the BM and migrate from the
BM to lymph nodes, mucosal-associated lymphoid tissues and spleen
in steady-state conditions. Furthermore, CMPs are capable of
differentiating into mDCs and pDCs in culture and in
vivo(33,34). Based on the evidence above, we
hypothesize that some pDCs may have mixed differentiation; however,
further investigation is required.
There are at least three factors in our culture
system: IL-4, GM-CSF and CpG ODN. GM-CSF is an essential growth
factor in the maturation of DCs. GM-CSF is important in the
negative regulation of the immune response through the induction of
myeloid suppressor Gr1+CD11b+ cells (29,35).
Although the exact role of GM-CSF has yet to be identified, the
system with or without CpG ODN may lead to different scenarios
following long stimulation with CpG ODN, which stimulates the
development and expansion of MDSCs.
A systemic expansion of MDSCs was observed in
infection-like polymicrobial sepsis in mice (14), infection with helminths (36–38)
and Candida albicans(39).
We also observed the accumulation of MDSCs and decreased levels of
DCs in the spleen following sustained infection with L.
monocytogenes. It has been reported that CpG ODN may also
induce resistance to L. monocytogenes infection following a
single injection of CpG ODN (26),
which is consistent with our data.
In conclusion, we demonstrated that BM precursor
cells differentiate into MDSCs and not DCs following long
stimulation with CpG ODN and sustained L. monocytogenes
infection. Our data may provide novel insights into the mechanism
of MDSC development during infection.
Acknowledgements
The authors appreciate the assistance of Dr Xuetao
Cao from the National Key Laboratory of Medical Immunology and Dr
Bing Yu from The Department of Cell Biology. The study was
supported by grants from the National Natural Science Foundation of
China (nos. 30872313 and 30271232)
References
|
1
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steinman RM: The dendritic cell system and
its role in immunogenicity. Annu Rev Immunol. 9:271–296. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steinman RM and Banchereau J: Taking
dendritic cells into medicine. Nature. 449:419–426. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar
|
|
5
|
Steinman RM and Nussenzweig MC: Avoiding
horror autotoxicus: the importance of dendritic cells in peripheral
T cell tolerance. Proc Natl Acad Sci USA. 99:351–358. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Littman DR and Rudensky AY: Th17 and
regulatory T cells in mediating and restraining inflammation. Cell.
140:845–858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang M, Tang H, Guo Z, et al: Splenic
stroma drives mature dendritic cells to differentiate into
regulatory dendritic cells. Nat Immunol. 5:1124–1133. 2004.
View Article : Google Scholar
|
|
8
|
Liu Q, Zhang C, Sun A, Zheng Y, Wang L and
Cao X: Tumor-educated CD11bhighIalow regulatory dendritic cells
suppress T cell response through arginase I. J Immunol.
182:6207–6216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bronte V and Zanovello P: Regulation of
immune responses by L-arginine metabolism. Nat Rev Immunol.
5:641–654. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodríguez PC and Ochoa AC: Arginine
regulation by myeloid derived suppressor cells and tolerance in
cancer: mechanisms and therapeutic perspectives. Immunol Rev.
222:180–191. 2008.PubMed/NCBI
|
|
11
|
Kusmartsev S, Nefedova Y, Yoder D and
Gabrilovich DI: Antigen-specific inhibition of CD8+ T cell response
by immature myeloid cells in cancer is mediated by reactive oxygen
species. J Immunol. 172:989–999. 2004.
|
|
12
|
Yang R, Cai Z, Zhang Y, Yutzy WH 4th, Roby
KF and Roden RB: CD80 in immune suppression by mouse ovarian
carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res.
66:6807–6815. 2006.PubMed/NCBI
|
|
13
|
Huang B, Pan PY, Li Q, et al: Gr-1+CD115+
immature myeloid suppressor cells mediate the development of
tumor-induced T regulatory cells and T-cell anergy in tumor-bearing
host. Cancer Res. 66:1123–1131. 2006.
|
|
14
|
Delano MJ, Scumpia PO, Weinstein JS, et
al: MyD88-dependent expansion of an immature GR-1(+)CD11b(+)
population induces T cell suppression and Th2 polarization in
sepsis. J Exp Med. 204:1463–1474. 2007.PubMed/NCBI
|
|
15
|
Liu C, Zhang C, Lu H, et al: Poly(I:C)
induce bone marrow precursor cells into myeloid-derived suppressor
cells. Mol Cell Biochem. 358:317–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsujimoto H, Efron PA, Matsumoto T, et al:
Maturation of murine bone marrow-derived dendritic cells with
poly(I:C) produces altered TLR-9 expression and response to CpG
DNA. Immunol Lett. 107:155–162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harty JT and Bevan MJ: Specific immunity
to Listeria monocytogenes in the absence of IFN gamma. Immunity.
3:109–117. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang H, Guo Z, Zhang M, Wang J, Chen G and
Cao X: Endothelial stroma programs hematopoietic stem cells to
differentiate into regulatory dendritic cells through IL-10. Blood.
108:1189–1197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia S, Guo Z, Xu X, Yi H, Wang Q and Cao
X: Hepatic microenvironment programs hematopoietic progenitor
differentiation into regulatory dendritic cells, maintaining liver
tolerance. Blood. 112:3175–3185. 2008. View Article : Google Scholar
|
|
20
|
Li Q, Guo Z, Xu X, Xia S and Cao X:
Pulmonary stromal cells induce the generation of regulatory DC
attenuating T-cell-mediated lung inflammation. Eur J Immunol.
38:2751–2761. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamamoto S, Yamamoto T, Kataoka T,
Kuramoto E, Yano O and Tokunaga T: Unique palindromic sequences in
synthetic oligonucleotides are required to induce IFN [correction
of INF] and augment IFN-mediated [correction of INF] natural killer
activity. J Immunol. 148:4072–4076. 1992.PubMed/NCBI
|
|
22
|
Krieg AM, Yi AK, Matson S, et al: CpG
motifs in bacterial DNA trigger direct B-cell activation. Nature.
374:546–549. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klinman DM, Yi AK, Beaucage SL, Conover J
and Krieg AM: CpG motifs present in bacteria DNA rapidly induce
lymphocytes to secrete interleukin 6, interleukin 12, and
interferon gamma. Proc Natl Acad Sci USA. 93:2879–2883. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hemmi H, Takeuchi O, Kawai T, et al: A
Toll-like receptor recognizes bacterial DNA. Nature. 408:740–745.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harty JT, Lenz LL and Bevan MJ: Primary
and secondary immune responses to Listeria monocytogenes. Curr Opin
Immunol. 8:526–530. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Krieg AM, Love-Homan L, Yi AK and Harty
JT: CpG DNA induces sustained IL-12 expression in vivo and
resistance to Listeria monocytogenes challenge. J Immunol.
161:2428–2434. 1998.PubMed/NCBI
|
|
27
|
Pan PY, Wang GX, Yin B, et al: Reversion
of immune tolerance in advanced malignancy: modulation of
myeloid-derived suppressor cell development by blockade of
stem-cell factor function. Blood. 111:219–228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sinha P, Clements VK, Fulton AM and
Ostrand-Rosenberg S: Prostaglandin E2 promotes tumor progression by
inducing myeloid-derived suppressor cells. Cancer Res.
67:4507–4513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Serafini P, Carbley R, Noonan KA, Tan G,
Bronte V and Borrello I: High-dose granulocyte-macrophage
colony-stimulating factor-producing vaccines impair the immune
response through the recruitment of myeloid suppressor cells.
Cancer Res. 64:6337–6343. 2004. View Article : Google Scholar
|
|
30
|
Bunt SK, Yang L, Sinha P, Clements VK,
Leips J and Ostrand-Rosenberg S: Reduced inflammation in the tumor
microenvironment delays the accumulation of myeloid-derived
suppressor cells and limits tumor progression. Cancer Res.
67:10019–10026. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gabrilovich D, Ishida T, Oyama T, et al:
Vascular endothelial growth factor inhibits the development of
dendritic cells and dramatically affects the differentiation of
multiple hematopoietic lineages in vivo. Blood. 92:4150–4166.
1998.
|
|
32
|
Greifenberg V, Ribechini E, Rössner S and
Lutz MB: Myeloid-derived suppressor cell activation by combined LPS
and IFN-gamma treatment impairs DC development. Eur J Immunol.
39:2865–2876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
D’Amico A and Wu L: The early progenitors
of mouse dendritic cells and plasmacytoid predendritic cells are
within the bone marrow hemopoietic precursors expressing Flt3. J
Exp Med. 198:293–303. 2003.
|
|
34
|
Karsunky H, Merad M, Cozzio A, Weissman IL
and Manz MG: Flt3 ligand regulates dendritic cell development from
Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic
cells in vivo. J Exp Med. 198:305–313. 2003.
|
|
35
|
Bronte V, Apolloni E, Cabrelle A, et al:
Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor
capable of activating or suppressing CD8(+) T cells. Blood.
96:3838–3846. 2000.
|
|
36
|
Terrazas LI, Walsh KL, Piskorska D,
McGuire E and Harn DA Jr: The schistosome oligosaccharide
lacto-N-neotetraose expands Gr1(+) cells that secrete
anti-inflammatory cytokines and inhibit proliferation of naive
CD4(+) cells: a potential mechanism for immune polarization in
helminth infections. J Immunol. 167:5294–5303. 2001.PubMed/NCBI
|
|
37
|
Gómez-García L, López-Marín LM, Saavedra
R, Reyes JL, Rodríguez-Sosa M and Terrazas LI: Intact glycans from
cestode antigens are involved in innate activation of myeloid
suppressor cells. Parasite Immunol. 27:395–405. 2005.
|
|
38
|
Brys L, Beschin A, Raes G, et al: Reactive
oxygen species and 12/15-lipoxygenase contribute to the
antiproliferative capacity of alternatively activated myeloid cells
elicited during helminth infection. J Immunol. 174:6095–6104. 2005.
View Article : Google Scholar
|
|
39
|
Mencacci A, Montagnoli C, Bacci A, et al:
CD80+Gr-1+ myeloid cells inhibit development of antifungal Th1
immunity in mice with candidiasis. J Immunol. 169:3180–3190.
2002.
|